Abstract
Background
Many indigenous communities reside in biodiverse environments replete with natural food sources but show poor access and utilization.
Methods
To understand the links between indigenous food access, dietary intakes, and biomarkers, we conducted a cross-sectional study among women of the Santhal Community (n = 211) from 17 villages in the Godda district of Jharkhand, India. Survey methods included household surveys, dietary intake assessment (24 HDR) and micronutrient and inflammatory biomarkers' estimation.
Results
The diversity in access to foods from different natural sources expressed as Food access diversity index was low. This led to poor consumption and thus a low Minimum Dietary Diversity. The mean nutrient intake was less than the estimated average requirement for all nutrients. Women with higher dietary diversity scores had higher nutrient intakes. Thiamine and calcium intakes were significantly higher in women consuming indigenous foods than non-consumers. One-fourth of the women had elevated levels of inflammatory biomarkers. The prevalence of iron deficiency was approximately 70%. Vitamin A insufficiency (measured as retinol-binding protein) was observed in around 33.6% women, while 28.4% were deficient. Household access to natural food sources was associated with specific biomarkers. The access to kitchen garden (baari) was positively associated with retinol-binding protein levels and negatively with inflammatory biomarkers, while access to ponds was positively associated with ferritin levels.
Conclusion
The findings highlight the role of access to diverse natural foods resources, including indigenous foods, for improving nutrition security in indigenous communities. Nutrition and health programs promoting indigenous food sources should include the assessment of biomarkers for effective monitoring and surveillance.
Similar content being viewed by others
Background
Micronutrient deficiency or hidden hunger is a crucial global health issue affecting more than 2 billion people worldwide [1]. It has many adverse health outcomes, including poor physical and mental development in children and loss of productivity and human potential [2]. Among the developing nations, the simultaneous manifestation of undernutrition, overnutrition, and micronutrient deficiency, also known as the triple burden of malnutrition, continues at high levels, and the progress in tackling the same has been unacceptably slow [3]. It leads to a vicious cycle of underdevelopment [4], which disproportionately affects vulnerable populations consisting of young children, adolescent girls, pregnant and lactating women, economically marginalized people, and indigenous populations [5, 6]. The current rates of anemia in India are alarming with the prevalence being 57% in women of reproductive age (WRA) and 67.1% in children 6 to 59 months [7]. If the current trends continue, India will have an excess of 22·8% anemia prevalence among the WRA group relative to WHO and UNICEF target of 2030, reiterating the need for more significant improvement rates [8].
India is home to 104 million indigenous people spread across 705 indigenous communities, constituting 8.6% of the country's population. These are designated as "Scheduled Tribes" by article 342 of the Indian Constitution [9]. Compared to their non-indigenous counterparts, the nutritional status of the indigenous population is significantly poorer. The prevalence of chronic energy deficiency (CED) and anemia in WRA are higher among the indigenous population (31.7% and 59.9%, respectively) as compared to the corresponding national Figs. (23% and 53.1%, respectively) [10]. This high prevalence of malnutrition is even though indigenous people hold guardianship of 80% of the global biodiversity and possess traditional ecological knowledgeFootnote 1 (TEK) about their natural food environment,Footnote 2 consisting of several indigenous foods (IFs), which are potentially rich sources of nutrition [11,12,13]. Indigenous foods are derived from plants, animals and fungi species, occurring in a particular geographic place accessed as part of traditional food systems from the natural food environment i.e., wild and cultivated food environments [14]. The rich TEK about the natural food environment can contribute to dietary diversity and can effectively address malnutrition [15].
Many programs for the development and protection of indigenous people exist globally. Likewise, in India, development of indigenous people has been at the center stage of every 5-year plan by the government. However, a wide gap exists in achieving desired nutrition and health indicators [16] owing to multiple factors such as food insecurity due to uncertainty of food supply; primitive agricultural practices; seasonal fluctuations and climatic disruptions; changing employment patterns and geographical remoteness [6, 17]. Other factors include socioeconomic vulnerability, poor household conditions, and inadequate public health infrastructure [16, 18].
National surveys play a crucial role in informing, designing, and implementing sound public health programs and monitoring the progress of ongoing programs, however these data sources rely primarily on self-reported dietary intake data [19]. More recently, the Comprehensive National Nutritional Survey (CNNS) 2016–18 provided robust nationally representative data by assessing biomarkers for micronutrient deficiencies and non-communicable diseases [19]. However, other than the CNNS, only a few studies have reported information on nutritional biomarkers in India owing to technical and logistic limitations such as special needs for specimen collection, handling, transport, and inadequate local infrastructure for testing. In addition, biomarker surveys have low participation rate due to the invasive nature of sampling, making them less feasible in large-scale nutrition surveys [20, 21].
Biomarkers of nutritional intake objectively and precisely quantify nutritional status and are not subject to measurement errors from recall bias and misreporting. They can be measured in multiple tissue types and help to validate traditional dietary assessment methods [20, 22]. Hence combining dietary biomarker data with self-reported data can strengthen the link between diet and disease and has potential to assist in the precise assessment of dietary intake [23, 24].
Jharkhand, a central-eastern state of India, is one of the most biodiverse regions having a large indigenous population constituting 26.3 percent of the state's total population. In Jharkhand, Santhal is the most populous indigenous community representing 34 percent of the total ST population, and is spread over vast areas of Giridih, Dumka, Purbi Singhbhum, Pakaur, and Sahibganj districts. The majority of the Santhals in Jharkhand are cultivators (48.6%) and agricultural laborers (38.4%) [25]. Many studies have documented a high prevalence of CED, undernutrition, and anemia among WRA, adult men, and children in the Santhal community residing in Jharkhand, Bihar, Orissa, and West Bengal [26,27,28,29]. Our previous studies have also indicated that Santhals have access to a biodiverse agroforestry environment consisting of natural food environment (forests, ponds/rivers, open fields) and cultivated food environment (agricultural land, kitchen gardens) consisting of around 103 diverse types of indigenous foods including a number of green leafy vegetables, other vegetables, fruits and meat and meat products [30].
The work described in this paper is a part of a larger project that examined the contribution of IF consumption towards dietary diversity and food security among WRA and children of indigenous communities of Jharkhand. This paper reports the iron and vitamin A levels along with the status of inflammation among the Santhal WRA group using selected biomarkers. Further, it explores the associations of biomarker levels with dietary intake, with a special emphasis on indigenous food intake and dietary diversity; nutritional intake; sociodemographic and economic factors; and access to different food sources.
Methodology
The present study is part of a larger project, with the comprehensive description and methodological approaches reported elsewhere [31]. In the present paper, we report the micronutrient and inflammatory biomarker status of the Santhal women and explore their association with the dietary, indigenous food, nutrient intake, and nutritional status in the context of their socioeconomic and demographic profile and food environment characteristics.
Study design and locale
This exploratory cross-sectional study was conducted in July–August 2018 in seventeen selected villages of Godda district of Jharkhand. The population of Santhal community in Godda district was 224,068 as per the census conducted in 2011 [32].
Sampling framework and study population
A two-stage cluster sampling was used wherein blocks (an administrative subdivision of a district) were purposively chosen (Sundarpahari, Boarijor, Pathargama, and Poriyahat), followed by random selection of a total of 17 villages (with a high concentration of Santhal population) using probability proportional to size (PPS) sampling from the selected blocks. This was followed by the second stage, in which a house listing of Santhal households (HH) was carried out, and a sampling frame of all eligible HHs was constructed. Based on the larger objective of the project, all HHs with at least one non-pregnant WRA group (15–49 years) and one child (6 to 54 months) were selected for the study. This paper reports the observations on all eligible women who fulfilled the inclusion criteria (age 18–49 years, not pregnant at the time of the study, had no self-reported morbidity that affected current dietary intake, and gave consent for participation). If more than one eligible woman was present in a HH, one woman was randomly selected using a Kish table [33].
Sample size calculation
The sample size for the present study is based on the objective of the larger study, which aimed at assessing the differences in micronutrient intake between IF consumers and IF non-consumers. The present study, however, did not analyze the data on the basis of IF consumers and non-consumers. Iron intake data from our previous study on same indigenous community [34] was used for sample size calculation based on the difference in mean dietary intake of iron of 4 mg/day (between the consumer of IFs and non-consumers) with a standard deviation of 7 mg/day between the two groups. In order to detect a suggested minimum difference in iron intake (80% power and 5% level of significance), a minimum of 97 women in each group was required. A design effect of 2.0 was considered to account for the loss in precision due to cluster sampling and deviation from normality. A sample size of 194 women was derived, and all the eligible HHs identified during HH listing were covered (n = 327). The HH survey could be completed in 275 HHs, a dietary survey in 266 women, and blood sampling could be completed in 238 women. After data collation, cleaning, and removal of outliers, a common pool of 211 women were included for analysis.
Study procedures
We conducted three different research activities, i.e., the Household survey, dietary survey, and collection of capillary blood samples to analyze biomarkers. An overview of the sampling framework and study procedures is given in Fig. 1.
Household (HH) survey
A pretested structured questionnaire developed on an electronic data capture (EDC) platform using the software CS Pro (Version 7.1) and Samsung tablets (Model SM‐T385) was administered at HH level to gather information on HH characteristics. At the HH level, any adult member of the family, preferably a woman in the HH, who had information on household food access and other HH characteristics, was administered the HH survey. This survey elicited information on socioeconomic and demographic characteristics such as age, educational and occupational status of HH members, type of house, number of rooms, presence of separate kitchen, access to water and sanitation facilities, and some other features. Besides this, information on availability and access to different food sources such as HH ownership of agricultural land and kitchen garden (baari), access to forests for gathering food, access to water bodies (pond/river), etc. was also collected using HH survey tool and agriculture diversity tool (agri-tool) (on a sub-sample).
Dietary survey
A 2-day, non-consecutive 24-h dietary recall (24 HDR) was conducted on the eligible woman from each HH to estimate nutrient intake. A food recall kit consisting of standard utensils and a food picture flipbook for portion size estimation was used and the participant was asked to recall the food items consumed in each meal over the past 24 h with their corresponding approximate amounts, the ingredients used, and the method of preparation followed for each recipe consumed. In order to minimize the error in self-reporting, a final probing was done to make the woman recall if any additional food was consumed but not reported. This information on the self-reported amounts of raw foods was used to calculate the daily intake of individual food groups. Nutritionists and nutrition interns administered a paper form after due training for dietary recalls.
Capillary blood collection and analysis
Sample collection, storage, and transport
A clinical biochemist provided training on sampling and processing procedures to the field worker and a laboratory technician. During the daily surveys, the capillary blood collection procedure included cleaning the ring finger of the non-dominant hand with an alcohol swab, pricking the finger using a lancet, collecting the blood in labeled microtainers, and storing them in an icepack lined icebox. Simultaneously, spotting of drawn blood (20 µl) on Whatman paper was also done to estimate hemoglobin from dried blood spots (DBS). At the end of each day's collection, serum of collected samples was separated by centrifugation; multiple aliquots were made and stored in cryoboxes in the freezer compartment of the refrigerator at the field site laboratory. These samples were then transported in dry ice to Kolkata via road and subsequently to the Bangalore laboratory (Certificate of incorporation no (CIN): U74900KA2015PTC079960; Start-up registration number: KBITS/SK-REGN/2016/165) and AIIMS, New Delhi via air shipment.
Biomarker estimation
The collected blood samples were analyzed for micronutrients and inflammatory markers- namely ferritin, soluble transferrin receptor (sTfR), Retinol Binding Protein (RBP), alpha-1-acid glycoprotein (AGP), C-Reactive Protein (CRP), and hemoglobin. An in-house assay was developed and validated for measuring these biomarkers in partnership with the laboratory at Bangalore. The method was developed using Luminex technology on a Luminex xMAP ® platform along with the drop array technology. More details have been included elsewhere [35]
Hemoglobin estimation
For Hemoglobin estimation, a colorimetric method based on the formation of cyanmethemoglobin (Benesphera™) was used as per manufacturers' protocol, and all DBS samples were processed in a single batch. The DBS was cut out, transferred to test tubes containing 5 ml of Drabkin's solution, and kept at room temperature (20–25°) for 6 h. After that, one mL of the Drabkin's solution was measured on a spectrophotometer at 540 nm (OD540). A standard curve was constructed using hemoglobin standards provided with the kit and used for calculating the hemoglobin concentrations in the DBS samples.
Analysis of the study variables
Socioeconomic status
A HH wealth index score was obtained using principal component analysis (PCA). Variables such as type of housing, ownership of selected assets, number of rooms, presence of separate kitchen, source of electricity, access to water and sanitation facilities, and monthly expense on food were used to generate HH Wealth Index. Depending upon the quantiles of the HH wealth index score, the HHs were classified into five groups.
Food environment assessment
A modified and adapted version of the crop diversity index (CDI) [36] namely the Food Access Diversity Index (FADI), was developed for measuring access to diverse natural food sources. Lower diversity in production and access to foods is indicated by lower values of FADI and vice versa. It was expressed as:
Where n is the total number of foods grown, gathered, or accessed and animals raised in a particular HH and N is the maximum possible number of foods grown, gathered, accessed, or raised in a specific village. Detailed information on FADI has been reported elsewhere [37].
Analysis of dietary intake data
Diet quality
The Minimum Dietary Diversity for Women (MDD-W) was calculated as per FAO and USAID guidelines[38]. It was calculated by adding the number of food groups (for food items with amounts ≥ 15 g) consumed by the women reported each day in the 2 days 24-HDR, and then the mean score for two days was calculated. Indigenous food consumption scores (IF score) were calculated from the 2 days 24-HDR by adding the number of indigenous foods from different food groups consumed by the women each day and then calculating the mean IF scores. If a woman consumed any IF over the recall period of 2 days, she got a score of ≥ 0.5 and if she did not consume any IF, she got a score of ‘0’.
Nutrient intake
A validated software, DietCal (Version 8.0; Profound Tech Solution), converted food intake data into nutrient intakes. As the data on nutrient intake was skewed, the box cox method was deployed to transform the distribution into a normal distribution. The transformed data was then used in the linear mixed effect regression (LMER) model, and the values thus obtained were back-transformed to calculate the 'usual nutrient intake' [39]. The estimated average requirement (EAR) cut-point approach was used to estimate the prevalence of nutrient adequacy using these usual intakes, except for iron, for which the full probability approach [40] was utilized to determine the adequacy. The reference values of EAR were taken from the latest nutrient requirement guidelines for Indians [41].
Analysis of biomarker levels
Table 1 provides information on cut-offs values taken and case identification based on the biomarkers. The ferritin and sTfR concentrations were adjusted for inflammation (CRP and AGP) using a linear regression model [42]. The linear regression model is used to predict the outcome variable, which is a continuous variable, given the number of predictors. In our study, ferritin was the outcome variable and predictors were CRP and AGP. Since these variables were not following normal distribution, these were log-transformed, and then a linear regression model was fitted. If CRP and AGP values are given then one can predict the value of ferritin using the given equation. The mathematical form of the model is Y = a + b1X1 + b2X2 + …. + bnXn, where, a = intercept, b1, b2, b3, …bn are regression coefficients and X1, X2, X3,..Xn are predictors. Thus, using log (CRP) and log (AGP), a linear regression model was developed to predict the log (ferritin) as the outcome. After that, the adjusted ferritin values were determined using the estimated regression coefficients in the following equation:
In order to obtain the ferritin values in ng/ml, antilog of log(ferritin)adj values were determined. Similarly, log (sTfR) was predicted deploying a linear regression model using log (AGP) and the sTfR values were obtained using the estimated regression coefficients in the following equation:
\(\log\left(\mathrm{sTFR}\right)\mathrm{adj}\;=\;\log\left(\mathrm{sTFR}\right)\;-\;0.241\;\ast\;\log\left(\mathrm{AGP}\right)\;-\;6.211)\) and then antilog of \(\log\left(sTFR\right)\;adj\) values was taken to obtain the sTfR values in µg/ml. As the data on all biomarkers was skewed, log transformation was deployed to transform the distribution into a normal distribution.
Statistical analysis
Data entry and collation were done using Microsoft Excel and Stata SE version 15.1 [43]. The categorical variables were reported as frequency and percentages, whereas the continuous variables were reported as mean and standard deviation. Bivariate analysis was done using Karl Pearson correlation coefficient, t-test, and one-way ANOVA along with post hoc test for association between exposures such as nutrient intake, IF score, MDDW, sociodemographic and economic variables, access to different food sources, and the outcome as biomarkers. The associations that were statistically significant at p-value < 0.05 were transferred to the Linear Mixed Effects Regression (LMER) model for multivariate analysis.
Ethical considerations
Ethical approval for the study was obtained from Institutional Ethics Committee at the Indian Institute of Public Health‐Delhi, Public Foundation of India, and The All India Institute of Medical Sciences, New Delhi. Administrative approvals were also taken from the authorities at the district level. Cluster level consent from the village leader was obtained before any data collection. Informed consent (verbal witnessed consent in case the participants could not read or write and signed non-witnessed written informed consent for literate participants) was taken before the administration of the interview, and collection of blood sample and for publication of the data. Participation in the study was voluntary and small incentives, procured from the local markets were given to the participants.
Results
Sociodemographic and economic profile of Santhal households
Table 2 presents the sociodemographic description of the study population (n = 211). The mean age of the women was 27.2 ± 7 years, with a majority having a primary or lower level of education (78.2%). The sample population was divided into five quintiles based on the HH wealth index, the mean wealth score of the lowermost, and the uppermost quintiles, were -2.8 ± 0.7 and 3.2 ± 1.4 respectively.
Household access to different food sources and water and sanitation
The households reported accessing various food sources such as agricultural land (92.9%), kitchen garden (baari) (71.6%), water bodies (ponds and rivers) (55.5%), and forests (54.5%). The food items accessed from different food sources are listed in Table 3 and 4. The mean quantitative estimate of foods accessed from different food sources expressed in terms of FADI was 0.3 ± 0.3, which suggests low diversity in production and access to foods from the natural environment. The FADI was found to be significantly associated with the family type (nuclear v/s joint) (p < 0.001) with higher mean value of FADI in joint family (mean = 0.39 ± 0.32,) as compared to nuclear (mean = 0.24 ± 0.23), suggesting higher diversity in access to different foods in HHs with joint family.
The main source of drinking water for a majority of households was tube well/hand-pump (63.5%), and a majority of them reported defecating in open field/jungle (91.5%) (Table 2).
Dietary intake pattern and nutrient intake of Santhal women
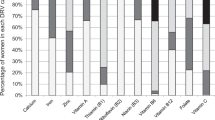
Table 5 provides a summary of food group wise intake among Santhal women based on the 2-days 24-h dietary recall. In addition to the cereals, the diet of Santhal women constituted of roots and tubers, other vegetable and pulses. Only a small proportion of women consumed flesh foods (14.2%), fruits (6.2%), and milk and milk products (1.4%).
Based on the 2 day-24 HDR, Table 6 provides a detailed description of mean usual nutrient intakes and the prevalence of nutrient inadequacy. Upon comparing the usual intake of each nutrient with the corresponding EAR, the mean intakes were found to be less than the EAR for all the nutrients.
Dietary diversity and IF consumption and their contribution to nutrient intake among Santhal women
The mean dietary diversity score obtained was 2.3 ± 0.6 with a median of 2 (range 1, 4) which is lower than the recommended minimum dietary diversity (MDD-W) of more than or equal to five food groups for the WRA group. The mean IF score obtained was 0.9 ± 0.7 with a median of 1.0 (range 0, 3.5).
There was a trend of increasing nutrient intake (all nutrients) with increasing MDD-W score (Table 7). The IF consumption scores were significantly associated with the intake of thiamine and calcium, indicating an increase in intake of these nutrients with a higher intake of indigenous foods.
Micronutrient status and status of inflammatory biomarkers of the Santhal women
Table 8 describes the mean biomarker levels. The mean CRP was found to be 3.1 ± 7.3 mg/L, and the mean AGP was 727.8 ± 259.7 mg/L, with one-fourth of the women reporting elevated levels of both CRP and AGP. While the prevalence of iron deficiency (< 30 ng/ml) based on the unadjusted ferritin values was 65.4% after adjusting the ferritin levels for inflammation using a linear regression model, the prevalence increased to 73%. The prevalence estimates based on the adjusted ferritin and sTfR levels were approximately similar (Table 9). Based on the hemoglobin levels, around 69.6% of the women were iron deficient or anemic, and 5% had severe anemia. Based on RBP levels 28.4% of the women were Vitamin A deficient, and 33.6% of the women had insufficient Vitamin A levels while 37.9% had normal levels.
Factors associated with the biomarker status of the Santhal women- Determinants of micronutrient status
The following section reports the association between various factors or determinants that contribute to the micronutrient status and the level of biomarkers that reflect the micronutrient status. We analyzed the associations aligned to the conceptual framework (Fig. 2) adapted from the UNICEF conceptual framework on determinants of maternal and child nutrition, 2020 [44]. It explored the association between enabling, underlying and immediate determinants of micronutrient status and the level of nutritional and inflammatory biomarkers as the outcome. The framework facilitated analyzing, organizing, and reporting of the data systematically.
Conceptual framework on enabling, underlying and immediate determinants of micronutrient status of Santhal women, Jharkhand, India adapted from UNICEF conceptual framework on determinants of maternal and child nutrition, 2020. MDD-W, Minimum Dietary Diversity for women. *HH wealth index was generated using household information on type of housing, number of rooms, presence of a separate kitchen, source of electricity and drinking water, ownership of selected assets and monthly expense on food
A. Enabling determinants of micronutrient status
Association between sociodemographic, economic profile and biomarkers
When a one-way ANOVA test or t-test was applied to find the association between sociodemographic variables and the biomarkers, no statistically significant difference was found in group means of the biomarker levels across the wealth quintiles (p > 0.05). However, a statistically significant difference between group means of RBP was observed between the two levels of education: primary and below (group 1) and above primary (group 2), wherein the mean RBP was higher in group 1 (mean = 1.19 mmol/L, p = 0.008) as compared to group 2 (mean = 0.92 mmol/L, p = 0.008). There were no significant differences in mean values of biomarkers among people with different drinking water sources and place of defecation (p > 0.05).
B. Underlying determinants of Micronutrient status
Association between access to different sources of food and the biomarkers
There was no statistically significant correlation between agroforestry diversity index-FADI and the biomarkers as determined by the Karl Pearson correlation coefficient test. Concurrently, when access to different food sources was compared with the biomarkers using t-test, it was found that access to agricultural land was significantly associated with hemoglobin levels (p = 0.01), and access to baari was significantly associated with the ferritin levels (p = 0.02), RBP levels (p = 0.02) and level of inflammatory biomarkers, i.e., CRP (p = 0.02) and AGP-1a (p = 0.01). Access to ponds and forests was significantly associated with the ferritin levels (p = 0.004 and p = 0.02 respectively for pond and forest).
C. Immediate determinants of micronutrient status
Association between minimum dietary diversity, indigenous food consumption, specific nutrient intake, and the biomarkers
No statistically significant correlation was found on exploring the correlation between MDD-W scores, indigenous food consumption, and biomarkers (Table 10). However, when the correlation between specific nutrient intake and the biomarkers was explored, it was found that the iron intake positively correlated with the ferritin level (r = 0.08, p = 0.25) and negatively correlated with the sTfR levels (r = -0.07, p = 0.32), but the correlation was not significant. The correlation between folate intake and ferritin (r = 0.18, p = 0.01) and folate intake and sTfR (r = -0.14, p = 0.03) was statistically significant. Intake of iron, folate, and vitamin C was also positively correlated with the hemoglobin level, with the correlation between folate intake and hemoglobin level being statistically significant (r = 0.18, p = 0.01) (Table 10). The energy intake was negatively correlated with the level of the inflammatory biomarker CRP (r = -0.14, p = 0.06), with the correlation being marginally significant.
D. Linear Mixed Effects Regression for assessing factors associated with biomarker status
When the variables that showed significant association in the bivariate analysis (p < 0.05) were transferred to the multivariate linear regression model, it was found that women having above primary level of education had 15.3% lower RBP level than women having a level of education of primary or below (p < 0.05, 95% CI: -27.82, -1.39). HH access to baari was positively associated with the RBP level and negatively associated with inflammatory biomarkers, while access to ponds was positively associated with the ferritin levels. The women who had HH access to baari had 18.53% higher level of RBP than those who did not access baari (p < 0.05, 95% CI: 2.33, 36.21). Some of the Vitamin A-rich foods accessed from the baari included mustard leaves, and pumpkin, accessed by 66.9%, and 22.5% of the HHs respectively as determined by the HH survey (Table 3). The women having HH access to baari had 38.12% lower level of CRP (p < 0.05, 95% CI: -58.85, -6.01) and 13.06% lower level of AGP-1a than those who did not have access to the same (p < 0.05, 95% CI: -22.97, -1.78). Women who had HH access to the pond had 27.12% higher ferritin levels than women who did not have access to the same (p = < 0.05, 95% CI: 1.82,58.57). Some iron-rich food items accessed from ponds included indigenous flesh foods like fish, snail, turtle, etc. (Table 4). Folate intake was positively associated with ferritin and hemoglobin levels, and as the folate intake changed by 1 unit, ferritin level also changed by 1.21% (p < 0.01, 95% CI:0.30,2.12) and hemoglobin changed by 0.28% (p < 0.05, 95% CI: 0.04,0.52). The folate intake was negatively associated with the sTfR levels and per unit increase in folate intake led to a 0.59% decrease in sTfR levels (p < 0.05, 95% CI: -1.19, -0.01).
Discussion
Our previous work [37, 45] among the indigenous communities of Jharkhand and their food environment suggests that while these communities have access to diverse natural food sources and possess TEK regarding IFs, the utilization of these are sub-optimal. Further, nutritional analysis of specific IFs revealed that these are nutrient-rich with the potential of contributing substantially to the nutrient intakes. Hence, we decided to explore the inter-links between nutritional status and dietary intake, focusing on IF consumption and their relationship with crucial micronutrient biomarkers among the Santhal community in Jharkhand. We also explored the inter-links between the biomarkers and access to different food sources. We specifically chose biomarkers of iron and vitamin A status and of inflammation for our study based on the commonly observed deficiencies in WRA in the region, and guided by the recent recommendations from expert groups [46, 47]. These were analyzed using a multiplex assay to minimize sample requirements and address logistic and technical challenges.
The food environment of the Santhal community consisted of diverse food sources. However, the actual diversity in access and utilization of food from these sources expressed as FADI, an agroforestry diversity index, was low. The dietary intake of the participating women also demonstrated low diversity expressed as MDD-W. Additionally, the mean nutrient intake was less than the EAR for each nutrient, thus reflecting inadequate nutrient intake. The women with higher MDD-W scores had a higher intake of all nutrients, and women -having higher IF scores had a significantly higher intake of thiamine and calcium. The biomarker status of the women revealed that one-fourth of the women had elevated levels of both CRP and AGP. The prevalence of iron deficiency was around 70%. Approximately 33.6% women were Vitamin A insufficient, while 28.4% were deficient. We found that some of the biomarkers were associated with HH access to food sources from the natural environment. The access to baari was positively associated with the RBP level and negatively associated with inflammatory biomarkers, while access to ponds was positively associated with the ferritin levels.
A high prevalence of iron deficiency among women in the reproductive age group is a persistent problem in the Indian population (IIPS and ICF 2021). Higher levels of iron deficiency ranging from 36 to 76% [48,49,50,51] are also reported among WRA in the various indigenous populations across Indian states, such as Uttarakhand, Kerala, Karnataka, and the north-eastern states. The NFHS-4 data also reports a high level of anemia (75.41%) among indigenous women of the Godda district [52], the region where the present study was conducted. Indigenous populations bear an additional burden of communicable diseases resulting in a high level of infection and inflammation [9]. One of the factors responsible for this could be the lack of sanitary facilities and open defecation, the prevalence of which was high in our study (91.5%). This is a consistent observation across rural India [53]. In this context of low intakes, high nutrient deficiency and increased inflammation, it is essential to note that studies have linked an increase in inflammatory markers with poor iron absorption [54, 55]. So, even if iron supplementation (a significant policy thrust area) and dietary diversification through indigenous foods are promoted in our study population, it is critical to simultaneously address underlying infections and inflammation to improve iron bioavailability and decrease anemia prevalence.
We did not find any significant correlations between the enabling determinants like sociodemographic and economic profiles and micronutrient status. Data from extensive global studies show a variable contribution of sociodemographic factors like education, household assets, healthcare access, sanitation and income quintiles on micronutrient and inflammatory biomarkers [56,57,58].
Indigenous food sources have demonstrably improved diet quality through dietary diversity [59]. The knowledge and routine consumption of several IFs accessed from the natural food environments is also reported among other indigenous communities worldwide [37, 60, 61]. Many of these foods have substantial quantities of micronutrients like iron, calcium, vitamin A, and zinc [30, 62] which are critical nutrients required for optimum health, especially for vulnerable populations like women and children. Despite the knowledge about diverse food sources, a low diversity in food access and utilization reflected in the low FADI score indicated underutilization of the IFs among Santhals. This has been a consistent observation across our work in the different indigenous communities [37, 61], who demonstrate this paradox of poor and diminishing consumption amidst collective understanding and historical usage of IFs. Other qualitative studies have documented reasons for this underutilization, including impact of climate change on natural food environments, resulting in decreased production and consumption of IFs; easy access to local markets for purchasing foods; and local agricultural organizations' promoting high yield hybrid varieties [61, 63]. However, it was interesting to note that, despite poor access, there is the utilization of some of the natural food sources, reflecting a habitual behavior towards food acquisition and consumption. Many of the food items routinely accessed from baari, e.g., mustard leaves and pumpkin, by a substantial number of HHs, have significant amounts of beta-carotene (2619 µg and 1449 µg of beta carotene per 100 g of mustard leaves and pumpkin respectively [64]. The observed correlation of RBP and ferritin levels with access to food from baari and ponds justified this premise.
Similarly, food items from ponds such as indigenous flesh foods like fish, snail, turtle, etc. have reasonable amounts of iron (ranging from 1- 2.3 mg/100 g in indigenous fish [45, 61], 2.29 mg/100 g in snail [65] and 311 mg/kg dietary matter in juvenile aquatic turtles [66]. Some studies have reported using biomarkers for testing whether food-related interventions for indigenous communities have had the desired effect [67, 68] concerning expected outcomes. Some have shown that geographical proximity to forest areas is a determinant of biomarker levels such as hemoglobin since location is a surrogate for access to fruits, vegetables, and animal foods from the natural food environment [69, 70]. Other studies have documented a positive impact of IF consumption on biomarker status [71, 72], including reduction of anemia and decrease in inflammation [69]. The latter was also an indirect observation in our study in which women accessing baari showed lower levels of anti-inflammatory markers. It is important to note that wild foods, especially green leafy vegetables, fruits, and mushrooms, have anti-inflammatory properties [73,74,75].
We did not observe any correlation between IF intake and MDD-W with biomarker levels. This finding could be ascribed to the generally low diversity without a significant gradient in intakes among the participants. However, we found some association between nutrient intake and biomarkers e.g., the dietary folate intake correlated negatively with a marker of inefficient erythropoiesis like sTfR. The role of folate as a nutrient necessary for normal hematopoiesis is well established, and since sTfR represents a state of insufficient iron availability, so the inverse correlation between the two is logical from a biological perspective.
Our findings re-affirm the potential of indigenous foods sourced from natural food environments like baari and water bodies to contribute to nutritional status. Strategies are required to promote the cultivation of these foods and increase awareness of the nutritional benefits of consuming these foods. The benefits of homestead food production programs coupled with nutrition education have been demonstrated in Bangladesh, Cambodia, Nepal, and the Philippines. They have been reported to increase food diversity and decrease the prevalence of anemia and night-blindness in children and WRA [76]. Projects conducted with William Treaties First Nation in Canada examined prospects of maintaining and using indigenous food systems and promoted traditional food acquisition activities. These included fishing, hunting, cultivating local foods, promoting community knowledge, skill-sharing, and building communal resources [77, 78]. Policy and program strategies incorporating conservation and promotion of indigenous seed varieties may effectively preserve and develop diversity [79]. Many factors affect the feasibility of maintaining/propagating the cultivation of these nutrition-rich foods, including seasonal availability, increasing availability of convenience foods, eroding TEK about their nutritional benefits, and changes in environmental regulations [80, 81]. These should be considered for designing contextual approaches for maximising impact.
Inference
Our study was conducted within the framework of recommendations from the BOND initiative and similar studies, and estimated appropriately selected nutrient biomarkers concurrently with inflammation markers to explore the impact of indigenous food consumption on biomarker status. Biomarkers were selected to incorporate the impact of multiple variables encompassing geography, access to samples, limited infrastructure, and modified biological responses due to high infection load. The use of a multiplex assay can reduce labor, cost, supplies, and sample volumes. The data also emphasizes the potential of IFs and their utilization in improving micronutrient status since a positive impact was seen on micronutrient biomarkers even with limited utilization. These findings suggest the necessity of including appropriate biomarkers in nutrition and health surveillance programs. Intervention programs need to incorporate strategies to promote routine utilization of diverse foods from the local environment to improve malnutrition in indigenous communities. Strategies such as promoting indigenous plants’ cultivation and rearing of indigenous animals and aquatic species can be incorporated into current agricultural policies. Activities to promote IF production through agricultural extension services can be undertaken. Incorporation of IFs into ongoing food security programs (targeted public distribution system) and food supplementation programs such as supplementary feeding initiatives under ICDS can be done. These activities can be strongly backed by behaviour change communication strategies including nutrition education programs, and activities to promote indigenous food consumption in the habitual diets of Santhal women.
Study limitations
Due to the cross-sectional nature of the study design, our findings represent only the associations, we cannot draw causal inferences from the same. There is also a possibility of unmeasured confounding factors in this study. Seasonal fluctuations, which are often known to affect nutrient consumption, were not included in the 24- hour DR of the respondent women. There may be some inaccuracy in reported portion size estimates, although standard food recall kits and a portion size estimation flipbook were employed during the survey. The nutrient calculation database used by us, calculates nutrients based on the nutritive value of raw foods using the Indian food composition table. However, it does not consider the nutrient retention factors ascribed to the effect of cooking on the nutrient content. The information on access to different natural food sources was gathered at the HH level, and it may not reflect the actual access/utilization of the women, although we made specific efforts to interview an adult woman during the HH survey. The markers of inflammation chosen by us could be affected by malaria and other infections. While we did ensure that only healthy, afebrile women were chosen as participants; we did not have biomarker-based information on recent malaria infection.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Notes
TEK refers to the in-depth knowledge base, beliefs and practices possessed by indigenous people about their natural food environment which have been passed down to them through generations by cultural transmission.
Natural Food environment refers to the subsistence food environment that include wild food (forests, open pastures, aquatic areas etc.) and cultivated food environment (fields, closed pastures, gardens from which consumers directly procure food.
References
Allen L, de Benoist B, Dary O, Hurrell R. Guidelines on food fortification with micronutrients. Geneva; Rome: WHO, FAO, UN; 2006.
Ritchie H, Roser M. Micronutrient Deficiency. Our World in Data. 2017. https://ourworldindata.org/micronutrient-deficiency. Accessed 10 Jun 2021.
Global Nutrition Report. Chapter 02 Inequalities in the global burden of malnutrition. Global Nutrition Report. 2020. https://globalnutritionreport.org/reports/2020-global-nutrition-report/inequalities-global-burden-malnutrition/#section-2-2. Accessed 8 Jun 2021.
Food and Agriculture Organization. Preventing micronutrient malnutrition a guide to food-based approaches - Why policy makers should give priority to food-based strategies. FAO, Rome. 2020. http://www.fao.org/3/x0245e/x0245e01.htm. Accessed 9 June 2021.
Dukhi N. Global Prevalence of Malnutrition: Evidence from Literature. IntechOpen. 2020.
Kapoor AK, Dhall M. Poverty, Malnutrition and Biological Dynamics among Tribes of India. Health Sci J. 2016;10;3:1–5.
IIPS and ICF. National Family Health Survey- 5, 2019–21. 2021. http://rchiips.org/nfhs/NFHS-5_FCTS/India.pdf. Accessed 14 Nov 2021.
India State-Level Disease Burden Initiative Malnutrition Collaborators. The burden of child and maternal malnutrition and trends in its indicators in the states of India: The Global Burden of Disease Study 1990–2017. Lancet Child Adolesc Health. 2019;3:855–70.
MoHFW. Ministry of Tribal Affairs. Report on Expert Committee on Tribal Health, Tribal Health in India, Bridging the Gap and a Roadmap for the Future. Government of India. 2020.
IIPS and ICF. National Family Health Survey (NFHS-4), 2015–16: India. 2017. http://rchiips.org/nfhs/pdf/NFHS4/India.pdf. Accessed 12 Nov 2021.
Downs SM, Ahmed S, Fanzo J, Herforth A. Food environment typology: advancing an expanded definition, framework, and methodological approach for improved characterization of wild, cultivated, and built food environments toward sustainable diets. Foods. 2020;9:532.
Kuhnlein HV, Erasmus B, Spigelski D. Indigenous Peoples’Food Systems: the many dimensions of culture, diversity and enviroment for nutrition and health. Rome: FAO, UN, Centre for Indigenous Peoples’ Nutrition and Environment; 2009.
Rai R, Nath V. The Role of Ethnic and Indigenous People of India and their Culture in the Conservation of Biodiversity. Paper presented in XII World Forestry Congress, 2003.
Kennedy G, Kanter R, Chotiboriboon S, Covic N, Delormier T, Longvah T, et al. Traditional and Indigenous Fruits and Vegetables for Food System Transformation. Curr Dev Nutr. 2021;5:nzab092.
FAO. News Article: Strengthening indigenous food systems is key to achieving a zero-hunger world. Rome: Food and Agriculture Organization of the United Nations (FAO); 2018.
Kumar MM, Pathak VK, Ruikar M. Tribal population in India: A public health challenge and road to future. J Fam Med Prim Care. 2020;9:508–12.
Bhattacharjee L, Kothari G, Priya V, Nandi BK. The bhil food system: links to food security, nutrition and health. In: Indigenous Peoples’ food systems: the many dimensions of culture, diversity and environment for nutrition and health. Rome: FAO of the UN, Centre for Indigenous Peoples’ Nutrition and Environment; 2009.
Singh KD, Alagarajan M, Ladusingh L. What Explains Child Malnutrition of Indigenous People of Northeast India? PLoS ONE. 2015;10:e0130567.
Ministry of Health and Family Welfare (MoHFW), Government of India, UNICEF and Population Council. Comprehensive National Nutrition Survey (CNNS) National Report. New Delhi; 2019.
Hedrick VE, Dietrich AM, Estabrooks PA, Savla J, Serrano E, Davy BM. Dietary biomarkers: advances, limitations and future directions. Nutr J. 2012;11:109.
Maruvada P, Lampe JW, Wishart DS, Barupal D, Chester DN, Dodd D, et al. Perspective: dietary biomarkers of intake and exposure-exploration with omics approaches. Adv Nutr. 2020;11:200–15.
Picó C, Serra F, Rodríguez AM, Keijer J, Palou A. Biomarkers of nutrition and health: new tools for new approaches. Nutrients. 2019;11:1092.
Freedman LS, Kipnis V, Schatzkin A, Tasevska N, Potischman N. Can we use biomarkers in combination with self-reports to strengthen the analysis of nutritional epidemiologic studies? Epidemiol Perspect Innov. 2010;7:1–9.
Gormley IC, Bai Y, Brennan L. Combining biomarker and self-reported dietary intake data: a review of the state of the art and an exposition of concepts. Stat Methods Med Res. 2019;29:617–35.
Office of the Registrar General, India. Jharkhand, Data Highlights: The Scheduled Tribes. Census of India. 2001. https://www.censusindia.gov.in/Tables_Published/SCST/dh_st_jharkhand.pdf. Accessed 12 Nov 2021.
Bose K, Banerjee S, Bisai S, Mukhopadhyay A, Bhadra M. Anthropometric Profile and Chronic Energy Deficiency Among Adult Santal Tribals of Jhargram, West Medinipur District, West Bengal, India: Comparison with Other Tribal Populations of Eastern India. Ecol Food Nutr. 2006;45:159–69.
Chowdhury SD, Ghosh T. The upper arm muscle and fat area of Santal children: an evaluation of nutritional status. Acta Paediatr. 2009;98:103–6.
Chakrabarty S, Pal M, Bharati S, Bharati P. Chronic energy deficiency among tribal communities of Orissa. India Tribes Tribals. 2008;2:95–101.
Rao T, Tuhina V. Malnutrition and anemia in tribal pediatric population of Purnia district (Bihar). Indian Pediatr. 2006;43:181.
Ghosh-Jerath S, Singh A, Phfi M, Kamboj P, Goldberg G. Exploring the Potential of Indigenous Foods to Address Hidden Hunger: Nutritive Value of Indigenous Foods of Santhal Tribal Community of Jharkhand India. J Hunger Environ Nutr. 2016;11:1–21.
Ghosh-Jerath S, Downs S, Singh A, Paramanik S, Goldberg G, Fanzo J. Innovative matrix for applying a food systems approach for developing interventions to address nutrient deficiencies in indigenous communities in India: a study protocol. BMC Public Health. 2019;19:944.
Census. Census of India Website : Office of the Registrar General & Census Commissioner, India. Office of the Registrar General & Census Commissioner, India. 2011. https://censusindia.gov.in/. Accessed 11 Nov 2021.
World Health Organization. World Health Survey-Survey Manual. 2002. https:// www.who.int/healthinfo/survey/whslongversionsurveymanual.pdf.
Ghosh-Jerath S, Singh A, Magsumbol MS, Lyngdoh T, Kamboj P, Goldberg G. Contribution of indigenous foods towards nutrient intakes and nutritional status of women in the Santhal tribal community of Jharkhand. India Public Health Nutr. 2016;19:2256–67.
Singh A, Baalasubramanian S, Kalaivani M, Kapoor R, Bhagwat K, Ghosh-Jerath S. Standardisation and application of a novel multiplex assay for estimating micronutrient status and inflammatory markers in women of Sauria Paharia and Santhal tribes of Jharkhand. Br J Nutr. 2022;128(12):2464–79.
Michler JD, Josephson AL. To specialize or diversify: Agricultural diversity and poverty dynamics in Ethiopia. World Dev. 2017;89:214–26.
Ghosh-Jerath S, Kapoor R, Singh A, Nilima, Downs S, Goldberg G, et al. Agroforestry diversity, indigenous food consumption and nutritional outcomes in Sauria Paharia tribal women of Jharkhand, India. Matern Child Nutr. 2021;17:e13052.
FAO. Minimum dietary diversity for women: An updated guide to measurement - from collection to action. Rome, Italy: FAO; 2021.
Tooze JA, Kipnis V, Buckman DW, Carroll RJ, Freedman LS, Guenther PM, et al. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med. 2010;29:2857–68.
Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Assessment. Washington, DC: National Academies Press; 2000.
ICMR, NIN. Short Report of Nutrients Requirement for Indians- A report of the expert group, 2020. NIN. 2020. https://drive.google.com/file/d/1j3umH5zcJAGNR_WUFwl3-0rBiemYw8DR/preview?usp=embed_facebook. Accessed 11 Nov 2021.
Namaste SM, Aaron GJ, Varadhan R, Peerson JM, Suchdev PS, BRINDA Working Group. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106 Suppl 1:333S-347S.
StataCorp. Stata Statistical Software: Release 15. 2017.
UNICEF Nutrition Section, Programme Division. UNICEF Nutrition Strategy 2020–2030. 2020. https://www.unicef.org/media/91741/file/UNICEF-Nutrition-Strategy-2020-2030-Brief.pdf. Accessed 11 Nov 2021.
Ghosh-Jerath S, Kapoor R, Singh A, Downs S, Barman S, Fanzo J. Leveraging Traditional Ecological Knowledge and Access to Nutrient-Rich Indigenous Foods to Help Achieve SDG 2: An Analysis of the Indigenous Foods of Sauria Paharias, a Vulnerable Tribal Community in Jharkhand. India Front Nutr. 2020;7:61.
Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, et al. Biomarkers of Nutrition for Development (BOND)-Iron Review. J Nutr. 2018;148 suppl_1:1001S-1067S.
Suchdev PS, Namaste SM, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project1234. Adv Nutr. 2016;7:349–56.
Dabral M, Kothiyal P. Prevalence of anemia among reproductive age group tribal women in Uttarakhand. India Indian J Pharm Sci Res. 2015;5:301–4.
De M, Halder A, Chakraborty T, Das U, Paul S, De A, et al. Incidence of anemia and effect of nutritional supplementation on women in rural and tribal populations of eastern and north-eastern India. Hematology. 2011;16:190–2.
Ismail IM, Kahkashan A, Antony A, Sobhith V. Role of socio-demographic and cultural factors on anemia in a tribal population of North Kerala, India. Int J Community Med Public Health. 2017;3:1183–8.
Kamath R, Majeed JA, Chandrasekaran V, Pattanshetty SM. Prevalence of anemia among tribal women of reproductive age in Udupi Taluk. Karnataka J Fam Med Prim Care. 2013;2:345.
Ministry of Tribal Affairs, IIPS, Piramal Swasthya. ompendium of District Factsheets Tribal Health and Nutrition. 2021.
Coffey D, Spears D. Where India Goes. HarperLitmus. 2017.
Pasricha S-R, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet Lond Engl. 2021;397:233–48.
Stoffel NU, von Siebenthal HK, Moretti D, Zimmermann MB. Oral iron supplementation in iron-deficient women: How much and how often? Mol Aspects Med. 2020;75:100865.
Borges MC, Buffarini R, Santos RV, Cardoso AM, Welch JR, Garnelo L, et al. Anemia among indigenous women in Brazil: findings from the First National Survey of Indigenous People’s Health and Nutrition. BMC Womens Health. 2016;16:7.
Rai A, Khan MN, Thapa S. Trends and determinants of anaemia in women of Nepal: a multilevel analysis. Matern Child Nutr. 2020;16:e13044.
Wirth JP, Woodruff BA, Engle-Stone R, Namaste SM, Temple VJ, Petry N, et al. Predictors of anemia in women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):416S-427S.
Powell B, Thilsted SH, Ickowitz A, Termote C, Sunderland T, Herforth A. Improving diets with wild and cultivated biodiversity from across the landscape. Food Secur. 2015;7:535–54.
Bacchetta L, Visioli F, Cappelli G, Caruso E, Martin G, Németh É, et al. Commentary A manifesto for the valorization of wild edible plants. J Ethnopharmacol. 2016;191:180–7.
Ghosh-Jerath S, Kapoor R, Barman S, Singh G, Singh A, Downs S, et al. Traditional Food Environment and Factors Affecting Indigenous Food Consumption in Munda Tribal Community of Jharkhand. India Front Nutr. 2021;7:600470.
Grivetti LE, Ogle BM. Value of traditional foods in meeting macro-and micronutrient needs: the wild plant connection. Nutr Res Rev. 2000;13:31–46.
Sheeth T. Changing food pattern of tribal women of Jharkhand state with special reference to Gumla district. Food Sci Res J. 2016;7:207–16.
Longvah T, Anantan I, Bhaskarachary K, Venkaiah K, Longvah T. Indian food composition tables. Ministry of Health and Family welfare. National Institute of Nutrition, Indian Council Med Res, Hyderabad. 2017.
Babalola O, Akinsoyinu A. Proximate composition and mineral profile of snail meat from different breeds of land snail in Nigeria. Pak J Nutr. 2009;8:1842–4.
Kienzle E, Kopsch G, Koelle P, Clauss M. Chemical Composition of Turtles and Tortoises. J Nutr. 2006;136:2053S-2054S.
Browne J, Lock M, Walker T, Egan M, Backholer K. Effects of food policy actions on Indigenous Peoples’ nutrition-related outcomes: a systematic review. BMJ Glob Health. 2020;5:e002442.
Slagman A, Harriss L, Campbell S, Muller R, McDermott R. Low proportions of folic acid deficiency after introduction of mandatory folic acid fortification in remote areas of northern Queensland, Australia: a secondary health data analysis. Biomark Biochem Indic Expo Response Susceptibility Chem. 2019;24:684–91.
Callister A, Gautney J, Aguilar C, Chan J, Aguilar D. Effects of Indigenous Diet Iron Content and Location on Hemoglobin Levels of Ghanaians. Nutrients. 2020;12:E2710.
Tata CY, Ickowitz A, Powell B, Colecraft EK. Dietary intake, forest foods, and anemia in Southwest Cameroon. PLoS ONE. 2019;14:e0215281.
Kenny T-A, Hu XF, Jamieson JA, Kuhnlein HV, Wesche SD, Chan HM. Potential impact of restricted caribou (Rangifer tarandus) consumption on anemia prevalence among Inuit adults in northern Canada. BMC Nutr. 2019;5:30.
Milman N, Byg KE, Mulvad G, Pedersen HS, Bjerregaard P. Iron status markers in 224 indigenous Greenlanders: influence of age, residence and traditional foods. Eur J Haematol. 2001;66:115–25.
Li Y, Zhang J-J, Xu D-P, Zhou T, Zhou Y, Li S, et al. Bioactivities and Health Benefits of Wild Fruits. Int J Mol Sci. 2016;17:1258.
Souilem F, Fernandes Â, Calhelha RC, Barreira JCM, Barros L, Skhiri F, et al. Wild mushrooms and their mycelia as sources of bioactive compounds: Antioxidant, anti-inflammatory and cytotoxic properties. Food Chem. 2017;230:40–8.
Tufts HR, Harris CS, Bukania ZN, Johns T. Antioxidant and Anti-Inflammatory Activities of Kenyan Leafy Green Vegetables, Wild Fruits, and Medicinal Plants with Potential Relevance for Kwashiorkor. Evid Based Complement Alternat Med. 2015;2015:e807158.
Talukder A, Haselow NJ, Osei AK, Villate E, Reario D, Kroeun H, et al. Homestead food production model contributes to improved household food security and nutrition status of young children and women in poor populations. Field Actions Sci Rep J Field Actions. 2010; Special Issue 1.
Asprilla-Perea J, Díaz-Puente JM, Martín-Fernández S. Estimating the potential of wild foods for nutrition and food security planning in tropical areas: Experimentation with a method in Northwestern Colombia. Ambio. 2021;Advance Online Publication:1–17.
Domingo A, Charles K-A, Jacobs M, Brooker D, Hanning RM. Indigenous Community Perspectives of Food Security, Sustainable Food Systems and Strategies to Enhance Access to Local and Traditional Healthy Food for Partnering Williams Treaties First Nations (Ontario, Canada). Int J Environ Res Public Health. 2021;18:4404.
Caritus India. Preserving bio-diversity of food system by conserving indigenous seed. Caritus India. 2013. https://www.caritasindia.org/preserving-bio-diversity-of-food-system-by-conserving-indigenous-seed/. Accessed 11 Nov 2021.
Farfán-Heredia B, Casas A, Rangel-Landa S. Cultural, economic, and ecological factors influencing management of wild plants and mushrooms interchanged in Purépecha markets of Mexico. J Ethnobiol Ethnomedicine. 2018;14:68.
Marushka L, Batal M, Tikhonov C, Sadik T, Schwartz H, Ing A, et al. Importance of fish for food and nutrition security among First Nations in Canada. Can J Public Health Rev Can Santé Publique. 2021;112(Suppl 1):64–80.
Acknowledgements
The authors would like to thank the team at “Ekjut,” a grassroots NGO in Jharkhand, especially Mr. Mohd. Sarfraz Ali, for their support throughout the conduct of the surveys. We would also like to acknowledge the help from Ms Kiran Kumari Pasi, Deputy Commissioner Godda District, Jharkhand who facilitated necessary permissions to conduct the study. We are grateful to Dr. RD Paswan, Civil Surgeon Godda for granting permission to conduct the study. The contribution of Awadesh Kumar, Nakul Sain, Johann Paharia and Haradhan in helping with sample collection, processing and communication with the participants is greatly appreciated. We would like to thank our field staff, and Anganwadi workers in the study villages for extending their warmth and cooperation. Finally, we are indebted to the Santhal families for agreeing to provide samples and for sharing their invaluable traditional knowledge and wisdom.
Funding
This work was supported by the DBT/ Wellcome India Alliance Fellowship (https://www.indiaalliance.org/) [IA/CPHI/16/1/502639] awarded to SGJ. The funding source had no role in the design, analysis or writing of this article.
Author information
Authors and Affiliations
Contributions
SGJ and AS contributed to the conceptualization, study design, methodology, data collection and supervision. AD, AB, RK and SB contributed to the methodology, formal analysis and investigation. SGJ, AS and AD were involved in original draft preparation and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Authors’ information
Archna Singh
AS is faculty at the Department of biochemistry, AIIMS, New Delhi. She has a doctoral degree from AIIMS, Delhi, and an MPH from the University of Michigan, Ann Arbor. Her research experience has converged on topics related to nutrition, metabolism and nutritional biochemistry in the past 15 years and aims to resolve the linkages between nutrition and metabolism. Her community nutrition work has included research on trans fatty acid consumption patterns among different communities. She has worked among indigenous people and is presently involved in assessing nutritional status of these communities and analyzing the diversity and composition of their local foods. Her patient related research explores the determinants of High-Density Lipoprotein quality and mitochondrial reprogramming in pediatric leukemias. Archna enjoys reading across genres and experimenting with different cuisines and hopes that these will enrich her scientific process and outputs.
Suparna Ghosh-Jerath
SGJ is Professor and Head, Community Nutrition at Public Health Foundation of India. Her research spans across areas like indigenous food systems of India, the role of agroforestry and dietary diversity in food security, health systems research, and food and nutrition policies to address malnutrition in India.
Ridhima Kapoor
RK is a public health nutritionist. She has completed her Masters in Food and Nutrition from University of Delhi, India. She currently works as a research fellow at Indian Institute of Public Health-Delhi, Public Health Foundation of India (PHFI). Her research interest centers around food systems and its impact on maternal and child nutrition in vulnerable communities.
Ayushi Dhasmana
AD is currently working as a research assistant at Public Health Foundation of India. She pursued her Masters in Food and Nutrition with specialization in Public Health Nutrition from University of Delhi, India. She works on evaluating the potential of indigenous foods for improving food security and nutritional status of vulnerable communities of Jharkhand, India. Her other research interest includes maternal and child nutrition; and impact of food-based approaches like fortification and biofortification on anemia level.
Ashish Bandhu
AB is an Assistant Professor in the school of Institute of Health Management Research at IIHMR University. He teaches Biostatistics, Data Management and Statistical Data Analysis. In various capacities, he has more than 7 years of experience in both domestic and international settings. He has worked as a Data Manager cum Data Analyst in Family Planning Project named PMA Agile with support from the Johns Hopkins Bloomberg School of Public Health, Baltimore, USA funded by Bill & Melinda Gates Institute for Population and Reproductive Health, USA. Apart from teaching and training, his research interests include Nutrition, Family Planning, Road Traffic Injuries and Facility Survey. His research work has appeared in national and international journals including “Sight and Life” and “Journal of Health Management”.
Sivasankar Baalasubramanian
SB is a scientist turned entrepreneur with over 20 years of global experience in innovative research in the areas of immunobiology of cancer, infectious diseases, and vaccines. He has a PhD in Immunology from All India Institute of Medical Sciences, New Delhi, India and has worked at School of Medicine, Cardiff University UK as part of his Wellcome Trust fellowship. He has worked as a Principal Investigator at the Singapore Institute of Clinical Sciences, Singapore before returning to India. Since, coming back to India, he serves as a co-founder for a biotech startup focused on developing cutting edge immunological tools to cater various industries nationally and globally.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval was obtained from the Institutional Ethics Committee at the Indian Institute of Public Health‐Delhi, Public Foundation of India, and All India Institute of Medical Sciences, New Delhi (March 2018; IEC-121/09.03.2018, RP-42/2018].). Informed consent was obtained from all participants. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Rawdataset.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Singh, A., Dhasmana, A., Bandhu, A. et al. Contribution of natural food environments to nutritional intake and biomarker status: insights from the women of indigenous santhal communities of Jharkhand, India. BMC Nutr 9, 20 (2023). https://doi.org/10.1186/s40795-023-00669-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-023-00669-1