Abstract
Background
This study aimed to evaluate the effect of vitamin D3 supplementation on body composition and anthropometric measures of nursing mothers.
Methods
In a double-blind, randomized clinical trial, 90 nursing mothers with overweight or obesity were randomized into three groups for 12 weeks: two groups of vitamin D3 supplementation (2000 IU/d (VD1), n = 32 and 4000 IU/d (VD2), n = 29) and placebo (PL) group (n = 29). The information on body composition was obtained using the body impedance analysis (BIA) method. Serum 25-Hydroxy vitamin D (25(OH) D), Intact Parathyroid Hormone (iPTH), calcium, and phosphorus were measured before and after the intervention. Data were analyzed based on the intention-to-treat (ITT) method. Two-way repeated measure ANOVA (mixed ANOVA) was applied to assess whether the mean changes in the results from baseline to 12 weeks differ in the three groups.
Results
There was a significant increase in the serum 25(OH) D concentration in the VD2 group compared to VD1 and PL groups (mean change (MC), 12.3 ng/ml; 95% CI, 9.4/15.0, p-value < 0.001). In addition, fat mass (MC, − 4.3 kg; 95% CI, − 7.0/− 1.1, p-value < 0.007), fat mass index (MC, − 1.6; 95% CI, − 2.6/− 0.5, p-value < 0.006) and body fat percentage (MC, − 8.1; 95% CI, − 12.0/− 4.2, p-value < 0.007) reduced in VD2 group as compared with VD1 and PL groups.
Conclusion
The intake of 4000 IU/d vitamins D3 supplementation would elevate circulating 25(OH) D concentrations in nursing mothers with overweight or obesity and improve some indices of body composition.
Trial registration
Iranian Registry of Clinical Trials (http://www.irct.ir: IRCT20140413017254N6) registered on 11-04-2018.
Graphical Abstract
The graphical abstract of this clinical trial, is a figure that explains the final results of the manuscript in a clear and attractive way

Similar content being viewed by others
Background
Nearly half of the nursing mothers in developed countries suffer from overweight or obesity during lactation [1]. This figure in Iran was reported as 31.7–37.3% [2, 3]. Factors such as unhealthy dietary intake, low physical activity [4], higher serum leptin concentration [5] or pre-pregnancy high body mass index (BMI) [6], and low serum 25(OH) D concentration [7] (< 20 ng/ml (50 nmol/l) [8,9,10]) may cause women to gain more weight during pregnancy, which may lead to obesity in lactation duration [11].
The global prevalence of a vitamin D deficiency in pregnant and nursing women was reported to be 21–85% [12]. In Iran, the Second National Survey on the status of micronutrients showed that 85% of pregnant women are exposed to vitamin D deficiency (≤ 30 ng/ml) or a severe deficiency (≤10 ng/ml) Therefore, a vitamin D deficiency may be high in nursing mothers too [13], which may be due to less frequent multivitamin intake during lactation as compared with pregnancy [14]. Other causes, such as an increase in the body’s need for bone mass [15], lack of adequate sunlight exposure [16], and a low intake of dietary vitamin D [12], can contribute to vitamin D deficiency [17, 18].
The concentration of serum 25(OH) D has an inverse association with body weight, BMI, and fat mass (FM) [19, 20]. After exposure to sunlight, the increase in serum concentrations of 25(OH) D was 43% less in obese individuals than non-obese participants [21]. However, the findings of a study reported that volumetric dilution causes a difference in serum 25(OH) D concentrations between non-obese and obese women, leading to vitamin D deficiency [22, 23].
Vitamin D supplementation may affect FM and body weight in overweight people [8, 9]. A systematic review reported that the effect of vitamin D supplementation on weight loss in participants with obesity was not conclusive [8, 9]. However, some of the interventions included in this review, investigated the combination therapy of vitamin D with other treatments such as energy restriction, calcium, or omega-3 supplements [8, 9]. Thus, the interpretation of the findings remains controversial. A meta-analysis provided evidence of an inverse association between FM and serum 25(OH) D. However. It does not support the hypothesis that vitamin D supplementation can augment body-fat loss [24]. The impact of different doses of vitamin D supplements on the body composition in nursing women with overweight or obese is unknown. Only one trial has previously investigated the impact of vitamin D supplementation during lactation on mothers with different weight status [25]. However, data on nursing mothers with obesity have not been reported separately. This study showed significant negative correlations between serum 25(OH) D and FM [25]. Therefore, more studies were needed to determine whether higher vitamin D intake might reduce body fat in nursing mothers. We hypothesized that the higher dose of vitamin D supplementation might improve serum 25(OH) D concentration and body composition. The present study aimed to investigate the effects of larger doses of vitamin D3 supplementation on serum 25(OH) D concentration and body composition in nursing mothers with overweight or obesity.
Methods
Participants and study design
The present study is part of a double-blind, randomized clinical trial, which aimed to investigate the effects of 2000 and 4000 IU/d of vitamin D3 supplementation on serum 25(OH) D concentration, body composition, and anthropometric measures in nursing mothers with overweight or obesity, as well as to assess the growth and risk of infection in their infants. Postpartum nursing mothers were recruited at the private hospital’s maternity ward in Qazvin province from November 2018 to March 2019 (autumn to winter). Inclusion criteria were participants aged 20–49 with a BMI 25–39.9 kg/m2 who delivered at term (gestational age of 37–42 weeks),and a birth weight appropriate for gestational age (2500–3900 kg). In addition, the mothers had to undertake to continue breastfeeding for the 3 months of the study. The exclusion criteria were having diagnosed gastrointestinal disorders interfering with bowel function, having severe hepatic, renal, inflammatory, cancer, diabetes, hypertension, epilepsy, and thyroid diseases, or taking any medication, a history of smoking or alcohol consumption in the past month and adhering to a specific diet during the past 12 weeks.
The sample size was calculated according to Roosta et al.’s study [26] by two mean comparison formulas, type I error (α) =0.05, type II error (β) =0.2, and mean (Standard Deviation) of waist circumference (WC) changes. Mean changes of WC after supplementation of vitamin D at the end of the study of Roosta et al. [26] were equal to 1.91 ± 1.7 cm and 0.55 ± 1.04 cm in the intervention and control groups, respectively. For power = 0.8 and (α) value = 0.05, the sample size was calculated to equal 18. Since there were 3 groups in this study, the calculated sample size was multiplied by √ (the number of groups) [27]. Finally, the required sample size for this study was 90 participants.
The protocol for the present study was published previously [28] and was registered in the Iranian Registry of Clinical Trials (http://www.irct.ir: IRCT20140413017254N6) on 11-04-2018. The Ethics Committee of Tehran University of Medical Sciences approved the study (IR.TUMS REC 1397429).
Outcomes
The outcomes of the study were WC, weight, BMI, body fat percentage (BFP), FM, fat-free mass (FFM), skeletal muscle mass, relative fat mass index (RFMI), fat mass index (FMI), serum concentrations of 25(OH) D, calcium, iPTH and phosphorus.
Randomization and intervention
Ninety nursing women with overweight or obesity were randomly allocated to three groups: two groups of vitamin D3 supplementation 2000 IU/d (VD1, n = 32), 4000 IU/d (VD2, n = 29) and placebo group (PL, n = 29). The participants were randomly assigned to groups with a 1:1:1 randomization ratio. Randomization was performed by an assistant using permuted block randomization method, and stratified randomization was employed to match the women based on age (20–34 and 35–49 years) and BMI (25–29.9 and 30–39.9 kg/m2). The intervention allocation was blinded for both investigators and participants. Vitamin D3 (cholecalciferol) and placebo (lactose) supplements were in the form of nano microcapsules, which have provided by the Nano Hayat Darou Industrial Co. (Tehran, Iran). Mothers are instructed to consume two nano micro capsules daily, one capsule with lunch and dinner. The capsules were identical in size, color, and shape. The vitamin D3 supplement and placebo were packed by the Pharmacy. The double-dummy method was used for the double-blind study. The intervention started approximately 3 days after delivery and continued up to 12 weeks. Mothers were called up every week. The number of returned capsules were recorded at the final visit to calculate compliance and adherence to the intervention.
Socio-demographic, sunlight exposure, physical activity measurement and dietary analysis
Questionnaire inquiries on socio-demographic data were completed by participants at the beginning of the study. The researcher asked sunlight exposures and physical activity at the beginning and the end of the study. Duration of outdoor activity and sunlight avoidance histories such as usage of umbrella and sunscreen were asked. Participants were also inquired about wearing long or short sleeves clothes, short or long pants and the traditional Islamic veil. This questionnaire was obtained from a previous study, which investigated the validity and reliability of the questionnaire [29]. The reliability of the questionnaire was calculated as a pilot and through (Test re Test) for 20 students. The coefficient of stability was obtained as 0.85 [29]. The validity of the questionnaire was determined by the opinion of ten faculty members of the Kurdistan University of Medical Sciences.
The short form of the International Physical Activity Questionnaire (IPAQ) was used to assess physical activity patterns during the previous week (http://www.ipaq.ki.se 2017). The questionnaire consists of seven questions to evaluate the intensity of activities. Scores less than 600 metabolic equivalents (MET) minutes per week show low activity, scores expressed in 600–3000 MET minutes per week show moderate physical activity, and scores more than 3000 MET minutes per week represent intense physical activity.
For the assessment of dietary intakes, two 24-hour food recalls were completed at the baseline and end of the study. One of the 24 -hour recalls was filled for one of the working days of a week and another one on holidays. The Nutritionist IV software version 4.1 (First Databank Division, the Hearst Corporation, and San Bruno, CA) was used to estimate dietary intake of nutrients.
Anthropometry and body composition assessment
Height was measured using a fixed stature meter (Model No.26 SM). The BMI was calculated as weight in kilograms divided by the square of the height in meters. Bioelectrical Impedance Analysis (BIA) was performed using In Body model 270 as a body composition analyzer (In Body Co., Ltd. Seoul, Korea), to assess weight, FM (kg), FFM (kg), skeletal muscle mass (kg), BFP (%), FMI as FM (kilogram) divided by the square of height (meter) [30], and RFMI for women calculated as 76 − (20 height × WC in meters) [31]. All measurements were done at 8–10 a.m.
Biochemical assay
After an 8-h overnight fasting, the registered staff nurses took the venous blood samples between 9 and 10 a.m. Then blood samples were centrifuged at 3000 rpm for 10 min at 4 °C to obtain the serum and were stored at − 21 °C until biochemical analyses. Serum was used for analyzing calcium, phosphorus, iPTH, and 25(OH) D concentrations at the baseline and end of the study. Assay performance was measured using the kit, and standard laboratory procedures, and performance were within acceptable limits. Calcium and phosphorus concentrations were measured by colorimetric enzymatic test (Pars Azmun Co., Tehran, Iran) by photometric UV test BILT1500. 25(OH) D and iPTH were measured by Enzyme-linked Immune Sorbent Assay (ELISA) (Monobind, Inc. Lake Forest, CA (92630), the USA) and (Biomerica, Inc. Irvine, CA (92614) USA), respectively. The inter- and intra-assay coefficients of variation (CV) for calcium, phosphorus, 25(OH) D, and iPTH were 1.04 and 2.01%, 1.61 and 2.22%, 3.87 and 4.55%, 4.5, and 3.9%, respectively.
Statistical analyses
All statistical analyses were performed by SPSS version 24 (SPSS Inc., Chicago, IL, USA). Analyses were done based on ITT analyses. The ITT population consisted of all the enrolled and randomized participants. In the ITT method, information on the baseline was considered a covariate. Multiple imputation methods were used to impute missing values. The missing data were imputed using a linear regression imputation method. The BMI and serum 25(OH) D concentrations in each study group were used for multiple imputations. The normal distribution of variables was tested and confirmed by Kolmogorov–Smirnov test. The baseline measurements and dietary intakes of participants in the three groups were compared using one-way analysis of variance (ANOVA) test for quantitative variables with normal distribution, the Kruskal Willis test for non-parametric, and the Chi-square test for qualitative variables. The differences between before and after values of body composition measures were computed; then, the independent t-student test was used to determine the relationship between the mean changes of body composition measures and serum 25(OH) D status.
Two-way repeated measure ANOVA (mixed ANOVA) with Bonferroni correction was applied to assess the time effect and time-by-treatment (two doses of vitamin D or placebo) interaction effect on all outcome measures. The models were adjusted for saturated fatty acids (SFA) intake that was different between treatment groups at the baseline with a p-value < 0.05. Since weight loss during lactation might complicate the findings relating to vitamin D supplementation, the body composition variables (except for weight and body mass index) were also adjusted for mean change of weight. P < 0.05 was considered statistically significant.
Results
Compliance was high, and more than 82, 93, and 89% of the capsules were consumed in VD1, VD2, and PL groups.
In the early days of the intervention, two women from the VD1 group complained of flatulence and diarrhea. However, no side effects were reported in VD2 and PL groups. The serum calcium concentrations in all three treatment groups were in the normal range (8.6–10.6 mmol/l) before and after treatment.
Characteristics of participants and dietary intake
Ten out of ninety participants did not complete the study for different reasons (two in the VD1 group, five in the VD2 group, and three in the PL group). One of them is taking calcium/vitamin D supplements due to a lumbar disc, two of them changed their medications. Four women could not continue the study due to the change in their husband’s working conditions, and one woman refused to take supplements at the beginning of the intervention after blood sampling, and two mothers did not respond to paging (Fig. 1).
There were no significant differences between the three groups in terms of general characteristics at the baseline and the end of the study (p-value> 0.05). All the participants took iron supplement after delivery. There were no significant differences regarding iron intakes between groups (p-value> 0.05) (Table 1). In addition, There were no significant differences among the three groups in terms of the intake of energy and nutrients, except for the intake of SFA, which was significantly higher in the PL group compared with the other two groups at the baseline of the study (p-value = 0.02) (Table 2).
Effect of vitamin D3 supplementation on serum concentrations of 25(OH) D, iPTH, calcium and phosphorus
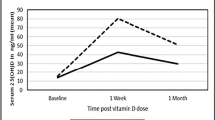
Serum 25(OH) D concentrations were significantly increased in the VD2 group (mean change (MC) 12.3 ng/ml; 95% CI, 9.4/15.0, p-value =0.001), but decreased in the VD1 and PL groups. A significant difference was observed between VD2 and VD1 (p-value = 0.001) and PL (p-value = 0.001) groups in terms of serum 25(OH) D changes. Vitamin D supplements did not affect serum calcium, iPTH, or phosphate measurements (p-value = 0.6) (Table 3).
Effect of vitamin D3 supplementation on body composition
FM (MC − 4.3 kg; 95% CI, − 7.0/− 1.1, p-value =0.005), BFP (MC -8.1; 95% CI, − 12.0/− 4.2, p-value =0.007) and FMI (MC -1.6; 95% CI, − 2.6/− 0.5, p-value =0.005) were significantly decreased in the VD2 group as compared with the VD1 and PL groups. After adjusting for intake of SFA and mean change of weight, the statistical difference of FMI (p-value = 0.006), FM (p-value = 0.007), and BFP (p-value = 0.007) in the three groups remained significant. A significant difference was found between VD2, VD1 (p-value = 0.02), and PL (p-value = 0.008) groups in FM changes. In addition, a significant difference was observed between VD2, VD1 (p-value = 0.05), and PL (p-value = 0.008) groups in BFP changes. Furthermore, FMI changes were different between VD2 and VD1 (p-value = 0.02) and VD2 and PL groups (p-value = 0.008). Vitamin D3 supplementation showed no significant effect on body weight, BMI, WC, FFM, skeletal muscle mass, and RFMI (Table 4).
The relationship between stratified baseline and achieved serum 25(OH) D status and mean changes of body composition measures
More than 50% of participants had vitamin D deficiency at the study’s baseline. However, after 12 weeks of vitamin D supplementation, 70.5% of women who achieved sufficient serum 25(OH) D, of whom 41.9% were in the 2000 IU and 58.1% in the 4000 IU group. Mothers with baseline insufficient serum 25(OH) D (< 20 ng/ml) showed higher reduction in BMI, WC, RFMI, FM, PBF, and FMI. In addition, mothers who achieved sufficient serum 25(OH) D (> = 20 ng/ml) showed a higher reduction in weight, BMI, FM, PBF, and FMI. However, these differences were not statistically significant except for FMI (p-value = 0.04) and FM, which was close to significant (p = 0.055) (Table 5).
Discussion
Supplementation with 4000 IU vitamin D3 decreased FM, BFP, and FMI in nursing mothers with overweight or obese as compared with 2000 IU and placebo. Nevertheless, vitamin D3 supplementation did not significantly affect body weight, BMI, WC, FFM, skeletal muscle mass, and RFMI.
Only one previous study evaluated the effect of vitamin D supplementation on weight status in nursing mothers [25], which showed no effect of 1200 IU/d vitamin D on body composition. The results of a meta-analysis reported that by decreasing BMI and WC, vitamin D3 supplementation had a desirable effect on weight loss. However, the adequate dosages and supplementation duration are unclear [32]. In addition, no data were provided on other measures of body composition, such as FM and BFP. Another meta-analysis reported vitamin D supplementation did not affect BFP [33]. Since, the effect of vitamin D supplementation on weight loss is influenced by a large number of factors [34]. The results of clinical trials investigating the effect of vitamin D supplementation on weight loss in subjects with overweight or obese are inconclusive [35,36,37]. The study population is one of the primary causes of the disparity between our clinical trial outcomes and those of others. A systematic review study reported that most clinical trials with the null effect of vitamin D on health were performed in populations without vitamin D deficiency [38]. So possible beneficial effects from vitamin D supplementation cannot be excluded. Besides, in most clinical trials, a combined treatment of vitamin D and other alternatives was used [21, 35, 39].
At the end of the present study, the mean serum 25(OH) D in 2000 IU and placebo groups was decreased. A decrease in serum 25(OH) D concentrations during lactation has been shown [13]. Postpartum deterioration of 25(OH) D status might be explained by less frequent multivitamin intake during lactation [14, 40]. Therefore, a decrease in serum 25(OH) D concentrations in the placebo group was expected. Since each 400 IU/d of 25(OH) D increases serum 25(OH) D concentrations by 2.8 ng/ml in individuals with a normal BMI [41], it was expected that vitamin D3 supplementation at the dose of 2000 IU/d also increased maternal serum 25(OH) D concentrations similar to other studies [42, 43]. There are probably several reasons for this finding. First, an increase in serum 25(OH) D concentrations after vitamin D supplementation has been reported to be 30% lower in obese individuals compared with non-obese ones [44]. Therefore, the Society of Endocrine Guideline recommends that adults who suffer from overweight or obesity need 2 to 3 times more vitamin D supplementation (6000–10,000 IU/d) to treat and prevent vitamin D deficiency [8, 9]. Second, the physiological condition of the mothers during breastfeeding is very important. Approximately 20% of the 25 (OH) D in mother’s milk is transferred daily to the infant through breast milk [45].
In the present study, as in the only similar previous study [42], the dose of 4000 IU/d seems still too low to fully replenish vitamin D insufficiency in all participants. As previously explained, this is probably because the response of serum concentration of 25(OH) D to vitamin D supplementation is low during lactation due to the increase in the body’s need for maintaining bone mass [45], the transfer of 25(OH) D to milk and also the presence of overweight or obesity in the participants [35].
Mothers who achieved sufficient serum 25(OH) D showed a higher reduction in body composition measures. Similar results have been reported in other studies [34, 46]. However, these differences were not statistically significant except for FMI and FM, which were close to significant. This might be related to insufficient sample size in each serum category 25(OH) D. There are various mechanisms by which vitamin D has important roles in fat tissue metabolism [47]. Cholecalciferol supports intestinal calcium absorption, which can contribute to weight loss [39]. In addition, 25(OH) D prevents the rise in PTH secretion, which is associated with a reduction in lipolysis [48]. 25(OH) D also increases the expression of the peroxisome proliferator-activated receptor gamma (PPAR-γ) gene, which improves fatty acid metabolism [49].
Our study had several strengths and limitations. This was the first study investigating the effect of supplementation with vitamin D3 at either 2000 IU/day or 4000 IU/day on body composition and serum 25(OH) D concentration in nursing mothers with overweight or obesity. Another strength was that this was a randomized, double-blind, placebo-controlled clinical trial with an appropriate sample size for determining a reasonable effect size. The primary limitation of our study was that we could not assess body composition by Dual-Energy X-Ray Absorptiometry (DEXA) as a gold standard methodology. However, BIA is a validated and reliable method to assess body composition [24]. The second limitation was that liver fat or separate visceral and subcutaneous body fat was not determined in participants. The third potential limitation was the short duration of the intervention. The fourth limitation was the lack of measuring lipid profile, blood glucose and insulin sensitivity.
In conclusion, vitamin D3 supplementation at a dose of 4000 IU/d in nursing mothers with overweight or obesity improved serum 25(OH) D concentration, which had a beneficial effect on FM, BFP, and FMI. However, future long-term studies with different doses are required to confirm the results and determine the impact of vitamin D3 supplementation on liver fat and separate visceral and subcutaneous body fat in nursing mothers with overweight or obesity.
Availability of data and materials
The datasets generated during the current study are available via the corresponding author (GS) on reasonable request.
Abbreviations
- ANOVA:
-
One-way analysis of variance
- BFP:
-
Body fat percentage
- BIA:
-
Body impedance analyses
- BMI:
-
Body mass index
- DXA:
-
Dual X-ray absorptiometry
- ECL:
-
Electro chemiluminescence
- ELISA:
-
Enzyme-linked immune sorbent assay
- FMI:
-
Fat mass index
- FM:
-
Fat mass
- FFM:
-
Fat free mass
- ITT:
-
Intention-to-treat
- MET:
-
Metabolic equivalents
- MC:
-
Mean change
- PTH:
-
Parathyroid hormone
- RFMI:
-
Relative fat mass index
- SD:
-
Standard deviation
- SFA:
-
Saturated fatty acids
- VD1:
-
Group of vitamin D3 supplementation (2000 IU/d)
- VD2:
-
Group of vitamin D3 supplementation (4000 IU/d)
- WC:
-
Waist circumference
- 25(OH) D:
-
25-Hydroxy vitamin D
References
Ota E, Haruna M, Suzuki M, Anh DD, Tho le H, Tam NT, et al. Maternal body mass index and gestational weight gain and their association with perinatal outcomes in Viet Nam. Bull World Health Org. 2011;89(2):127–36. https://doi.org/10.2471/BLT.10.077982.
Abedini Z, Ahmari Tehran H, Khorrami Rad A. Calorie intake and the related factors in lactating mothers referring to health centers. J Mazandaran Univ Med Sci. 2012;21:271–8.
Akbarzade M, Rafiee B, Asadi N, Zare N. Correlation between Maternal Body Mass Index, Non-stress Test Parameters and Pregnancy Outcomes in Nulliparous Women. Women’s Health Bull. 2014;1(3):1–5. https://doi.org/10.17795/whb-23649.
Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201(58):e1–58 e8.
Lacroix M, Battista MC, Doyon M, Moreau J, Patenaude J, Guillemette L, et al. Higher maternal leptin levels at second trimester are associated with subsequent greater gestational weight gain in late pregnancy. BMC Pregnancy Childbirth. 2016;16:62.
Olson CMMS, Strawderman. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc. 2003;103:48–54.
McAree T. Obesity and vitamin D deficiency–current concepts on their impact on pregnancy. Eur Endocrinal. 9:125. https://doi.org/10.17925/EurEndocrinal..2013;09.02.125.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. https://doi.org/10.1210/jc.2011-0385.
Zuk A, Fitzpatrick T, Rosella LC. Effect of vitamin D3 supplementation on inflammatory markers and glycemic measures among overweight or obese adults: a systematic review of randomized controlled trials. PLoS ONE. 2016;11:e0154215. https://doi.org/10.1371/journal.pone.0154215.
Spiro A, Buttriss JL. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr Bull. 2014;39(4):322–50. https://doi.org/10.1111/nbu.12108.
Gilmore LA, M Klempel-Donchenko LM. Redman. Pregnancy as a window to future health: excessive gestational weight gain and obesity. Semin Perinatol. 2015:296–303.
Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(429):e1–429 e9.
Milman N, Hvas A-M, Bergholt T. Vitamin D status during normal pregnancy and postpartum. J Perinat Med. 2012;40:57–61.
Wawrzyniak A, Hamułka J, Gorzel K. Assessment of vitamins and minerals intake with supplements during breast-feeding. Roczniki Panstwowego Zakladu Higieny. 2009;60:35356.
Abdollahi M, Mohammadi F, Houshiar-Rad A, Ghaffarpur M, Ghodsi D, Kalantari N. The Comprehensive Study on Household Food Consumption Patterns and Nutritional Status of I.R.Iran, 2001–2003. Ann Nutr Metab. 2005;49:72.
Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417.
Pannu K, Zhao Y, Soares MJ. Reductions in body weight and percent fat mass increase the vitamin D status of obese subjects: a systematic review and metaregression analysis. Nutr Res. 2016;36:201–13.
Schlabritz-Loutsevitch NE, Comuzzie AG, Mahaney MM, Hubbard GB, Dick EJ, Kocak J, et al. Serum vitamin D concentrations in Baboons (Papio spp.) during pregnancy and obesity. Comp Med. 2016;66:137–42.
Hamułka J, Wawrzyniak A, Pawłowska R. Assessment of vitamins and minerals intake with supplements in pregnant women. Rocz Panstw Zakl Hig. 2010;61:269–75.
Samuel L, Borrell LN. The effect of body mass index on optimal vitamin D status in U.S. adults: the National Health and Nutrition Examination Survey 2001-2006. Ann Epidemiol. 2013;23(7):409–14. https://doi.org/10.1016/j.annepidem.2013.05.011.
Basile LA, Taylor SN, Wagner CL, Horst RL, Hollis BW. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breast Med. 2006;1:27–35.
Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25OHD in thin and obese women. J Steroid Biochem Mol Biol. 2013;136:195–200.
Drincic AT, Armas LA, Van Diest EE, Heaney R. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring). 2012;20:1444–8.
McLester CN, Nickerson BS, Kliszczewicz BM, McLester JR. Reliability and agreement of various inbody body composition analyzers as compared to dual-energy x-ray absorptiometry in healthy men and women. J Clin Densitom. 2020;23(3):443–50. https://doi.org/10.1016/j.jocd.2018.10.008 2018 Nov 3. PMID: 30472111.
Czech-Kowalska J, Latka-Grot J, Bulsiewicz D, Jaworski M, Pludowski P, Wygledowska G, et al. Impact of vitamin D supplementation during lactation on vitamin D status and body composition of mother-infant pairs: a MAVID randomized controlled trial. PloS One. 2014;9:e107708. https://doi.org/10.1371/journal.pone.0107708.
Roosta S, Kharadmand M, Teymoori F, Birjandi M, Adine A, Falahi E. Effect of vitamin D supplementation on anthropometric indices among overweight and obese women: A double blind randomized controlled clinical trial. Nutr DOC. 2018;12:537–41.
Haghdoost A, Baneshi MR, Marzban M. Review article: How to estimate the sample size in special condition? (Part two). Iran. J Epidemiol. 2011;7(2):67–74 [In Persian].Available from https://www.sid.ir/en/journal/ViewPaper.aspx?id=259495.
Gerveieeha Z, Siassi F, Qorbani M, Ziaeian F, Sotoudeh G. the effect of different amounts of vitamin D supplementation on serum, anthropometric status, and body composition in overweight or obese nursing women: a study protocol for a randomized placebo-controlled clinical trial. Trials. 2019;20:542. https://doi.org/10.1186/s13063-019-3622-y.
Zehni K, Ashjaardalan A, Bagherisaweh MI, Rokhzadi MZ. The serum level of 25 hydroxy vitamin D and factors affecting its level among students in Kurdistan University of Medical Sciences in 2015. BUMS. 2015;20:1–10.
Peltz G, Aguirre MT, Sanderson M, Fadden MK. The role of fat mass index in determining obesity. Am J Hum Biol. 2016;22:639–47. https://doi.org/10.1002/ajhb.21056.
Woolcott, Bergman RN. Relative fat mass (RFM) as a new estimator of whole-body fat percentage─ A cross-sectional study in American adult individuals. Sci Rep. 2018;8(1):1–11.
Perna S. Is Vitamin D supplementation useful for weight loss programs? A systematic review and meta-analysis of randomized controlled trials. Jur Medicina. 2019;55:368.
Golzarand M, Hollis BW, Mirmiran P, Wagner CL, Shab-Bidar S. Vitamin D supplementation and body fat mass: a systematic review and meta-analysis. Eur J Clin Nutr. 2018;72(10):1345–57. https://doi.org/10.1038/s41430-018-0132-z Epub 2018 Mar 21. PMID: 29563638.
Salehpour A, Hosseinpanah F, Shidfar F, Vafa M, Razaghi M, Dehghani S, et al. A 12-week double-blind randomized clinical trial of vitamin D 3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:1–8.
Zhu W, Cai D, Wang Y, Lin N, Hu Q, Qi Y, et al. Calcium plus vitamin D3 supplementation facilitated fat loss in overweight and obese college students with very-low calcium consumption: a randomized controlled trial. Nutr J. 2013;12:8. https://doi.org/10.1186/1475-2891-12-8.
Ghavamzadeh S, Mobasseri M, Mahdavi R. The effect of vitamin D supplementation on adiposity, blood glycated hemoglobin, serum leptin and tumor necrosis factor-α in type 2 diabetic patients. Int J Prev Med. 2014;5:1091.
Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur J Endocrinol. 2008;159(6):675–84. https://doi.org/10.1530/EJE-08-0339.
Rejnmark L, Bislev LS, Cashman KD, Eiríksdottir G, Gaksch M, Grübler M, et al. Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS One. 2017;1:–e0180512.
Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipid Health Dis. 2012;11:1–9.
Specker BL, Tsang RC, Ho ML. Changes in calcium homeostasis over the first year postpartum: effect of lactation and weaning. Bio J Obstet Gynecol. 1991;78:56–62.
Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Ame J Clin Nutr. 2003;77:204–10.
Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. Journal of bone and mineral research. J Bone Miner Res. 2011;26:2341–57. https://doi.org/10.1002/jbmr.463.
Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Ame J Clin Nutr. 2004;79:717–26.
Zakharova I, Klimov L, Kuryaninova V, Nikitina I, Malyavskaya S, Dolbnya S, et al. Vitamin D insufficiency in overweight and obese children and adolescents. Front Endocrinol. 2019;10:103. https://doi.org/10.3389/fendo.2019.00103.
Hollis BW, Wagner CL, Howard CR, Ebeling M, Shary JR, Smith PG, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. Erratum in: Pediatrics. 2019;7:144(1). https://doi.org/10.1542/peds.2015-1669.
Lotfi-Dizaji L, Mahboob S, Aliashrafi S, Vaghef-Mehrabany E, Ebrahimi-Mameghani M, Morovati A. Effect of vitamin D supplementation along with weight loss diet on meta-inflammation and fat mass in obese subjects with vitamin D deficiency: a double-blind placebo-controlled randomized clinical trial. Clin Endocrinol (Oxf). 2019;90(1):94–101. https://doi.org/10.1111/cen.13861 Epub 2018 Nov 12. PMID: 30246883.
Taheri E, Saedisomeolia A, Djalali M, Qorbani M, Madani CM. The relationship between serum 25-hydroxy vitamin D concentration and obesity in type 2 diabetic patients and healthy subjects. J Diabetes Metab Disord. 2012;11:16. https://doi.org/10.1186/2251-6581-11-.
Saedisomeolia A, Taheri E, Djalali M, Moghadam AM, Qorbani M. Association between serum level of vitamin D and lipid profiles in type 2 diabetic patients in Iran. J Diabetes Metab Disord. 2014;13:1–5. https://doi.org/10.1186/2251-6581-13-7.
Wimalawansa S. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol. 2018;175:177–89. https://doi.org/10.1016/j.jsbmb.
Acknowledgements
The authors appreciate the participation of the mothers in this study.
Funding
This study has been supported by Tehran University of Medical Sciences (Grant No. 97–02–161-38838(. The funder has no role in study design, collection, analysis and interpretation of data, writing of paper, or decision to submit for paper.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: GS, FS and MQ contributed to the conception, design, statistical analysis and approval of the final version of the manuscript. MHA supervised the biochemical assay. ZG and RSH organized participant management, data collection and data interpretation. ZG drafted the manuscript and all authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Tehran University of Medical Sciences approved the study protocol (IR.TUMS REC 1397429). All methods were performed in accordance with the Helsinki Declaration. Also, the study procedure was explained to each participant and written informed consent form was signed by the participants at the beginning of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gerveieeha, Z., Siassi, F., Qorbani, M. et al. The effect of vitamin D supplementation on body composition in nursing mothers with overweight or obesity: a randomized double-blind placebo-controlled clinical trial. BMC Nutr 9, 1 (2023). https://doi.org/10.1186/s40795-022-00664-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-022-00664-y