Abstract
Objective
The current study was aimed to evaluate the effects of active form of vitamin D on TGF- β, NF-κB and MCP-1 in heart tissue of obese rats.
Methods
Forty rats were allocated into groups of normal diet and high fat diet for sixteen weeks; then each group was divided into two groups that received either 500 IU/kg vitamin D or placebo for five weeks. Biochemical parameters were assessed by ELISA kits.
Results
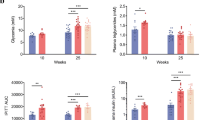
Vitamin D reduced TGF-β in obese rats supplemented with vitamin D compared with other groups (P = 0.03). Moreover, vitamin D reduced MCP-1 concentrations in the heart tissues of both vitamin D administered groups compared to placebo one (P = 0.002). NF-κB in the heart of HFD + vitamin D group was significantly lower (P = 0.03). Current study also showed that vitamin D improves glycemic status and reduce insulin resistance significantly in HFD group (P = 0.008).
Conclusion
Vitamin D was a potential anti- inflammatory mediator of cardiovascular disease and markers of glycemic status in obese rats. Further investigations are needed to better identify the therapeutic role of this vitamin in CVD and to elucidate the underlying mechanisms.
Similar content being viewed by others
Introduction
Overweight and obesity lead to adverse metabolic consequences like hypertension, hypercholesterolemia, and hypertriglyceridemia and insulin resistance [1, 2]. In fact, the mortality and morbidity of cardiovascular disease (CVD) is meaningfully higher among obese individuals. The possible reason is that the enlarged adipose tissue mass in obese individuals is a potent source of numerous peptides or nonpeptides that play a role in cardiovascular homeostasis; these molecules including interleukin (IL)-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, monocyte chemoattractant protein (MCP)-1 and nuklear factor kappa (NF-κB) are secreted from adipose tissue and have inflammatory actions against cardiac health [3]. It is clear that several inflammatory mediators have a critical role in the pathogenesis of obesity-related cardiovascular events and blockage in their expression is considered as a target of CVD treatment. TGF-β is expressed at high concentrations in the heart tissue of fetus and adults. In the rat heart, TGF-β is located in cardio-myocytes and extracellular matrix [4]. It is involved in the cardiac valve morphogenesis and is a potent activator of fibroblasts [5, 6]. TGF-β involves in myocardial injury [7] and contributes in unresolved cardiac pro-fibrotic remodeling in heart failure [8,9,10]. Moroever, TGF-β and MCP-1 act in the pathogenesis of cardiac events and myocardial infarction both together; MCP-1 stimulates TGF- β production in infracted heart. Moreover, MCP-1 induces TGF- β secretion, stimulates collagen synthesis and increases fibrogenic potential of mature macrophages [11]. MCP-1, attracts mononuclear cells and is involved in the atherosclerosis [12]. Blocking the MCP-1 expression and secretion reduces the severity of myocardial inflammation in myocardial events [13].
NFκB, another inflammatory protein involved in the expression and production of MCP-1, is a potent stimulator of inflammatory process in cardiovascular disease; its activation stimulates the transcription of several inflammatory genes such as MCP-1, TNF-α, TGF-β and IL-6 and promotes plaque formation [14]. NFκB in cardiac myocytes is activated in response to IL-1β, hydrogen peroxide and myocardial ischemia and promotes cardiac hypertrophy and nitric oxide production and cardiovascular events [15,16,17]. Above mentioned explanation highlighted the role of integrated inflammatory molecules including MCP-1, NF-κB and TGF-β in the pathogenesis of CVD; therefore, inhibition of their production and blocking their expression could be a therapeutic approach in treatmet of CVD.
Beside the classic role of vitamin D in the growth and mineralization of bone, this steroid endocrine hormone has other health benefits [18, 19]. Several evidence show that vitamin D deficiency can be associated with cardiovascular morbidity and mortality [20, 21]. It has been shown that, 25-OHD deficiency and parathyroid hormone (PTH) excess are associated with risk of cardiovascular diseases through divergent pathways among older adults [22, 23]. Similarly, Valcour et al. pointed to a smoothly decreasing relationship between vitamin D and PTH level; increasing serum 25-OHD levels are associated with decreasing PTH levels [24].
A follow-up study revealed higher death rate among vitamin D –deficient individuals [25]. Vitamin D receptor (VDR) is expressed in cardiovascular system [26] and clinical evidence have reported that vitamin D exerts a beneficial role in cardiac remodeling and heart failure survival [27,28,29]. Vitamin D inhibits TGF-β -induced cardiac tissue atrophy [10] and pro-fibrotic effects of TGF-β [30] and reduces TGF-β and MCP-1 expression in vivo [31].
Although there are some inconsistent results about the effects of vitamin D supplementation on cardiovascular survival; however, a meta-analysis in this regard elucidated that vitamin D supplementation inhibits ventricular remodelling and improves cardiac function in patients with heart failure [32]. Similarly, C D’Amore’s et al. review reported that vitamin D deficiency may favor the onset and/or progression of heart failure and left ventricular remodeling and is associated with more adverse prognosis in heart failure patients [33]. Also, in the study of Gostman et al. vitamin D deficiency was highly prevalent in heart failure patients and was a significant predictor of reduced survival. While, vitamin D supplementation was associated with improved outcome [34]. As far as we know, the pivotal role of vitamin D in CVD is not understood well. In the previous published work of the current project [35], we observed the significant potential effects of vitamin D on reducing TNF-α and oxidative stress. In the current study, we aimed to evaluate the role of vitamin D on glycemic status and cardiac tissue concentrations of TGF-β, MCP-1 and NF-κB in obese rats.
Methods
Animals
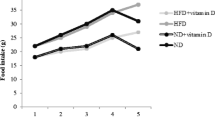
We purchased forty rats with 200–220 g of weight from the Pasteur institute animal care center (Karaj, Iran). In the standard condition of temperature and ad libitum food, animals were housed. The study procedure was in accordance of National Institutes of Health ethical guidelines. The ethics committee registration code is TBZMED.REC.1400.068. The study is also reported in accordance with ARRIVE guidelines. Rats were fed a standard diet for one week, then were randomly assigned into two groups of normal diet (ND) or high fat diet (HFD) for four months [36]. Then after 4 months, both groups were randomly allocated in to subgroups of ND, ND + vitamin D, HFD and HFD + vitamin D [e.g. 500 IU/kg/d vitamin D or Migliol as placebo (Sigma Adrich, USA)] for five weeks.
Rats’ anesthesia procedure was performed after one week acclimation period to prevent stress-induced response. After 24 h fasting, rats were anesthetized with peritoneal injection of 6.6 mg/kg Ketamin and 0.3 mg/ kg of Xylazine. Cardiac puncture blood samples were obtained and centrifuged for sera extraction. Consequently, the rats were sacrificed by decapitation under guillotine consisting of a metal frame and a sharp blade, and being operated by one hand. After decapitation of animals, the hemisphere of heart samples were removed for further assays.
ELISA
After homogenization of heart tissues in the phosphate-buffered saline and centrifuging them, the clear supernatants were collected for evaluation of total protein, MCP-1, NF-κB and TGF-β concentrations (e.g. Pars Azmun, Karaj and Hangzhou Eastbiopharm, China). Fasting serum glucose were assessed by UV-VIS spectrophotometer (Ultra-spec 2000, Pfizer, USA). Vitamin D and insulin was determined by ELISA kits (Eastbiopharm, China). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as below:
Statistical assay
SPSS software version 16 was used for statistical assays. Kolmogorov–Smirnov test was used to check the normality of variables. Data were presented as the mean ± SD. One-way analysis of variance (ANOVA) followed by the post hoc Tukey’s tests was used to compare the values between multiple groups. Paired sample t test was used to compare the parameters before and after supplementation. P values < 0.05 were considered as significance level.
Results
The mean value of measured parameters are presented in Table 1 in all study groups. Post hoc analysis illustrated a significant difference in terms of TGF-β in the heart tissues of vitamin D-administered obese rats between HFD + vitamin D and HFD and ND + vitamin D groups (P = 0.03). Also, intergroup comparisons showed a significant difference between HFD and HFD + vitamin D groups (P = 0.002) and between ND and ND + vitamin D groups (P = 0.03). Accordingly, cardiac tissue concentrations of NF-κB in the vitamin D-administered obese rats was lower compared with HFD group (P = 0.03). Also, the NF-κB concentrations in HFD group was significantly higher than ND group (P = 0.04). The correlation matrix between inflammatory parameters in the cardiac tissue and markers of glycemic status (Table 2) showed a strong and significant association between cardiac TGF-β and insulin concentrations (r = 0.63, P = 0.04). No significant changes in fasting serum glucose in HFD compared to ND group was observed while there was an increase in serum insulin concentrations (P = 0.04) and HOMA-IR index (P = 0006) in HFD induced obese rats compared to ND group. Moreover, vitamin D-administered group of obese rats revealed reduced insulin (P = 0.01) and HOMA-IR values (P = 0.008) compared to HFD group. However, no significant changes was shown for glucose level after vitamin D administration (P > 0.05) (Table 1).
Discussion
In the present study, five weeks administration of vitamin D had meaningful effects in reducing TGF-β, MCP-1 and NF-κB concentrations in the heart tissue of obese rats. Vitamin D decreased TGF-β concentrations in HFD + D group even more than ND group; this might be explained by vitamin D structure as a fat soluble vitamin which has a better absorption and metabolism in a high-fat diet. Although, HFD could not induce TGF-β elevation in comparison to ND, but the beneficial role of vitamin D in reducing TGF-β in HFD + vitamin D shouldn’t be ignored. Similar to our report, Sousa-Pinto, et al. characterized the patterns of TGF-β expression in fat depots of rats fed by a high fat diet in comparison to control group and evaluated the mRNA expression levels of TGF-β in all study groups by real-time polymerase chain reaction (PCR). They reported lower expression of TGF- β in retroperitoneal and epididymal adipose tissue in spite of feeding with high fat diet [37]. One possible explanation, is the differential effects of dietary carbohydrate versus dietary fat on pro-inflammatory markers; as we explained before, the composition of normal diet was 60% of carbohydrate, 30% of protein and 10% of dietary fat while high fat diet comprised of 59% of fat, 30% of carbohydrate and 11% of protein. In other published data from the current project, we demonstrated organ-specific effects of dietary carbohydrate and fat in producing inflammation in the human body; for example, high carbohydrate diet but not high fat diet, exerted strong effect in increasing TNF- α in cardiac tissue; whereas, the role of HFD in inducing pro-inflammatory response was more pronounced in renal tissues of rats [38] even though, in several human studies, dietary carbohydrate exerted more strong inflammatory response compared with dietary fat; in the study by Karimi E et al. [39] similar to our finding, high carbohydrate diet was positively associated with serum TGF-β and MCP-1 concentrations compared with dietary fat among 360 obese women while high fat diet was in negative association with TGF-β levels (β= -0.95, P = 0.05); possible role of carbohydrate in vascularization and angiogenesis might be attributed to the central role of it in TGF-β induction. Accordingly, the borderline non-significant increase in NF-κB activity in ND + vitamin D group compared with HFD group can be attributed to the higher carbohydrate amount of ND diet. This finding is in agreement of several previous studies that reported the potent positive role of dietary carbohydrate in increasing the receptor activator of the nuclear factor kappa-B ligand (RANKL) pathway [40, 41]. Moreover, vitamin D exerted reduced TGF-β concentrations in the cardiac tissue of HFD + vitamin D rats in our work. The role of TGF-β in myofibroblastic activation, increased collagen deposition and fibrosis in the myocardial injury has been reported previously [8, 9]. It has been suggested that gene therapy against TGF-β signaling pathways attenuates heart failure, left ventricular remodeling, and modulates infarcted tissue dynamics [8]. Vitamin D is an inhibitor of TGF-β expression, reduces cardiac myofibroblast activation, alters TGF-β pathway and reduces its bioavailability [10, 42, 43]. The anti-inflammatory role of vitamin D has also been shown previously [44, 45].
Vitamin D also demonstrated its anti-inflammatory properties against MCP-1 concentrations in HFD + vitamin D group. In agreement with our results, vitamin D caused a decline in the expression of MCP-1 in monocytes and down-regulated the lipopolysaccharide (LPS)-induced MCP-1 production in macrophages [46]. MCP-1 and its CCR2 receptor are considered as main targets of gene therapy for inflammation [47].
In the study by Mousa et al. [48] vitamin D had no effects on inflammatory parameters or NF-κB activity in peripheral blood mono-nuclear cells (PBMCs) of obese individuals. The possible reason for this controversy is the difference in the study procedure and design; the Moussa’s study was performed in vitamin D-deficient individuals and probably the dosage of vitamin D was insufficient to represent its anti-inflammatory actions; sixty-five overweight/obese, vitamin D-deficient (25-hydroxyvitamin D [25(OH)D] ≤ 50 nmol/L) adults were randomized to a single 100,000 IU bolus followed by 4,000 IU daily cholecalciferol or matching placebo for 16 weeks. Although in-vitro studies reported anti-inflammatory effects of vitamin D, this study didn’t show any effects of vitamin D supplementation on inflammatory markers or NFκB activity in-vivo in humans. However; more investigations are needed to reveal the underlying mechanisms of these controversies. Reduction of NF-κB after vitamin D supplementation in the current work could be explained by binding of vitamin D to VDR and suppressing NF-κB activation by direct interaction with IKKβ [49]. It has been shown that vitamin D reduces NF-kB translocation to the nucleus and reduces smooth muscle cell proliferation [50]. In the current study, vitamin D also improved markers of glycemic status. In our previous work, vitamin D represented amelioration in lipid abnormalities [35].
Conclusion
This work for the first time, revealed the ameliorative effects of vitamin D against inflammation in the cardiac tissue of obese rats. Further investigation in human models are warranted to confirm the findings of the current study.
Limitations
The results of the current study could not be generalized to human. Therefore, similar studies in human models are warranted. Moreover, it is suggested to evaluate histologic changes in cardiac tissue of rats for better clarification of underlying mechanisms.
Availability of data and materials
The data that support the findings of this study are available from corresponding author but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of corresponding author.
Abbreviations
- ANOVA:
-
Analysis of variance
- CAD:
-
Coronary artery disease
- CVD:
-
Cardiovascular disease
- HDL:
-
High density lipoprotein cholesterol
- LDL:
-
Low density lipoprotein cholesterol
- MDA:
-
Malondialdehyde
- NADH:
-
Nicotinamide adenine dinucleotide
- TC:
-
Total cholesterol
- TGF-β:
-
Transforming growth factor β
- TNF-α:
-
Tumor necrosis factor α
- MCP-1:
-
Monocyte chemoattractant protein 1
- IL-6:
-
Interleukin 6
References
Organization WH. Obesity: situation and trends. Global Health Observatory (GHO) data: Risk factors; 2017.
Gortmaker SL, et al. Changing the future of obesity: science, policy, and action. The Lancet. 2011;378(9793):838–47.
Poirier P, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Circulation. 2006;113:898–918.
Thompson NL, et al. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989;108:661–9.
Heine U, et al. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987;105:2861–76.
Kane CJ, et al. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157–73.
Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta. Mol Genet Metab. 2000;71:418–35.
Okada H, et al. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–7.
Ikeuchi M, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004;64:526–35.
Meredith A, et al. 1,25 Dihydroxyvitamin D3 inhibits TGFβ1- mediated primary human cardiac myofibroblast activation. PLoS ONE. 2015;10(6):e0128655.
Dobaczewski M, Frangogiannis N. Chemokines and cardiac fibrosis. Front Biosci (Schol Ed). 2010;1:391–405.
Aukrust P, et al. Elevated circulating levels of C–C chemokines in patients with congestive heart failure. Circulation. 1998;97:1136–43.
Goser S, et al. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation. 2005;112:3400–7.
Zhuo JL. Monocyte chemoattractant protein-1: a key mediator of angiotensin II-induced target organ damage in hypertensive heart disease? J Hypertens. 2004;22(3):451–4.
Norman DAM, Yacoub MH, Barton PJR. Nuclear factor NF-kB in myocardium: developmental expression of subunits and activation by interleukin-1b in cardiac myocytes in vitro. Cardiovasc Res. 1998;39:434–41.
Zelarayan L, et al. NF-kB activation is required for adaptive cardiac hypertrophy. Cardiovasc Res. 2009;84:416–24.
Bond M, et al. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and – 9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50(3):556–65.
Janda M, et al. Knowledge about health benefits of vitamin D in Queensland Australia. Prev Med. 2010;50(4):215–6.
Hewison M. An update on vitamin D and human immunity. Clin Endocrinol. 2012;76(3):315–25.
el Maaty MAA, Gad MZ. Vitamin D deficiency and cardiovascular disease: potential mechanisms and novel perspectives. J Nutri Sci Vitaminol. 2013;59(6):479–88.
Jorge AJL, et al. Vitamin D deficiency and cardiovascular diseases. Int J Cardiovasc Sci. 2018;31:422–32.
Kestenbaum B, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–41.
Jassal SK, et al. Vitamin d, parathyroid hormone, and cardiovascular mortality in older adults: the Rancho Bernardo study. Am J Med. 2010;123(12):1114–20.
Valcour A, et al. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metabolism. 2012;97(11):3989–95.
Murr C, et al. Vitamin D deficiency parallels inflammation and immune activation, the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chem Lab Med. 2012;50(12):2205–12.
Chen S, et al. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106–12.
Rahman A, et al. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–9.
Argacha JF, et al. Vitamin D deficiency-induced hypertension is associated with vascular oxidative stress and altered heart gene expression. J Cardiovasc Pharmacol. 2011;58(1):65–71.
Gode S, et al. Effect of vitamin D deficiency on the development of postoperative atrial fibrillation in coronary artery bypass patients. J Cardiovasc Thorac Res. 2016;8(4):140–6.
Ramirez AM, et al. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor β1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010;118(3):142–50.
Zeng X, et al. Effects of 1, 25-dihydroxyvitamin D3 on expression of TGF-β1, CD68 and MCP-1 in type 2 diabetic nephropathy rat. Biomed Res. 2017;28(11):4797–802.
Zhao J-D, et al. Effect of vitamin D on ventricular remodelling in heart failure: a meta-analysis of randomised controlled trials. BMJ open. 2018;8(8):e020545.
D’Amore C, et al. Vitamin D deficiency and clinical outcome in patients with chronic heart failure: a review. Nutr Metabolism Cardiovasc Dis. 2017;27(10):837–49.
Gotsman I, et al. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail. 2012;14(4):357–66.
Farhangi MA, et al. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat-diet induced obese rats. BMC Cardiovasc Disord. 2017;17(1):161–7.
Sobesky JL, et al. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1β, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun. 2014;42:22–32.
Sousa-Pinto B, et al. Characterization of TGF-β expression and signaling profile in the adipose tissue of rats fed with high-fat and energy-restricted diets. J Nutr Biochem. 2016;38:107–15.
Farhangi MA, Mesgari-Abbasi M, Shahabi P. Cardio-renal metabolic syndrome and pro-inflammatory factors: the differential effects of dietary carbohydrate and fat. Acta Endocrinol. 2019;15(4):436–41.
Karimi E, et al. High carbohydrate intakes may predict more inflammatory status than high fat intake in pre-menopause women with overweight or obesity: a cross-sectional study. BMC Res Note. 2021;14:279–85.
Sirjani M, et al. The effects of high fat, low carbohydrate and low fat, high carbohydrate diets on tumor necrosis factor superfamily proteins and proinflammatory cytokines in C57BL/6 mice. Iran J Allergy Asthma Immunol. 2014;13(4):247–55.
Mousavi SN, et al. Comparison of maternal isocaloric high carbohydrate and high fat diets on osteogenic and adipogenic genes expression in adolescent mice offspring. Nutr Metab. 2016;13(1):69–72.
Irani M, et al. Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: a randomized placebo-controlled trial. J Clin End Met. 2015;100(11):4307–14.
Aschenbrenner JK, et al. 1, 25-(OH2) D3 alters the transforming growth factor β signaling pathway in renal tissue. J Surg Res. 2001;100(2):171–5.
Calton EK, et al. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS ONE. 2015;10(11):e0141770.
Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annual Rev Pharm Toxicol. 2011;51:311–36.
Wang YC, et al. Effect of vitamin D3 on monocyte chemoattractant protein 1 production in monocytes and macrophages. Acta Cardiol Sinica. 2014;30(2):144.
Matoba T, Egashira K. Anti-inflammatory gene therapy for cardiovascular disease. Curr Gene Ther. 2011;11:442–6.
Mousa A, et al. Effect of vitamin D supplementation on inflammation and nuclear factor kappa-B activity in overweight/obese adults: a randomized placebo-controlled trial. Sci Rep. 2017;7:15154.
Chen Y, et al. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem. 2013;288(27):19450–8.
Agrawal DK. Vitamin D and immunomodulation in coronary artery disease. In: Department of Clinical and Translational Science. United States of America: Creighton University; 2017.
Acknowledgements
We are thankful from Student Research Committee, Tabriz University of Medical Sciences for their financial support (Grant number: 67559).
Funding
This research has been performed by a grant from Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
MAF and FE was the main researcher, designed the project, wrote the manuscript and performed the statistical analysis, revised the manuscript and supervised the project. MM was involved in data collection, analysis and also revision, MMA and AZT were involved in laboratory works and experimental design of the work and revision. FJ was involved in hypothesis generation and designing the first project. Also, she was involved in revision. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animal experiments were conducted in conformity with the National Institutes of Health ethical guidelines for the care and use of laboratory animals (NIH; Publication No. 85 − 23, revised 1985) and approved by the veterinary ethics committee of the Tabriz university of medical sciences (Registration number: IR.TBZMED.VCR.REC.1400.068). The study is also reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ebrahimzadeh, F., Farhangi, M.A., Tausi, A.Z. et al. Vitamin D supplementation and cardiac tissue inflammation in obese rats. BMC Nutr 8, 152 (2022). https://doi.org/10.1186/s40795-022-00652-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-022-00652-2