Abstract
Background
Goiter remains one of the major public health problems particularly among young children in economically disadvantaged countries like Ethiopia. The aim of the study was to assess the prevalence of goiter and its associated factors among children aged 6–12 years in Chole district, Arsi Zone, Eastern Ethiopia.
Methods
A school based cross-sectional study was conducted in February, 2017 among 422 primary school children in Chole district, eastern Ethiopia. The schools and study subjects were randomly selected. A structured, pretested and interviewer- administered questionnaire was used to collect the required data. It was conducted after getting due consents from the school administration and assent from caregiver/parent. Spot testing kits were used to estimate the level of iodine in salts. Descriptive statistics, cross-tabulations for chi-square test, and bivariate and multivariate logistic regression models were used to show the magnitude of goiter and its associated factors. Odds ratios with 95% confidence intervals were computed to determine the presence and strengths of associations.
Results
From the 422 study participants, 407 (96.4%) completed the questionnaire. Of these 205(50.3%) were female. The mean age of participant school children was 9.87(SD ± 1.6) years. The prevalence of goiter among study subjects was 36.6% (95% CI, 31.6–40.8%). History of goiter in the family (AOR = 6.80; 95% CI: 3.34–13.84), cabbage consumption (AOR = 2.52; 95% CI: 1.38–4.60) and living with family in a single room (AOR = 2.30; 95% CI: 1.13–4.67) were positively associated with the development of goiter among primary school children in Chole district, eastern Ethiopia. But consuming milk (AOR =0.37; 95% CI: 0.23–0.59) was found to be negatively associated or protective against the development of goiter among the study subjects.
Conclusions
Iodine deficiency was found to be significant public health problem in the study area. Consuming milk was found to be protective, whereas consuming goitrogenic foods like cabbage were found to be the risk factors for the development of goiter among school -aged children. Thus, ensuring the consumption of iodized salt and promoting iodine rich food items among the community in Chole district and other similar settings in Ethiopia are strongly recommended.
Similar content being viewed by others
Background
Goiter remains as one of the major health problems particularly among the young children and pregnant women worldwide [1]. Iodine is a crucial constituent of hormones formed by the thyroid gland and goiter is the most visible sequel of iodine deficiency in human life [2]. Globally, two billion people are at risk of Iodine deficiency disorders (IDD) due to insufficient intake of iodine and about 266 million school-aged children are at risk of insufficient iodine intake related health problems [3].
Goiter is one of the most pathological manifestations of long term depletion of iodine storage in human body especially among children living in iodine deficient areas [4]. It is caused mainly by inadequate intake of iodine containing foods and high consumption of goitrogenic foods such as cabbage, maize, sweet potato and millet. The goitrogenic foods contain thiocyanate and isothiocyanate that restrain thyroid iodide transport [4, 5]. Insufficient production of thyroid hormone in the body is also a risk factor for goiter formation, besides inadequate iodine intake [1].
Iodine deficiency during childhood would reduce cognitive, somatic growth and motor function [6, 7]. It has multiple adverse effects in humans, and these effects are best explained as iodine deficiency disorders. The most common outcome of iodine deficiency during childhood is developmental delay [8].
Despite the fact that the World Health Organization (WHO) stated goiter as the sole most preventable cause of mental retardation and brain damage its prevalence among the population of the world is still estimated to be 15.8% [4, 9]. The burden of goiter is more prevalent in low income countries like Ethiopia [2].
A complete review on Iodine deficient disorder which was conducted in 2012 reported that the Africa continent harbors the highest burden of goiter rate which was between 4.7 to 28% [4].
As a study shows about 62% of the total population of Ethiopia are at risk of Iodine deficiency disorder. In addition, an estimated 12 million school -age children in the country were exposed to in adequate iodine intake related health problems [10]. Thus, Ethiopia is considered as one of the countries in the globe with most people vulnerable to in sufficient iodine intake.
This can be explained further by the 14% increase in the prevalence rate of goiter among the total population of Ethiopia between 1980 to 2009,i.e. from 26 to 40%. The high IDD related peri- natal death of about 50,000 per year in the country is another important indication for the severity and magnitude of IDD in the country [11,12,13].
Furthermore, the proportion of children with goiter ranged from 15 to 30% in different regions of the country. The accessibility of iodine that is supposed to have effect on the prevalence of iodine deficiency disorder in iodine deficient areas in Ethiopia are believed to be caused by many ecological and nutritional predictors. This could be further explained by the mountainous landscape of many of the regions in the country which could result in the washing away of important nutrients like iodine because of the likely occurrences of repeated erosions in the areas for many years [14].
As a result, crop growing of the areas could be either with very low or no iodine content. For instance, as the findings of a study conducted in Bale Zone of Oromia region on iodine content of edible salt, cereals and drinking water showed there was very low amount of iodine and high concentration of other goitrogenic minerals in drinking water of the area [15].
Among the most vulnerable population group, school age children are particularly important for the assessment of IDD due to their high vulnerability. Studies reported that the prevalence of goiter among school age children of a certain area is an indicator for the status of iodine consumption in the community [16,17,18,19,20,21,22].
Other studies conducted previously in different countries reported that the prevalence of goiter among school children aged from 6 to 12 years varies from country to country. For instance, it was reported as 19.8% in the Karnataka, India, 35% in Pakistan and 48.3% at Kashmir Valley, in India [16, 21, 22]. As a study conducted in 2007 reported the prevalence of goiter among school children aged 6–12 years in Ethiopia was 39.9% [13].
Universal salt iodization (USI) is one of the most cost effective development attempts that can have paramount importance in improving the economic and social development of a country. As the World Health Organization (WHO) report indicated, about70% of the population of the world had access to iodized salt at household level [7, 20]. But the Ethiopian Demographic Health Survey (EDHS) of 2011 report indicated that only 15% of the households had access to an adequate amount of iodized salt in the country [23, 24].
In addition, WHO recommended that if the Total Goiter Rate (TGR) which is equivalent to the number of goiters of grades 1 and 2 detected in a population divided by the total number of individuals examined is 5% and above among children aged 6–12 years, it is considered as public health significant problem [7, 13]. Moreover, the daily recommended dietary allowance of iodine for school aged Child or 6–12 years old in order to prevent goiter is about 120 μg [25, 26].
However, most of the people in Ethiopia including our study area, Chole district live in the mountainous areas that are more vulnerable to erosions and flooding as well as subsequent risk of prevalent iodine deficiency in the community.
Cognizant of the problem, the Government of Ethiopia had launched a five –year national plan to eradicate iodine deficiency and associated health problems by the year 2015 through the achievement of utilization of adequately iodized salt to 90% of the population in the country [10, 12, 23]. In addition, the Federal Ministry of Health designed a National Nutrition Program and Micronutrient Guideline, and endorsed a proclamation for ensuring the availability of iodized salt [12, 24], though significant changes have not been attained and updated data are still scarce [10, 24, 27, 28].
Furthermore, even though many efforts were undertaken to minimize the problem, goiter is still prevalent in Ethiopia mainly among children and women living in high land areas including our study area or Chole district. Assessing the burden of ID among the most at risk population segment or school children might have paramount significance to clearly understand the progress of the current interventions and also to plan for sound actions in the future based on timely and research- based evidence.
Therefore, this study was designed to fill the current research gap about the prevalence and associated factors of goiter among school children (6–12 years) in Chole District, Oromia region, Ethiopia.
Methods
Study area/setting
The study was conducted in Oromia Regional State from February 5 to 25, 2017 with the aim to assess the prevalence and associated factors of goiter among primary school children aged 6–12 years.. Over 60% of the area of Chole district is highland. The estimated total population of the district for the year 2016 was 113,798, and of which 57,791 (50.8%) were female [29]. There were four health centers, 18 health posts and six different categories of private clinics, 3 Kindergartens (KGs), 44 primary and 5 secondary schools during the year 2016/17 in Chole district [30].
Study design and population
A school based cross-sectional study was conducted among selected primary schools children in Chole district using systematic random sampling technique. Although the study focused on all the school children in the district aged 6–12 years, who were available during the study period, few students who were unable to communicate effectively because of some of the health problems of hearing and speaking during the data collection period were excluded.
Sample size and sampling technique
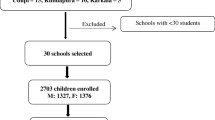
The sample size of the study was calculated using a single population proportion formula. It was calculated considering the proportion (P) of goiter among school children based on the finding from the similar study done previously in Goba town, Eastern Ethiopia which was 50.0% [28]. In addition, 95% Confidence Interval (CI), 5% desired precision, and 10% contingency for the non response rate were considered during the sample size calculation. Accordingly, the calculated total sample size was 422 primary school children.
As the sampling technique was considered, firstly, the number of schools in the district was obtained from Chole district education office. Secondly, among the primary schools in the study area during the study period, four primary schools were selected using simple random sampling technique. Thirdly, the sample size was allocated to each of the four primary schools proportionately. Fourthly, grades of school children were selected using simple random sampling technique. Finally, systematic sampling technique was used to select all the targeted 422 students from four primary schools. Students who were selected but absent on the day of data collection were replaced by the next student from the same class.
Study variables and measurement
The outcome variable of this study was goiter status and its presence or absence was assessed using inspection and palpation techniques of the physical examination [28, 30,31,32].
The risk factors used to determine associations with goiter among school children were some of the selected socio-demographic characteristics of the study participant children and their parents. The socio- demographic characteristics or variables were chosen based on theoretical backgrounds or findings of previous similar studies. In addition, children’s dietary or eating practices in their respective households, focusing on the recall of at least a week proceeding the data collection period were assessed and computed for their level of significance with presence or absence of goiter among the study participants.
Data collection methods and quality control
An interviewer –administered, structured and pre-tested questionnaire was used to collect the data. Pre-testing of the questionnaire was carried out in a primary school that had similar characteristics with the schools selected for the actual data collection and then necessary corrections on the questionnaire were made accordingly. The data collection process was conducted by four senior health professionals with experiences in similar data collection procedures after being trained for two days on the concepts and contents of this study. Two experts in nutrition and the investigators of this study supervised all the process of data collection closely to maintain the required quality.
As studies indicated the prevalence of goiter among children of a certain area can be taken as an indicator for the status of iodine consumption in the community or the likelihood of iodine deficiency disorder in the area [28, 32].
Accordingly, at the day of physical examination for determining the status of goiter among schoolchildren, the participants were instructed to bring a handful of salt used by their home. The iodine content of the sample salt was tested by rapid iodized salt test kit. The salt sample was taken in a teaspoon and then a drop of the test solution was poured on the salt. Salt samples with less than 15 ppm of iodine content were classified as inadequate iodine [10, 28].
Since 1960, inspection and palpation methods have been used for assessment of the severity of endemic goitre in populations [2]. Accordingly, in this study the physical examination of children were done by experienced two health officers using inspection and palpation techniques to determine thyroid size or goiter. The goiter was graded when a student was in a normal position as 0, 1 and 2. Consequently, Grade 0 (neither palpable nor visible goiter), grade 1 (palpable goiter but not visible) and grade 2 (a swelling in neck that is clearly visible). Then, the total goiter rate (TGR) was calculated by adding grade 1 and 2 and dividing to the total number of children examined to express in percentages ([17, 19, 21, 22] 281).
Furthermore, the collected data on socio-demographic characteristics and dietary practices of school children and their respective parents as well as the status of goiter among school children were checked first for their completeness and then cleaned and checked for accuracy, consistencies and missed values before conducting analysis.
Statistical analysis of the data
The data analysis was done using SPSS computer software version 20.0. Descriptive statistics like frequency distribution and percentage were employed using tables and figures. Bivariate and multivariate logistic regression models were performed to identify factors associated with goiter among primary school children in study area.
Variables that have shown statistically significant associations in bivariate analyses or P- value less than 0.05 as well as those variables with the theoretical associations were entered in to multiple logistic regression models to assess for their independent contributions. The variables with theoretical associations refer to the variables age, consuming maize, and iodine content of the salt that didn’t show statistically significant associations with the status of goiter among school children during bivariate analysis of this study despite the fact that they were reported as risk factors for the development of goiter among school –aged children in some previous similar studies [5, 33,34,35]. The presence of association was determined by the odds ratio and level of significance (P-value < 0.05).
Results
Socio- demographics characteristics of the study participant children
From the total 422 study participants 407(96.6%) completed the questionnaire. All the students who completed the questionnaire were involved in the analysis. The mean age of the study subjects was 9.87 (+ 1.6) years. Among the study participants 205 (50.4%) were female, 216(53.1%) were Oromo and the rest 191 (46.9%) were Amhara by ethnicity. The majority of the study subjects 263(64.6%) were Orthodox Christians Table 1.
Dietary factors associated with goiter among school children
Goitrogens foods were frequently eaten by children. From the total 407 children included in the study, 322 (79.1%) were consuming cabbage, 327(80.3%) were consuming maize, 147(36.1%) were consuming millet and 76(18.7%) were consuming sweet potato at least once during the previous week. In addition, of the study participants 269(66.1%) were consuming milk which is non goitrogenic at least once during the previous week.
Of all the 407 study subjects who were asked to bring the sample of salts from what their respective family is consuming during the data collection period, 387(95.1%), 8(2.0%) and 12 (2.9%) brought salts with no iodine (ppm = 0), inadequate iodine content (ppm < 15 and sufficient amounts of iodine (ppm > 15, respectively (Table 2).
Prevalence of goiter among the study participants
The observed prevalence of goiter among study participants was 36.6%. Of these 29% were with grade one (palpable) goiter and 7.6% were with grade two (visible) goiter. The prevalence of goiter was higher among female students than males. It was 38.0 and 35.1% among females and males, respectively.
Factors associated with goiter in bivariate analysis
In bivariate analysis, history of goiter in the family (COR = 6.68;95 CI: 3.43–13.01), living with family in a single room (COR = 2.21; 95 CI: 1.15–4.27), consuming cabbage (COR = 2.17; 95 CI: 1.26–3.76) and consuming milk (COR = 0.40, 95% CI: 0.26–0.62) were found to be factors associated with development of goiter among school children in Chole district (Table 3, Annexed on Page 23).
Factors associated with goiter in multivariable analysis
The variables history of goiter in the family (AOR =6.80;, 95% CI: 3.34–13.84), living together with parents in a single room (AOR = 2.30; 95% CI: 1.13–4.67), consuming cabbage (AOR = 2.52; 95% CI: 1.38–4.60) and, consuming milk (AOR = 0.37; 95% CI: 0.23–0.59) at least once in a week or more frequently had shown statistically significant associations with the outcome variable or occurrences of goiter among school children by controlling for potential confounders (Table 4, Annexed on page 25).
Discussion
As the findings of this study showed, the prevalence of goiter among primary school students aged 6–12 years in the study area was 36.6%, and it was in line with the national prevalence (39.9%), and that of the finding of a study conducted among children aged 6–12 years in rural northwest Ethiopia (37.6%) [5, 10, 12]. However, this prevalence was lower than the finding of a study conducted in Shebbe Sonbo District, Jimma Zone with higher altitude and rain fall because of its topography [31]. The difference could be explained in terms of variations in altitude and annual rain fall between the two study areas and the subsequent likely erosions of the top soil rich with iodine content [31]. But the observed prevalence of goiter among the participant school children of this study was higher than the prevalence of goiter among school –aged children reported in studies conducted in India [21, 22]. The possible explanations for higher prevalence of goiter among children in our study comparing with the findings of other similar studies in Asia (Karnataka, Kashmir Valley and Pakstan) could be due to the mountainous nature of Ethiopia and poor soil conservation for long period of time which might have contributed to the removal of iodine rich top layer of the soil [12, 13, 16, 21, 22, 31].
The findings of this study also revealed that, the prevalence of goiter was higher in the older group of children. It was also in agreement with the findings of a study that was done in Goba town where the prevalence of goiter among age group 6–8 was reported 39% and among those aged 9–12 it was 53.7% [36]. The increase in prevalence of goiter with age may be because of the amount of iodine required by body as the age increases [6].
In addition, our findings indicated that the prevalence of goiter was higher among females than males. Higher prevalence of goiter among females may be due to the fact that iodine requirement for female children are higher than males especially at the beginning of pubertal age [32]. The females are more vulnerable to goiter due to physiological reasons like early puberty, which starts about 2 years earlier than males. Nonetheless, sex as a variable didn’t show statistical significance with the status of goiter in bivariate analysis which concurs with the findings of a similar study that was conducted among children in Wolaita, Ethiopia [32].
School -aged children who reported family history of goiter were about six times more likely to have goiter compared to their counterparts. This finding is in agreement with the previous study that was done in North West Ethiopia [5]. The consistency in findings regarding the associations between family history and goiter among school children in Ethiopia could be explained in terms of a number of environmental and dietary factors associated with iodine deficiency among different segments of population that are inter-generational in many parts of the country due to either poverty or low coverage of national salt iodization program [11,12,13,14,15]. Children who lived in a single room together with their parents were more likely to develop goiter when compared with those who lived in a house with two or more rooms. This finding is also consistent with the finding of a study conducted in Goba town [28]. It could be explained in terms of the variation in nutritional status or quality of daitry intake of a community or a family‘s iodine intake [28].
Children who consumed cabbage at least once in a week or more frequently were more likely to develop goiter than those who had not. It was also consistent with findings of similar other studies that were conducted previously in different parts of the country i.e. in Goba Town, in Shebbe Senbo district, Jimma zone and Wolayita Soddo Town [28, 31, 32]. The possible explanation for this could be due to cabbage being a natural goitrogens food that contains thiocyanate and isothiocyanate, which inhibit thyroid iodide transport. The inhibited thyroid iodide transport in our body, may result in the enlargement of thyroid gland [32]. Thus, continuous efforts through health education should be done to ensure the consumption of iodized salt among the residents of Chole district and other similar settings through increased awareness of community on the benefits of iodine rich diets such as cow milk,butter and cheese. In addition, more efforts to make the iodized salt accessible to the community can have paramount importance as a solution.
Those study participant children who were consuming milk at least once in a week were less likely to develop goiter comparing with their counterparts This finding also concords with the reports from different sources that indicated cow milk contains useful nutrient for prevention of goiter [37, 38]. Even though foods from animal origin have less amount of iodine than the foods from plant origin, milk and dairy are fairly good source of iodine [37, 38].
Furthermore, as salt is frequently consumed by everyone, consumption of iodized salt is the easiest and cheapest modality of IDD prevention and control. The Federal Democratic Republic of Ethiopia (FDRE) passed a mandatory salt regulation and monitoring requiring all salt meant for human consumption to be iodized since March 2011 [36, 39].
However, our study findings indicated that only 2.9% of the families of school children who participated in this study had sufficient levels of iodine in their salt (> 15 ppm). The finding of our study was lower than the findings of Ethiopian Demographic and Health Survey or EDHS 2011 which reported 15% of the households’ access iodized salt [23]. The accessibility of sufficient (15 ppm) iodized salt in this study was also lower than the study conducted in Northwest Ethiopia and Goba town which were 29 and 29.9%, respectively [10, 28]. This could be explained in terms of low coverage of iodization of salt to the community which might be improved through an efficient universal salt iodization national program with due emphasis to the most iodine deficient districts like Chole, our study area [23, 24, 36, 39].,
Furthermore, as recommended by the World Health organization(WHO), 90% of the households in a population should have access to use salt that contains the iodine level of > 15 ppm for the effective elimination of iodine deficiency disorder or goiter [17, 19]. Additionally, the proportion of people who are consuming food items that contain adequate iodine such as cow milk in our study area was found to be very low. This could be explained in terms of low income among many families in the study area which could also have resulted in the observed high prevalence of goiter among the study participant school children.
Thus, periodic assessment of locally available food items with fairly high amount of iodine and the content of house hold salts for ensuring the recommended content of iodized salt to the community can have paramount importance in decreasing the prevalence of goiter and its effects among school children.
Limitation
In this study urinary iodine level of the study subjects that could reveal recent iodine intake status and could also help for treatment and monitoring was not tested due to resource constraints. Since the study was conducted in the school, the storage of the salt in the home wasn’t observed which might also contribute as factor of goiter.
In addition, as this study was a cross –sectional study design, determining the causality of goiter is not strong. Furthermore, some of the information like family history of goiter that was collected from school children might lack accuracy and need to be cross checked with the information from the students’ respective parents.
Conclusions
The study revealed that primary school children in the study area were severely affected by iodine deficiency, and the area can be classified as goiter endemic. Family history of having goiter, consuming cabbage, living together with family in a single room and inability to consume milk at least once in a week were found to be the likely risk factors for having goiter among school children. Supplying iodized oil capsule to school children, promoting iodine rich foods and ensuring iodized salt through effective universal salt iodization program in the community are believed to play significant role in decreasing the prevalence of goiter and its associated health effects among primary school children. Finally, periodic assessment of iodine content in house hold salts for ensuring the recommended content of iodized salt to the community can also have paramount importance in decreasing the prevalence of goiter and its effects among school children in Chole district and other similar settings in low income countries including Ethiopia.
Abbreviations
- AOR:
-
Adjusted Odds Ratio
- CI:
-
Confidence Interval
- COR:
-
Cumulative Odds Ratio
- EDHS:
-
Ethiopian Demographic and Health Survey
- FDRE:
-
Federal Democratic Republic of Ethiopia
- IDDs:
-
Iodine Deficiency Disorders
- IRB:
-
Institutional Review Board
- KGs:
-
Kindergartens
- PPM:
-
Parts Per Million
- SD:
-
Standard Deviation
- SPHMMC:
-
St Paul’s Hospital Millennium Medical College
- SPSS:
-
Statistical Package for Social Sciences
- TGR:
-
Total Goiter Rate
- USI:
-
Universal Salt Iodization
- WHO:
-
World Health Organization
References
Hetzel B. The Story of Iodine Deficiency: An International Challenge in Nutrition. New York: Oxford University Press; 1989. https://www.amazon.com/Story-Iodine-Deficiency-International-publications/dp/0192618660.
FAO/WHO. Vitamin and mineral requirements in human nutrition. 2nd ed. Geneva: World Health Organization; 2005. http://whqlibdoc.who.int/publications/2004/9241546123.pdf
Benoist B, McLean E, Andersson M, Rogers L. Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull. 2008;29:195–202. PubMed.
Biswa M, Rath K, Divya A, Sanjay K. Goitre :A Complete Review Iodine deficient disorder. International Journal of Pharma and Bio Sciences. 2012;3(3): ISSN 0975-6299,
Mesele M, Degu G, Gebrehiwot H. Prevalence and associated factors of goiter among rural children aged 6-12 years old in Northwest Ethiopia. BMC Public Health. 2014. https://doi.org/10.1186/1471-2458-14-130.
Anderson M, Takkouche B, Egli I, Allen HE, Benoist B. Iodine status worldwide: WHO global database on iodine deficiency Department of Nutrition for health and development. Geneva: WHO; 2004. Available at: whqlibdoc.who.int/publications/2004/9241592001.pdf
Zimmermann M. Iodine deficiency Endocrine Rev. 2009; 30: 376–408 View ArticlePub Med
Zimmermann M. "Key Barriers to Global Iodine Deficiency Disorder Control." Human Nutrition Laboratory, Swiss Federal Institute of Technology Zürich (Ethz) .2007;GHS-A-00-05-00012-00.
World Health Organization. The World Health Report: Reducing Risks, Promoting Healthy Life.Geneva; 2002. Available at: https://groups.nceas.ucsb.edu › ... › Supplemental readings from the Reader.
Adish A, Chuko T, Abay A, et al. Ethiopia breaking through with a new iodized salt law. IDD Newsletter. 2013;39(4):7–8 www.iccidd.org/newsletter/idd_nov13_ethiopia.pdf.
Takele L, Belachew T, Bekele T. Iodine concenteration in salt at household and retail shop levels in Shebe town, Southwest Ethiopia. E Afr Med J. 2003;80:532–9 Google Scholar.
International Council for control of Iodine Deficiency Disorders /ICCIDD/. Salt iodization in Ethiopia: new partnerships give children a brighter future. IDD Newsletter. 2009;33(3):13–4 http://www.iccidd.org/cm_data/IDD-NL-2009-3.pdf.
Abuye C, Berhan Y. The goitre rate, its association with reproductive failure, and the knowledge of iodine deficiency disorders (IDD) among women in Ethiopia: Cross-section community based study. BMC Public Health. 2007;7:316 PubMed Central Cross Ref Pub Med Google Scholar.
Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr. 2007;10:1571–80 Cross Ref Pub Med Google Scholar.
Duressa F, Mohammed Y, Feyissa R, Tufa T, Siraj K. Comparative analysis of iodine concentration in water, soil, cereals and table salt of Horaboka, Mio and Besaso Towns of Bale Robe, South East Ethiopia. J Environ Pollut Human Health. 2014;2:27–33 Google Scholar.
Veena G, George J, Ayushi A, et al. Prevalence of goiter and its associated factors in a coastal district of Karnataka. Indian J Pediatr. 2015;27(Supp 01).
World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers; 2007 [cited 2017 Dec 11]. Available from: http://apps.who.int/iris/bitstream/10665/43781/1/9789241595827_eng.pdf.
Pandav CS, Arora NK, Krishnan A, Sankar R, Pandav S, Karmarkar MG. Validation of spot-testing kits to determine iodine content in salt. Bull World Health Organ. 2000;78(8):975–80.
World Health Organization. Goitre as a determinant of the prevalence and severity of iodine deficiency disorders in populations. 2014 [cited 2017 Dec 11]. Available from: http://www.who.int/vmnis/indicators/goitre_idd/en/
Andersson M, Takkouche B, Egli I, Allen HE, de Benoist B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005;83:518–25 [PMC free article] [PubMed].
Fazli S, Muhammad J, Mehmood K, et al .Prevalence of goiter and iodine status among 6-12 years school age children in district Kohat, Pakistan: South East Asia Journal Of Public Health 2014; 42–46,2014.
Pandit MI, Raja W, Hussain R, Khan MS. Prevalence of Goiter in School Age Children (6–12 years) in a Rural District (Bandipura) of Kashmir Valley. Int J Sci Res (IJSR) ISSN (Online). 2015;4:2319–7064.
Central Statistical Agency of Ethiopia. Ethiopia demographic and Health Survey report. Addis Ababa: ECSA; 2011. Available at : https://www.dhsprogram.com/pubs/pdf/GF26/GF26.pdf; https://dhsprogram.com/pubs/pdf/fr255/fr255.pdf
ICCIDD. Children in Northern Ethiopia are iodine deficient. IDD News letter 2014. Available at: http://ign.org/cm_data/idd_may14_ethiopia.pdf
WHO. Vitamin and mineral requirements in human nutrition. 2nd ed. Geneva: World Health Organization; 2005. http://whqlibdoc.who.int/publications/2004/9241546123.pdf
WHO. In: 1, editor. Recommended iodine levels in salt and guidelines for monitoring their adequacy and effectiveness, vol. 74. Geneva: WHO; 1996. Available at: https://www.ncbi.nlm.nih.gov/books/NBK254239/ .
Berhanu N, Michael KW, Bezabih M. Endemic goiter in school children in southwestern Ethiopia. Ethiop J Health Dev. 2005;18(3):175–8. https://doi.org/10.4314/ejhd.v18i3.9956.
Demelash H, Ketema E, Zemedkun G, Melese A. Prevalence of goiter and associated factors among primary school children aged 6-12 years old in Goba town, south east, Ethiopia. Int J Nutrition and Food Sci. 2015;4(3):381–7.
Central Statistical Agency. National Census Report of Ethiopia. 2007:2009 Available at: https://searchworks.stanford.edu/view/10252781unstats.un.org/unsd/censuskb20/Attachment489.aspx?AttachmentType=1.
Annual Report of Chole District ,Oromia region, Ethiopia; 2016 [Desk review].
Mezgebu Y, Mossie A, Rajesh P, Beyene G. Prevalence and severity of iodine deficiency disorder among children 6–12 years of age in Shebe Senbo District, Jimma zone. Southwest Ethiopia Ethiopia Journal Health Science. 2012;22(3):196–204.
Eskinder W, Solomon S, Sibhatu B. Epidemiological study of risk factors for goiter among primary schoolchildren in southern Ethiopia;2014.Available at: journals.sagepub.com/doi/abs/https://doi.org/10.1177/156482651403500103
Evaluation of Goitre and its Socio demographic Risk Factors among Rural School Children of Kancheepuram. The Journal of Clinical and Diagnostic Research. 2018 ;DOI: https://doi.org/10.7860/JCDR/2018/34477.11638
Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012;142(4):744–50.
Zhao W, Han C, Shi X, Xiong C, Sun J, Shan Z, Teng W. Prevalence of goiter and thyroid nodules before and after implementation of the universal salt iodization program in mainland China from 1985 to 2014: a systematic review and meta analysis. PloS one. 2014;9(10):e109549.
Ethiopia Universal Salt Iodization [https://www.nutritionintl.org/2009/04/salt-iodization-ethiopia/.
Phillips DI. Iodine, milk and elimination of endemic goiter in Britain. J Epidemiol Community Health. 1997; Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1060507/.
Goiter: Causes ,treatment and symptoms .Medical News Today ;2017.Available at: https://www.medicalnewstoday.com/articles/167559.php
Ethiopian public Health Institute (EPHI). National salt iodization coverage towards Prevention of Iodine Deficiency Disorder in Ethiopia. 2014.Available at: http://www.ephi.gov.et/images/pictures/USI presentation_Forum_October_25_2014.pdf.
Acknowledgments
The authors are pleased to thank Arsi zonal health office for providing us with iodine salt field test kits.
Our acknowledgements also go to all the staff members of Chole District Health and Education Offices as well as the study participant schools for their cooperation and support during data collection.
Finally, we would like to thank all the study subjects and data collectors for their great contributions that were vital for making this study real.
Funding
This research was partially funded by St Paul’s Hospital Millennium Medical College in Addis Ababa, Ethiopia and the remaining budget was covered from the very limited personal sources of the researchers themselves. In addition, the financial support from the College was used for some of the payments done to the data collectors and supervisors, as well as to the related stationery and secretarial services. But there was no direct involvement of the funding body in the design of the study, data acquisition, analysis, interpretation and writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on agreement with the co-author up on reasonable request.
Author information
Authors and Affiliations
Contributions
AB contributed significantly in inception and design of the study, data collection, analysis and interpretation as well as drafting and revising the manuscript. TM contributed significantly in inception and design of the study, data analysis and interpretation, drafting and critically revising the manuscript for important intellectual content. In addition, both authors have read the final manuscript and then approved it for possible publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the Institutional Review Board (IRB) of St Paul’s Hospital Millennium Medical College.
Letters of permission were also secured before the field work from Arsi Zone Education and Health Departments, and Chole District education Office. Moreover, informed oral consents were obtained from the selected school principals and concerned teachers. Informed written consents were obtained from the respective parents and/or legal guardians of participating children before data collection.
Ethical considerations were well observed and the objectives of the study were clearly communicated to the parents and/or guardians. In addition, before obtaining the oral consents and written assents accordingly, the study subjects and parents /guardians were assured that their names and the names of their respective children wouldn’t be mentioned; and the data will be used only for the study purpose. Furthermore, privacy and confidentiality of the collected information were also ensured at all levels.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bekele, A., Adilo, T.M. Prevalence of goiter and its associated factors among primary school children in Chole District, Arsi Zone, Ethiopia: a cross- sectional study. BMC Nutr 5, 5 (2019). https://doi.org/10.1186/s40795-018-0267-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-018-0267-2