Abstract
Background
Resistance exercise and protein intake are both strong stimuli for muscle protein synthesis. The potential for a protein to acutely increase muscle protein synthesis seems partly dependent on absorption kinetics and the amino acid composition. The aim of this double-blinded randomized cross-over study was to compare time dependent changes in blood amino acid concentrations after ingesting 20 g of five distinct high quality dairy protein supplements (native whey, whey protein concentrate 80, hydrolysed whey, microparticulated whey, and milk proteins). Furthermore, we investigated whether differences in time dependent changes in blood amino acid concentrations affected acute blood glucose and urea responses, and recovery of muscle function after a bout of strength training.
Methods
Ten young healthy, recreationally active men ingested different milk protein supplements after a whole-body strength training session on five occasions in a randomized manner. Blood concentrations of amino acids, glucose and urea was measured before and 0, 30, 45, 60, 90, 120 min, and 22 and 30 h post-exercise. Maximal voluntary isometric knee extension and counter movement jump were assessed before, immediately after, 6, 22 and 30 h after exercise.
Results
Intake of native whey induced a faster and higher leucinemia than the other protein supplements (p < 0.001). All whey protein supplements showed faster time dependent changes in blood amino acid concentrations for total essential and branched chain amino acids compared to milk. There were no major differences between trials in blood concentrations of glucose or urea, or recovery of muscle function after exercise.
Conclusion
Native whey induced the strongest leucinemia and appears to have a greater potential for stimulating muscle protein synthesis than other whey supplements and milk. There were no meaningful differences in blood glucose, urea or recovery of muscle function after the supplements. Future studies should investigate whether the increased leucinemia with native whey translates into greater muscle protein synthesis and muscle mass accretion over time. (NCT02882386, August 16, 2016).
Similar content being viewed by others
Background
Increasing or maintaining muscle mass is of great importance for populations ranging from athletes to patients and elderly. Resistance exercise and protein ingestion are two of the most important stimuli of muscle protein synthesis (MPS). After ingestion of protein, the increased appearance of circulating essential amino acids stimulates MPS through intracellular kinases [1]. The anabolic response to protein intake is further potentiated by resistance exercise [2–4]. Both the physical characteristics of proteins (e.g., different digestion rates of whey and casein) and the amino acid composition, affect the potential of a certain protein to stimulate MPS [5–7].
Milk contains two protein fractions, the readily digestible and so called “fast” whey fraction, and the slowly digestible and so called “slow” casein fraction. Although both fractions are considered to be of high quality, containing all essential amino acids (EAA), the difference in digestion rate, splanchnic extraction and amino acid profile gives them distinctive properties. Studies on muscle responses show that whey intake induces a large but transient rise in MPS, whereas casein intake produces a moderate more persistent increase in MPS [8]. The leucine threshold hypothesis suggests that a certain intracellular leucine concentration is needed in order to robustly increase MPS [9, 10]. Given its superior ability to rapidly increase blood leucine concentrations to high levels, whey is often considered the most effective protein source to stimulate MPS [11].
Knowledge about how the manufacturing processes of protein containing foods or supplements affects the amino acid profile, absorption kinetics and anabolic potential is important when optimizing diets of e.g. athletes or elderly. Whey protein concentrate (WPC-80) is a by-product of cheese production and thereby subjected to both heat and acidification, denaturing the protein to some extent. Proteins can be further degraded by hydrolysation or microparticulation. Protein hydrolysates are commonly produced by adding proteolytic enzymes, resulting in a complex mixture of peptides with differing lengths, and free amino acids [12]. Hydrolysed casein may be more rapidly digested than intact casein [7, 13, 14]. Grimble and colleges found that hydrolysing whey proteins to mainly di- and tri-peptides improved absorption in the perfused human jejunum [15], however, other studies did not find any effect on absorption rates by hydrolysing whey proteins [13, 16] or a slowed absorption rate [17]. By the use of filtration, heat and shear forces, it is possible to microparticulate proteins to very small particles, giving them distinct properties resembling those of emulsified fat droplets [18]. As a consequence, microparticulated dairy proteins are often used as fat replacements in food. Native whey protein is produced by filtration of unprocessed milk. Because of the direct filtration of raw milk, native whey is a more intact protein compared to WPC-80, and the amino acid profile may also be slightly different (claimed by producers to have higher leucine content than WPC-80 [19]). Consequently, native whey may have characteristics that are positive for stimulation of MPS, but whether these distinct characteristics translate into a more favourable aminoacidemia compared to WPC-80, hydrolysed whey, and microparticulated whey remains to be answered.
The aim of the current study was to compare the time dependent changes in blood amino acid concentrations after ingestion of 20 g of five high quality but distinct dairy proteins; WPC-80, hydrolysed whey (WPH), microparticulated whey (MWP), native whey, and milk. Twenty grams of protein were chosen based on studies showing that 20 g of high quality protein is sufficient to maximally stimulate MPS [4, 20]. We hypothesised that native whey would induce a more rapid and higher leucinemia than WPC-80 due to the higher leucine content (claimed by the producers [19]). Furthermore, because of the rapid absorption rates of amino acids from intact WPC-80, we hypothesised that hydrolysation or microparticulation of WPC-80 would not enhance time dependent changes in blood amino acid concentrations of this product.
Methods
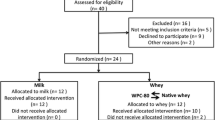
Thirteen healthy male subjects (age: 26.6 ± 7.4 years, height: 180.8 ± 6.3 cm, weight: 80.8 ± 6.3 kg) were recruited to this single blinded, randomized, five-way cross-over, controlled study. Two participants withdrew before the start due to reasons not related to the study. One participant had to withdraw due to knee pain during the training sessions. The results in this article are from the remaining 10 participants. Participants were sport science students recruited through student e-mails and flyers posted around the campus area. Participants were recreationally active and performed strength training 1–3 times per week. All were deemed healthy based on responses to a routine health-screening questionnaire. Resistance trained participants were included to minimize the exercise induced muscle damage, which can occur in untrained individuals, and to avoid learning effects during the study. One week before the start of the study all participants performed a familiarization workout to establish the maximal load they could lift for 10 repetitions (10RM). Performance tests were also practiced during this session. Written informed consent was obtained from all participants before the start of the study. The study was evaluated by the Regional Committee for Medical and Health Research Ethics (REC South East).
Study design
The study protocol is outlined in Fig. 1. All participants completed five study days consisting of a bout of strength exercise and intake of a protein supplement. All measures except maximal voluntary contraction (MVC) and counter movement jump (CMJ), which were only done after native whey and milk, were conducted in the same way with all supplements. Further, the days with native whey and milk had two additional time points of measurements at 22 and 30 h after exercise. Thus, participants and investigators were able to distinguish the days with native whey and milk form other testing days, but not whether they received native whey or milk on these days. The drinks were produced in colour-coded bottles in order to blind the participants and the investigators as much as possible within the design of the study. Training sessions were separated by at least five days, and participants were instructed to refrain from exercise for 72 h before each study day. Participants were instructed to maintain their habitual diet for the whole study period. If consuming any nutritional supplements, participants had to stop using these supplements at least two weeks before the start of the study. Due to short shelf life, all participants consumed the microparticulated whey the first study day. The participants but not the investigators were blinded for this. The other protein supplements were consumed in a randomized order on the following test days.
Participants met in the lab at 07:00 am on each study day after an overnight fast. They received a standardized breakfast consisting of oatmeal and a glass of orange juice (1855 kJ: 9.2 g fat, 69.3 g carbohydrates and 16.6 g protein). Participants were provided water ad libitum. Two hours after breakfast, the participants performed the standardized resistance exercise session in 40 min and consumed one of the five protein supplements within 6 min after the end of the session. Blood serum and plasma samples were collected at 0, 30, 45, 60, 90, and 120 min after ingestion of the protein supplement. Additional blood samples were collected at 22 and 30 h on the study days when milk and native whey were ingested. During the study days with milk and native whey, recovery of muscle function was measured as changes in maximal isometric voluntary contraction knee extensions (MVC), counter movement jump (CMJ) 30 min prior to, and 0, 6, 22 and 30 h after the exercise session. The 0 h time point was immediately after the workout and about 20 min after the last set of leg exercise.
Supplements
Bottles with the supplements were produced and delivered by Tine SA (Oslo, Norway). Each serving consisted of 636 ml and contained 3.3% protein, 1.2% fat, 4.7% lactose and 2% sucrose (Table 1). All supplements were adjusted with cream (TINE SA, Oslo, Norway) and lactose (VARIOLAC® 992, Arla, Vidby J, Denmark) to match milk on macronutrients. The rationale behind this adjustment was to be able to isolate differences to protein composition alone. Proteins in all supplements, except milk, consisted of 100% whey. Milk protein was 80% casein and 20% whey. Sucrose and artificial flavour was added to make supplements similar in taste.
In this study, microparticulated whey was produced by microfiltration of WPC-80 at high temperatures and high shear forces, with an end product of micro particles between 1 and 10 μm. Whey hydrolysate was produced using proteolytic enzymes and had a 10% degree of hydrolysis.
Exercise protocol
Training sessions consisted of 4 sets of 10RM repetitions of leg press and knee extensions, and 3 sets of 10RM repetitions of bench press and seated rowing. Warm-up sets of 10 repetitions at 50 and 80% of the 10RM weights were carried out in each exercise. A new set started every two minutes, while 3 min of rest was given between exercises. On the first study day, participants were allowed to make adjustments to the training load, whereas on the following four study days the training load could not be changed and was identical to the first study day. Each session was initiated by 10 min walk/easy jog on a treadmill.
Tests and measurements
Blood serum samples were clotted at room temperature for 30 min before being centrifuged at 1300 × g for 10 min at 4 °C. Blood plasma samples were collected in lithium heparin tubes and immediately centrifuged at 1300 x g for 10 min. Blood samples were stored at −80 °C until analyses. Serum samples were analysed for urea (analytic CV: 2.2%), and glucose (CV: 1.7%) at Fürst Medical Laboratory (Oslo, Norway).
Amino acid concentration was measured in plasma using the Phenomenex EZ:faast free (physiological) GC-MS analysis kit (Phenomenex®, Torrance, CA, USA). Samples were concentrated by solid phase extraction, derivatized, and separated by liquid-liquid extraction as directed by the EZ:faast kit. Derivatized samples were then analyzed by gas chromatography–mass spectrometry using a Shimadzu QP-2010 Ultra GCMS (Shimadzu Scientific Instruments, Columbia, MD, USA) equipped with the EZ:faast amino acid analysis column (10 m × 0.25 mm x 0.25 μm). The injection was 2 μl at 300 °C at a constant helium carrier gas flow of 1.1 ml/min with a split ratio of 1:15. The initial oven temperature was 110 °C and was raised to 320 °C at 30 °C/min. The MS interface was 320 °C and the EI source was 240 °C. Mass spectra were analyzed from 45–450 m/z at 4 scans/s. Total run time was 7 min. Duplicate sample injections were performed to reduce injection variability. A 3 point standard curve was produced for each measured amino acid (0–200 nmol/ml). The concentration of each amino acid was determined using its standard curve and was then corrected for injection differences using a norvaline internal standard (200 nmol/ml). Total amino acid (Total AA), essential amino acid (EAA), and branched-chain amino acid (BCAA) concentrations reported were the sum of the individual amino acid concentrations in each group.
Unilateral MVC for the knee-extensors was tested using a custom-made knee-extension apparatus (Gym2000, Geithus, Norway). Participants were seated in a chair with a four-point belt fixing the chest and hips, with 90° in hip and knee joints. Three attempts of 5 s with 1 min rest between were given to reach MVC. Force was measured with a force transducer (HMB U2AC2, Darmstadt, Germany). MVC was tested after 5 min warm up on a cycle ergometer, except for immediately after the workout. CMJ was tested using a force plate (HUR Labs Oy, Tampere, Finland). Participants were instructed to keep their hands at the hips and drop down to about 90° in the knee joint, before immediately jumping as high as possible. Three attempts were given, and if the third jump was the highest, participants were given a fourth attempt. A rest period of 30 s was given between each jump. The test-retest CV for jump height in this test is less than 3% in our lab.
Statistics
A two-way ANOVA with repeated measures was used to compare the five experimental trials (milk, MWP, WPH, WPC-80 and native whey) and sampling time points for all dependent variables (variable). A one-way ANOVA was used to compare the area under the curve of individual and total BCAA, EAA and total amino acids between protein supplements. Significant F ratios were further analysed using Tukey´s pairwise comparison. Statistical analyses were made using Prism Software (Graphpad 6, San Diego, CA, USA). All results are expressed as means ± SD. Statistical significance level was set at p ≤ .05.
Results
There were no reported or observed adverse effects of any of the protein supplements. Baseline values for all dependent variables were not significantly different between studies, except for serum urea, which was higher in the morning of the WPC-80 study.
Blood amino acid profiles
Total amino acid concentration in blood demonstrated a treatment by time effect (p < 0.0001; Fig. 2a) revealing an increased amino acid concentration at 30 min after ingesting native whey compared to milk. Blood EAA, branched chain amino acids (BCAA) and leucine showed a similar treatment by time effect (p < 0.0001; Fig. 2b-d). Native whey reached the highest values for EAA and BCAA, primarily driven by higher leucine concentration, which was higher than after all other protein supplements during the first hour after intake of protein (Fig. 2d). Blood concentration of isoleucine was higher after intake of WPC-80, MWP, WHP and native whey than milk (see Additional file 1A). For valine, the blood concentrations were only different between native whey and milk from 30 to 45 min, and native whey, WPC-80, MWP compared to milk at 60 min (see Additional file 1B).
Blood concentrations of total amino acids (a), essential amino acids (b), branched chain amino acids (c) and leucine (d) before and after a bout of strength training and intake of 20 g of protein from milk, microparticulated whey (MWP), whey protein hydrolysate (WPH), whey protein concentrate 80 (WPC-80) or native whey (NW) in young men. Values are means ± SD (only shown for highest and lowest values), n = 10 in each group. Milk and WPH were higher than baseline at 30 and 45 min, MWP, WPC-80 and NW were higher than baseline at time point 30, 45 and 60 min in A, B, C and D (not indicated in the figures; p < 0.05). & NW significantly greater than milk at the same time point, p < 0.05; # all whey proteins significantly greater than milk at the same time point, p < 0.05; $ NW significantly greater than all other whey proteins at the same time point, p < 0.05
The area under the curve did not differ between protein supplements for total AA, EAA or BCAA (see Additional file 2). For leucine, the area under the curve revealed higher values for native whey than for WPC-80, WPH and milk, and higher values for MWP than milk (see Additional file 2 D).
Blood leucine peak was reached at 45 min for most participants when consuming native whey, WPH and WPC-80. After consuming MWP, blood leucine peak was reached after 60 min, whereas leucine peak was reached at 30 min after milk intake for most participants. Regardless of time point, the leucine peak was higher after intake of native whey compared to all other supplements.
Blood glucose and urea
The ANOVA revealed a main effect of time for serum glucose (p < 0.001), which for WPC-80, MWP, WPH and native whey, was lower than baseline at 30, 45, 60 and 90 min (Fig. 3a). At time point 30 min, milk was significantly higher than native whey, WPC-80, WPH and MWP (p = 0.03).
Glucose (a) and urea (b) concentrations in serum before and after a bout of strength training and intake of 20 g of protein from milk, microparticulated whey (MWP), whey protein hydrolysate (WPH), whey protein concentrate 80 (WPC-80) or native whey (NW) in young men. Only NW and milk have values at 22 and 30 h. Values are means ± SD (only shown for highest and lowest values), n = 10 in each group. # milk is significantly different from WPH, WPC-80 and native whey
There was a main effect of time for serum urea, revealing a significant increase in concentration at the 90 and 120 min time points (p < 0.001; Fig. 3b). WPC-80 had significantly higher values than MWP, WPH and milk at baseline (p = 0.04). This difference was maintained during the trial. At 22 and 30 h, urea levels for milk and native whey were not significantly different from baseline.
Recovery of performance
Both MVC (Milk: −11.1 ± 3.4%, native whey: −6.2 ± 5.7%; Fig. 4a) and CMJ (Milk: −6.4 ± 4.0%, native whey: −4.0 ± 3.6%; Fig. 4b) performance demonstrated a treatment by time effect (p < 0.001) indicating muscular fatigue post-exercise at 0 and 22 h after exercise. There were no differences between protein supplements.
Discussion
The main finding of the current study was that 20 g of native whey induced a significantly faster increase and higher peak values in blood leucine concentrations than 20 g of MWP, WPH, WPC-80 and milk after a bout of strength training. All whey protein supplements had significantly faster increases and higher peak values of leucine, BCAA and EAA plasma concentrations than milk. Despite these differences in time dependent changes in leucine, BCAA and EAA blood concentrations, we were not able to show any differences in recovery of muscle function after consumption of native whey compared to milk after a bout of heavy load strength training. Finally, hydrolysation or microparticulation of WPC-80 did not increase the rise in blood concentrations of the investigated amino acids (see Additional file 1, 3 and 4).
Increasing extracellular availability of amino acids, especially BCAA and leucine, have been shown to increase protein synthesis both at rest and after exercise [2, 5, 21, 22]. Our data show minimal differences in total amino acid concentration in blood between protein supplements, whereas the whey supplements lead to more favourable time dependent changes in blood concentrations of EAA, BCAA and leucine compared to milk. Our results are in line with other studies showing a more rapid and greater aminoacidemia after whey protein intake compared to casein [5, 8, 16, 23]. Reitelseder and colleagues showed that a rapid aminoacidemia induced by whey was associated with a greater acute (0–3 h post exercise) stimulation of MPS, however when measuring MPS for a longer period of time (6 h), there were no differences between whey and casein [8]. To isolate the effects of aminoacidemia from protein type and amino acid content, West and colleagues provided participants with a bolus (25 g) or a pulse (10 × 2.5 g every 20 min; mimicking casein) of whey protein after resistance exercise [24]. The bolus resulted in a rapid rise in blood concentrations of amino acids and a greater MPS compared to pulse for 5 h post-exercise, indicating that aminoacidemia affects the anabolic response. However, in the rested state a bolus of 15 g EAA did not provide an anabolic advantage over a pulse of 4×3.75 g of EAA with regards to FSR, leading to the suggestion that the anabolic response is mostly dependent on the total dose [25]. Based on these results one could argue that it seems like a certain peak in blood concentration of EAAs is required to kick-start the protein synthesis, whereas maintenance of increased protein synthesis is relying on the amount of amino acids available over time [6]. Nonetheless, leucinemia has yet to be regarded as an exclusive predictor of MPS. Studies have shown leucine to be an important stimulus for the intracellular kinases regulating MPS, such as mTOR and p70S6K [26, 27]. Based on these studies the high content of leucine and BCAA, and its rapid aminoacidemia suggest a great potential for native whey to increase acute post-exercise intracellular anabolic signalling and MPS. However, whether differences in acute post-exercise MPS translate into long-term effects is more uncertain [28], as the results of Reitelseder and colleagues suggest that the initial differences in MPS between whey and casein might diminish with time [8].
There were no differences between WPC-80, WPH and MWP in time dependent changes in blood amino acid concentrations. Thus, our data are in accordance with others [13, 16, 17] suggesting that, in contrast to casein, there is a limited potential to increase the absorption rate of whey proteins by degradation. We showed that native whey gave the highest leucinemia. As we do not have direct data on absorption kinetics, we are unable to differentiate whether the increased leucinemia after intake of native whey is due to faster absorption, higher leucine content or a combination of these factors. However, when comparing the increases in blood concentrations of amino acids with similar amount in native whey and WPC-80 (e.g., glutamic acid, glycine, methionine and serine), there were no differences between supplements, indicating that differences in amino acid content, and not a faster absorption, was the main cause of differences in blood concentrations.
For total AA, EAA, BCAA and leucine concentrations in blood, some participants reached higher values than others for all drinks. This individual difference in aminoacidemia was not directly related to body mass, which is reasonable to assume correlates with lean mass, as all participants were lean trained young men (see Additional file 5A). Nevertheless, the lighter participants did to some extent reach higher leucine levels with native whey than heavier participants. This observation is in line with a recent study finding no differences in the time dependent changes in blood leucine concentrations after intake of 20 g of whey protein in a group with low lean mass (<65 kg) to a group with high lean mass (>70 kg) [29]. Combined these findings might suggest that a certain amount of leucine is needed on order for leucine kinetics to be affected by body mass. Another interesting observation is the fact that some subjects had no difference in leucine peak or area under the curve after whey proteins compared to milk, whereas other participants increased their values considerably with the whey proteins compared to milk (see Additional file 5A and B). This suggests individual differences in blood leucine concentration response to whey vs. milk proteins.
In contrast to the differences in time dependent changes in blood amino acid concentrations, there were not many differences in blood glucose and urea responses to the various protein supplements. Nevertheless, serum glucose was higher with milk than WPH, WPC-80 and native whey at 30 min. Previous studies have shown a greater stimulation of insulin secretion by certain amino acids compositions and a rapid aminoacidemia compared to a slow and more sustained aminoacidemia [8, 30, 31]. As all drinks in the current study contained the same amount and composition of carbohydrates, the faster and greater aminoacidemia, together with the amino acid composition of the whey proteins could result in higher blood insulin levels and a greater transport of glucose into muscle. Serum urea increased after protein consumption for all groups at 90 and 120 min, indicating that a portion of the ingested amino acids was rapidly oxidized. The differences in absolute urea values between studies were mainly due to a higher baseline value for the WPC-80 trial. The relative change in urea concentration from baseline was higher after intake of WPH than after intake of milk, MWP, WPC-80 at 60 min, and WPC-80 and native whey at 120 min. As the total urea production, aminoacidemia and absolute values were similar between supplements; this relative difference in urea production at two time points probably has no physiological importance.
Our strength training protocol induced a small reduction (0–16%) in maximal force generating capacity, which recovered rapidly (within 22 h), indicating only mild muscular stress during the strength training session [32]. Earlier studies have indicated an attenuated strength decline when consuming whey protein after eccentric exercise compared to carbohydrates or placebo [33, 34]. As the current study involved a less strenuous workout and more comparable supplements a difference in recovery from exercise was less likely than in the previous studies. A more strenuous workout resulting in greater loss of force and increased muscle damage could possibly lead to differences between drinks. However, the workout applied in the current study is considered a normal workout, and thus more representative of typical training, in contrast to a workout of eccentric muscle actions. Moore and colleagues found the protein effect on MPS in young subjects to be maximized after dosages of approximately 20 g of high quality protein [4, 20]. One can speculate whether MPS reached a ceiling effect in the current study. Consequently, reducing the protein dose to a suboptimal level could be a stronger design to investigate these differences [22, 35]. A greater initial MPS after intake of whey would be considered an advantage for a quick recovery; however, this advantage might be short lived as the MPS after casein intake catches up after the first hours of recovery [8]. Looking at effects over time during an intensive training period can be an alternative approach, as the evidence of beneficial effects of protein on markers of muscle damage seems stronger when protein is consumed over time after daily training sessions [36].
The cross-over design of the current study is considered a strength, as it allows for the participants to be their own control, making the statistical comparison between drinks stronger. Participants and investigators in this study were not completely blinded, which could limit the conclusions. Yet, the authors consider it unlikely that blinding would affect the primary outcome of blood aminoacidemia. Due to short shelf life, all participants consumed MWP during the first training session. In the case of adaptations to the workout during the study this could affect the comparisons between MWP and the other protein supplements. However, by using strength-trained participants, the adaptation to the workout during the study period was minimized. As we did not use stable isotope tracers, we can only assume that the observed changes in blood amino acid concentrations was due to the accelerated appearance of amino acids from the ingested protein source, and not due to an increase in endogenous release from body tissues. Furthermore, it is not possible to know whether the increased amino acid concentration in blood after intake of native whey resulted in a greater anabolic intracellular signalling or increased MPS compared to the other whey supplements or milk, as we did not collect biopsies. The current study included only young trained men. Future studies should include other populations; especially elderly persons might show a greater benefit of increased leucinemia after intake of native whey. Acute studies should include biopsies to measure anabolic signalling and MPS. In order to fully investigate the potential of native whey on muscle mass and strength, long-term supplementation alone or in combination with strength training interventions should be conducted.
Conclusions
Native whey intake induced a greater leucinemia than intake of WPC-80, WPH, MWP or milk the first hour after strength training in young men. All whey supplements led to higher blood peak concentrations for total essential amino acids, BCAA and leucine than milk the first hour after ingestion. In contrast, there were no differences between effects of supplements on glucose, urea or muscle recovery, and time dependent changes in blood amino acid concentrations were not changed by hydrolysation or microparticulation of WPC-80. Future studies should investigate whether the differences in amino acid and leucine kinetics translate into greater anabolic intracellular signalling, MPS and muscle growth.
Abbreviations
- ANOVA:
-
Analysis of variance
- BCAA:
-
Branched chain amino acids
- CMJ:
-
Counter movement jump
- EAA:
-
Essential amino acids
- GCMS:
-
Gas chromatography mass spectrometer
- MPS:
-
Muscle protein synthesis
- MVC:
-
Mean voluntary contraction
- MWP:
-
Microparticulated whey protein
- NW:
-
Native whey protein
- RM:
-
Repetition maximum
- SD:
-
Standard deviation
- WPC-80:
-
Whey protein concentrate 80
- WPH:
-
Whey protein hydrolysate
References
Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, et al. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–23.
Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–9.
Pennings B, Boirie Y, Senden JMG, Gijsen AP, Kuipers H, van Loon LJC. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005.
Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. The American Journal of Clinical Nutrition American Society for Nutrition. 2013;99:86–95.
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107:987–92.
Churchward-Venne TA, Burd NA, Mitchell CJ, West DWD, Philp A, Marcotte GR, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590:2751–65.
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–5.
Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, et al. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. AJP: Endocrinology and Metabolism American Physiological Society. 2011;300:E231–42.
Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11:222–6.
Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care. 2009;12:66–71.
Devries MC, Phillips SM. Supplemental Protein in Support of Muscle Mass and Health: Advantage Whey. J Food Sci. 2015;80:A8–A15.
Manninen AH. Protein hydrolysates in sports nutrition. Nutr Metab (Lond) BioMed Central. 2009;6:38.
Calbet JAL, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr Steinkopff-Verlag. 2004;43:127–39.
Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. The American Journal of Clinical Nutrition American Society for Nutrition. 2009;90:106–15.
Grimble GK, Guilera Sarda M, Sessay HF, Marrett AL, Kapadia SA, Bowling TE, et al. The influence of whey hydrolysate peptide chain length on nitrogen and carbohydrate absorption in the perfused human jejunum. Clin Nutr. 1994;13:46.
Farup J, Rahbek SK, Storm AC, Klitgaard S, Jorgensen H, Bibby BM, et al. Effect of degree of hydrolysis of whey protein on in vivo plasma amino acid appearance in humans. Springerplus. 2016;5:532. Springer International Publishing.
Farnfield MM, Trenerry C, Carey KA, Cameron-Smith D. Plasma amino acid response after ingestion of different whey protein fractions. Int J Food Sci Nutr. 2009;60:476–86.
Wijayanti HB, Bansal N, Deeth HC. Stability of Whey Proteins during Thermal Processing: A Review. Comprehensive Reviews in Food Science and Food Safety. 2014;13:1235–51.
Lactalis. Prolacta: An excellent aminogram. http://ginobio.com/wp-content/uploads/2013/11/%E6%99%AE%E4%B9%90%E5%AE%83%EF%BC%88Prolacta%EF%BC%89.pdf. Accessed 19 May 2016. 2013. p. 1–12.
Moore DR, Robinson MJ, Fry JL, Tangx JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89(1):161–68.
Bohé J, Low A, Wolfe RR, Rennie MJ. Human Muscle Protein Synthesis is Modulated by Extracellular, Not Intramuscular Amino Acid Availability: A Dose‐Response Study. J Physiol. 2003;552:315–24. Blackwell Publishing Ltd.
Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women, at rest and after exercise. AJP: Endocrinology and Metabolism. 2015;308:E1057–65.
Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr. 2012;108:958–62.
West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr. 2011;94:795–803.
Mitchell WK, Phillips BE, Williams JP, Rankin D, Lund JN, Smith K, et al. A Dose- rather than Delivery Profile-Dependent Mechanism Regulates the “Muscle-Full” Effect in Response to Oral Essential Amino Acid Intake in Young Men. The Journal of nutrition American Society for Nutrition. 2015;145:207–14.
Apró W, Moberg M, Hamilton DL, Ekblom B, Rooyackers O, Holmberg H-C, et al. Leucine does not affect mechanistic target of rapamycin complex 1 assembly but is required for maximal ribosomal protein s6 kinase 1 activity in human skeletal muscle following resistance exercise. FASEB J Federation of American Societies for Experimental Biology. 2015;29:4358–73.
Moberg M, Apró W, Ohlsson I, Pontén M, Villanueva A, Ekblom B, et al. Absence of leucine in an essential amino acid supplement reduces activation of mTORC1 signalling following resistance exercise in young females. Appl Physiol Nutr Metab. 2013;39:1–12.
Mitchell CJ, Churchward-Venne TA, Cameron-Smith D, Phillips SM. What is the relationship between the acute muscle protein synthetic response and changes in muscle mass? J Appl Physiol. 2014;118:495–7.
Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, et al. The response of muscle protein synthesis following whole‐body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep Physiological Reports. 2016;4:e12893.
Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sport Exer. 2004;36:2073–81.
Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest American Society for Clinical Investigation. 1966;45:1487–502.
Paulsen G, Mikkelsen UR, Mikkelsen UR, Raastad T, Peake JM, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev. 2012;18:42–97.
Cooke MB, Rybalka E, Stathis CG, Cribb PJ, Hayes A. Whey protein isolate attenuates strength decline after eccentrically-induced muscle damage in healthy individuals. J Int Soc Sports Nutr BioMed Central. 2010;7:30.
Buckley JD, Thomson RL, Coates AM, Howe PRC, DeNichilo MO, Rowney MK. Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J Sci Med Sport. 2010;13:178–81.
Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr. 2014;99:276–86.
Pasiakos SM, Lieberman HR, McLellan TM. Effects of Protein Supplements on Muscle Damage, Soreness and Recovery of Muscle Function and Physical Performance: A Systematic Review. Sports Med. 2014;44:655–70.
Acknowledgements
The authors would like to thank Hege Østgaard at the Norwegian School of Sport Sciences for excellent lab work. The participants are acknowledged for their great contribution.
Funding
The study was funded by TINE SA. TINE SA was not involved in design of the study, data collection, analysis, interpretation of data or writing of the manuscript. EB and MC are partially supported by USDA-ARS-6026-51000-010-05S, Arkansas Biosciences Institute (the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000), NIH-AG033761 and NIH-P30AG028718.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
HH contributed with data analysis, drafting and write-up of the manuscript, JALL participated in study protocol development and data collection, TR contributed in protocol development, data collection and write-up of the manuscript. GP contributed in planning and review of the manuscript, MC contributed to in sample analysis and review of the manuscript, EB contributed in planning, sample analysis and review of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Written informed consent was obtained from all participants before the start of the study. The study was evaluated by the Regional Committee for Medical and Health Research Ethics (REC South East), Oslo, Norway. Reference number 2014/834.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Blood concentration of total isoleucine (A) and valine (B) before and after a bout of strength training and intake of 20 g of protein from milk, microparticulated whey (MWP), whey protein hydrolysate (WPH), whey protein concentrate 80 (WPC-80) or native whey (NW) in young men. Values are means ± SD (only shown for highest and lowest values), n = 10 in each group. All supplements increased valine concentrations at time points 30, 45 and 60 min and isoleucine at 30, 45, 60 and 90 min, p < 0.05. # milk significantly lower then all other drinks at the same time point, p < 0.05; & NW significantly higher than milk at the same time point, p < 0.05; ϕ NW, WPC-80 and MWP significantly higher than milk at the same time point, p < 0.05; θ NW, WPC-80 and WPH significantly higher than milk and MWP, p < 0.05. (TIFF 1117 kb)
Additional file 2:
Area under the curve for blood total amino acids (A), EAA (B), BCAA (C) and leucine (D) concentrations after a bout of strength training and intake of 20 g of protein from milk, microparticulated whey (MWP), whey protein hydrolysate (WPH), whey protein concentrate 80 (WPC-80) or native whey (NW) in young men. Individual and mean (± SD) data are shown. * indicate significant difference between groups, p < 0.05. (TIFF 1305 kb)
Additional file 3:
Blood concentration of total histidine (A), lysine (B), methionine (C), phenylalanine (D), threonine (E) and tryptophan (F) before and after a bout of strength training and intake of 20 g of protein from milk, microparticulated whey (MWP), whey protein hydrolysate (WPH), whey protein concentrate 80 (WPC-80) or native whey (NW) in young men. Values are means ± SD (only shown for highest and lowest values), n = 10 in each group. & NW significantly higher than milk at the same time point, p < 0.05. (TIFF 1236 kb)
Additional file 4:
Blood concentration of total alanine (A), glutamic acid (B), glutamine (C), glycine (D), proline (E), serine (F), tyrosine (G) and aspargine (H) before and after a bout of strength training and intake of 20 g of protein from milk, microparticulated whey (MWP), whey protein hydrolysate (WPH), whey protein concentrate 80 (WPC-80) or native whey (NW) in young men. Values are means ± SD (only shown for highest and lowest values), n = 10 in each group. (TIFF 1823 kb)
Additional file 5:
Individual blood leucine concentration peak values (A) and individual area under the curve values for blood leucine concentrations (B). Each participant is indicated by individual symbols. Filled symbols indicate the five heaviest participants (>80 kg) and open symbols indicate the lightest participants (<80 kg). (TIFF 1088 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hamarsland, H., Laahne, J.A.L., Paulsen, G. et al. Native whey induces higher and faster leucinemia than other whey protein supplements and milk: a randomized controlled trial. BMC Nutr 3, 10 (2017). https://doi.org/10.1186/s40795-017-0131-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-017-0131-9