Abstract
Background
Verticillium wilt of olive (VWO), caused by Verticillium dahliae Kleb, is one of the most threatening diseases affecting olive cultivation. An integrated disease management strategy is recommended for the effective control of VWO. Within this framework, the use of biological control agents (BCAs) is a sustainable and environmentally friendly approach. No studies are available on the impact that the introduction of BCAs has on the resident microbiota of olive roots. Pseudomonas simiae PICF7 and Paenibacillus polymyxa PIC73 are two BCAs effective against VWO. We examined the effects of the introduction of these BCAs on the structure, composition and co-occurrence networks of the olive (cv. Picual) root-associated microbial communities. The consequences of the subsequent inoculation with V. dahliae on BCA-treated plants were also assessed.
Results
Inoculation with any of the BCAs did not produce significant changes in the structure or the taxonomic composition of the ‘Picual’ root-associated microbiota. However, significant and distinctive alterations were observed in the topologies of the co-occurrence networks. The introduction of PIC73 provoked a diminution of positive interactions within the ‘Picual’ microbial community; instead, PICF7 inoculation increased the microbiota’s compartmentalization. Upon pathogen inoculation, the network of PIC73-treated plants decreased the number of interactions and showed a switch of keystone species, including taxa belonging to minor abundant phyla (Chloroflexi and Planctomycetes). Conversely, the inoculation of V. dahliae in PICF7-treated plants significantly increased the complexity of the network and the number of links among their modules, suggestive of a more stable network. No changes in their keystone taxa were detected.
Conclusion
The absence of significant modifications on the structure and composition of the ‘Picual’ belowground microbiota due to the introduction of the tested BCAs underlines the low/null environmental impact of these rhizobacteria. These findings may have important practical consequences regarding future field applications of these BCAs. Furthermore, each BCA altered the interactions among the components of the olive belowground microbiota in idiosyncratic ways (i.e. PIC73 strongly modified the number of positive relations in the ‘Picual’ microbiota whereas PICF7 mostly affected the network stability). These modifications may provide clues on the biocontrol strategies used by these BCAs.
Similar content being viewed by others
Background
The cultivated olive tree (Olea europaea L. subsp. europea) is a long-living woody plant of unquestionable economic, social and environmental significance. It is extensively grown in Mediterranean-type climate regions worldwide [1]. The intensification of olive cultivation observed in the last decades, mostly due to a growing demand for olive oil [2] may explain, among other reasons, the increase in both incidence and severity of olive pests and diseases [1, 3]. Among the biotic constraints affecting olive cultivation, the soil-borne fungus Verticillium dahliae Kleb., causal agent of Verticillium wilt of olive [VWO], is considered the most threatening disease in many areas where this tree is cultivated [4]. The effective control of VWO is very difficult due to a multiplicity of factors [5, 6], and available measures have so far proven unsuccessful when implemented individually. Therefore, an integrated disease management strategy is recommended [4]. The use of biological control agents [BCAs] to reduce the impact of VWO on susceptible cultivars or increase the durability of tolerant genotypes is a sustainable and environmentally friendly management approach in this context [7,8,9,10,11].
Our previous works showed the effectiveness of some olive rhizobacteria belonging to the genera Pseudomonas [12, 13] and Paenibacillus [7] to control VWO. One of the best performing BCA against VWO is Pseudomonas simiae PICF7 [14, 15], a versatile endophytic rhizobacteria isolated from olive roots (cultivar Picual) that displays biocontrol and plant-growth promoting (PGP) abilities in different plants [3, 7, 16,17,18,19]. This strain can colonize and endure within olive root tissues, triggering a broad range of defence responses in both roots [20] and above-ground tissues [3], although niche (i.e., roots/rhizosphere) competition seems to play a key role in the effective biocontrol of V. dahliae [21]. Another effective rhizobacteria controlling VWO is Paenibacillus polymyxa PIC73, originating from ‘Picual’ roots as well [7]. This strain also showed in vitro inhibition ability against a broad range of olive pathogens (i.e., V. dahliae, Rosellinia necatrix, Phytophthora cinnamomi, Pseudomonas savastanoi pv. savastanoi, Colletotrichum nymphaeae and C. godetiae) [7]. Several strains of Paenibacillus spp. are able to produce antimicrobials active against both bacteria and fungi, and a range of hydrolytic enzymes for the degradation of cellulose-containing cell wall components [22, 23].
Recent studies showed that inoculation with plant-associated beneficial bacteria may perturb indigenous microbial populations [24,25,26]. Even though the soil microbial community might have the ability to reorganize and return to the original state (resilience) after the disturbance provoked by the inoculation, the potential ecological impacts of microbial inoculants on the soil resident communities remain largely unknown. Indeed, the quick disappearance of a bacterial inoculum does not necessarily imply the lack of a lasting legacy over the soil indigenous community [25]. Biocontrol agents do not only affect the host (e.g., triggering genetic defence responses) and the pathogen (e.g., antibiosis), but they also have an effect on the plant-associated microbiome [27,28,29,30,31]. The impact of introducing a BCA on the natural pre-existing microbiota can take place at different levels, namely the structure and composition of the microbial communities, the functioning of their components, and/or the co-occurrence interaction networks. These outcomes have been recently highlighted in the case of the interaction of P. simiae PICF7 with the banana (Grand Naine Cavendish cultivar) root-associated microbiome [32]. Yet, our knowledge about the impact that BCA application has on the indigenous plant microbial communities is very limited, particularly in the case of the interactions taking place among their components. Understanding the microbe–microbe interactions is crucial in order to predict the consequences of these interactions for plant performance and physiology [33, 34]. The analysis of microbial co-occurrence interactions can also provide useful information about relevant members of the plant microbiota in order to improve plant health and counteract potential threats more effectively.
No studies are available on the impact that the introduction of BCAs has on the resident root microbial community of olive plants. Therefore, the main objectives of this work were: (I) to evaluate whether, and to what extent, the inoculation with two well-known BCAs, P. simiae PICF7 and P. polymyxa PIC73, modify the structure, the composition and the co-occurrence interactions of the belowground microbial communities associated to the olive cultivar Picual (VWO-susceptible); and (II) to assess the effect that the inoculation with V. dahliae has on the root-associated microbiota of ‘Picual’ plants previously bacterized with these BCAs. We tested the hypothesis that the introduction of PICF7 or PIC73 produces distinctive modifications in the composition and network topology of the ‘Picual’ root microbial community. The in-depth analysis of the changes produced in the resident microbiota may provide clues on the biocontrol strategies used by these BCAs.
Methods
Plant material and bacteria inoculation
Two hundred and fifty olive plants (cv. Picual, 2-year old) purchased in a commercial nursery (Córdoba, Spain) were grown in pots (11 × 11 × 12 cm), containing a peat-based substrate [35]. Prior to bacterial treatment, plants were acclimated in a greenhouse for one month under natural lighting and day/night temperature of 26 ± 2 °C, and relative humidity ranging from 40% (day) to 80% (night). Pseudomonas simiae PICF7 and P. polymyxa PIC73 inoculum were prepared as previously described [7, 36]. Bacterial inoculum consisted of 150 ml of a cell suspension (2.1 × 108 cfu/mL in the case of PICF7 or 5.5 × 105 cfu/mL for PIC73) that were added (by irrigation) to each pot accordingly to the experimental design (see below). Non-inoculated plants (control) were just drenched with 150 ml of sterile MgSO4·7H2O 10 mM. One week after bacterization, plants were inoculated with V. dahliae V937I, an isolate representative of the defoliating (D) pathotype [37], by adding 150 ml per pot of a conidia suspension (1 × 106 conidia/mL) prepared as previously described [21]. Plants of the control treatment (MgSO4·7H2O) were drenched with 150 ml of water in this step.
Experimental design
The bioassay consisted of the following treatments: (1) 40 control, non-inoculated plants (CON); (2) 30 plants bacterized with P. polymyxa PIC73 (PIC73); (3) 30 plants treated with P. simiae PICF7 (PICF7); (4) 30 plants inoculated with V. dahliae V937I (Vd); (5) 30 plants bacterized with PIC73 and subsequently inoculated with V937I (PIC73_Vd); and (6) 30 plants treated with PICF7 and then inoculated with V937I (PICF7_Vd) (Fig. 1). Additionally, 10 plants for each treatment were used to monitor the presence of VWO symptoms during the experiment. The study was performed at three different levels separately: (i) “Treatment” (i.e., bacterized, inoculated with V. dahliae, bacterized + inoculated with V. dahliae and non-inoculated/non-bacterized), (ii) “Time” (t0 and 15, 30 and 50 days after bacterization, DAB), and (iii) “Treatment-Time” (all treatments at each single sampling time). Root tissues were sampled at 0 (only for control plants), and at 15, 30 and 50 (ten plants per time-point and treatment) DAB. Root samples were collected and washed gently under tap water to remove the excess substrate without losing particles firmly attached to the root. The roots were then stored at -80 degrees Celsius until further processing (Fig. 1).
At the first sampling time (i.e., at 0 days after bacterization, DAB) 10 non-inoculated (control) plants were sampled and 120 plants were treated with microorganisms as follows: (i) 60 plants were bacterized with Paenibacillus polymyxa PIC73 (i.e., plants of the PIC73 and PIC73_Vd treatments), and (ii) 60 plants were bacterized with Pseudomonas simiae PICF7 (i.e., plants of the PICF7 and PICF7_Vd treatments). At the second sampling time (i.e., 7 DAB) 90 plants were inoculated with Verticillium dahliae V937I (i.e., plants of the Vd, PIC73_Vd and PICF7_Vd treatments, 30 plants per treatment). At the remaining sampling times (i.e., at 15, 30 and 50 DAB) ten plants of each treatment were sampled. Samples consisted of the entire root system and the potting substrate firmly attached to it. At each sampling time, collected samples were immediately frozen and stored at -80° C. At the end of the experiment all samples were lyophilized, ground to a fine powder and subjected to the DNA extraction procedure.
DNA extraction and illumina sequencing
Lyophilized root samples were ground to a fine powder using sterile mortars and pestles. DNA from 100 mg of ground root tissues per sample was extracted using the Maxwell RSC (Rapid Sample Concentrator) with the ‘PureFood GMO and Authentication’ Kit (Promega Corporation, Madison, WI, USA), according to the manufacturer’s instructions. DNA quality was then checked as previously described [32]. The DNA from root tissues was sequenced using the 2 × 300 PE technology in Illumina MiSeq platform at the genomics service of the Institute of Parasitology and Biomedicine “López Neyra” (CSIC), Granada, Spain. In the first run, prokaryotic libraries were constructed by amplifying the hyper-variable V3-V4 regions of the 16 S rRNA gene. In the second run, eukaryotic libraries were constructed by amplifying the ITS2 region. Detailed information about the sequencing strategy has been previously reported [34].
Illumina data processing
Raw reads were processed using DADA2 [38]. The Micro4all tutorial for 16 S rRNA and ITS2 amplicons processing was followed [39]. Only two samples that did not reach the 10.000 reads (i.e., one belonging to the group of plants bacterized only with PICF7 and another bacterized with PICF7 and then inoculated with V. dahliae, both at 15 DAB) had to be eliminated from the analyses. The classification of bacterial and fungal amplicon sequence variants (ASVs) was achieved using the assignTaxonomy command against the Ribosomal Database Project II, training set v.18 [40] and the UNITE v.7.2 dynamic database, respectively [41]. All ASVs classified as mitochondria, chloroplast and unknown sequences were removed. ASVs accounting for less than 0.0168 and 0.005% of the total sequences were removed for bacteria and fungi, respectively. These percentages were calculated according to the MOCK community used (ZymoBIOMICS Microbial Community Standard II (Log Distribution), ZYMO RESEARCH, CA, United States) and to the recommendations by Bokulich et al., [42].
Statistical analysis
All analyses were performed following the above-mentioned tutorial [39]. Alpha diversity indices [observed richness, Shannon, inverse of Simpson diversity (InvSimpson) and Evenness (Pielou index) were compared in rarefied samples with one-way Analysis of Unbalanced Variance using the R package car [43] and with the Kruskal–Wallis test. The Tukey honestly-significant-difference and Wilcoxon signed rank were used as post hoc tests. For the beta diversity, the normalized data, obtained using the Bio-Conductor package edgeR [44], were considered to perform the permutational analysis of variance (PERMANOVA) using three different distance methods: Bray-Curtis, Unifrac and Weighted-Unifrac. A permutational analysis of multivariate homogeneity of groups dispersions (BETADISPER) was also performed [34]. Pairwise differences between groups were assessed with the function pairwise.adonis (R package pairwiseAdonis). The PERMANOVA significant results were plotted by Non-metric MultiDimensional Scaling analysis (NMDS) and Principal Coordinates Analysis (PCoA) using all the three distance methods mentioned above. Biologically relevant microbial phyla and genera were obtained, according with their statistically significant differences in relative abundance, using the R package ANCOMBC [45].
Network construction
Microbial (bacterial and fungal) networks were separately constructed for each treatment (control, PICF7-bacterized, PIC73-bacterized, V. dahliae-inoculated, PICF7/V. dahliae-inoculated and PIC73/V. dahliae-inoculated) considering all sampling times together (n = 40 for the control, n = 30 for PIC73-bacterized and PIC73/V. dahliae, n = 29 for PICF7-bacterized and PICF7/V. dahliae-inoculated). The networks were built and drawn by using the MENAP website and Cytoscape v.3.9.1 as previously described [34].
Results
Characteristics of sequencing datasets
A total of 9,936,065 (bacterial) and 5,566,966 (fungal) good quality reads were retained after the clustering. To avoid an overestimation of the diversity, ASVs with less than 0.005% of the high-quality reads were discarded. Therefore, a total of 693 bacterial and 511 fungal ASVs were considered.
Alpha and beta diversities of the ‘Picual’ root microbiota are not significantly affected by the presence of the BCAs or the inoculation with Verticillium dahliae
Concerning alpha diversity, bacterial and fungal communities showed similar trends for all the indices considered. No significant difference was found considering the factor “Treatment”, and both communities showed a significant increase over time for the number of ASVs (Observed richness) and Shannon index (p < 0.001) (Table 1). Interesting to note the significant difference (p < 0.05 Dunn post-hoc test) observed between the first (t0 for the control and 15 DAB for the other treatments) and the last sample time (50 DAB) for all treatments, except for PIC73 (Figures S1 and S2, Additional file 1).
Regarding to beta diversity the bacterial and fungal communities showed different trends during the experiment (Table 2). For bacteria, despite the fact that the PERMANOVA test showed statistically significant differences for all the factors considered, pairwise comparisons and the PCoA analysis performed on Bray–Curtis distances (Figures S3, S4, S5, Additional file 1) did not confirm such differences. Concerning the fungal community, and in spite of statistically significant PERMANOVA results for the factor “Treatment” (p < 0.05) (Table 2), pairwise comparisons did not show relevant differences. In contrast, the PCoA analysis confirmed the results of PERMANOVA that showed the influence of time on the mycobiome (Figure S6, Additional file 1). Indeed, the fungal community displayed differences between 0 and 50 DAB regardless the treatment.
The composition of the ‘Picual’ root microbiota only shows minor significant alterations upon bacterization with the BCAs and inoculation with V. dahliae
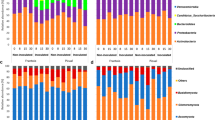
The bacterial taxonomic profile was dominated by Proteobacteria, representing no less than 55% of the relative abundance, followed by Actinobacteria (21%) and Bacteroidetes (15%). Few significant differences were found among treatments considering both all sampling times altogether and separately. At the “Treatment” level, only a significant decrease of Verrucomicrobia and Acidobacteria was observed in PICF7 compared with the control (Fig. S7A, Additional file 1). Considering the treatments at each sampling time most of the significant differences were detected for minor phyla (relative abundance < 0.1%) (Figure S8A, Additional file 1; Additional file 2). At genus level, most of the significant changes observed also affected minor taxa (relative abundance < 1%) (Additional file 3). Nevertheless, some major taxa (relative abundances > 1%) showed differences that are worth mentioning. For instance, considering only the treatments, increases of Streptacidiphilus in all PICF7-treated samples (i.e., in the presence and absence of the pathogen) (ANCOMB p = 0.049 and 0.008 for PICF7 and PICF7_Vd, respectively) and of Rhizobium in the PIC73 treatment were detected (ANCOMB p = 0.019) (Fig. 2A). Furthermore, decrease of Dyella was observed in V. dahliae-inoculated plants compared with all treatments (Fig. 2A) (ANCOMB p < 0.05). Taking into account also the time, the most noticeable change for major taxa was observed at 15 DAB (Additional file 4). At this sampling time, plants treated with PICF7 presented a significant increase of Streptacidiphilus compared with the control (ANCOMB p < 4.79•10− 6), and plants inoculated with PIC73 showed increments in the relative abundance of Rhizobium and Flavobacterium (ANCOMB p = 7.11•10− 4 and 2.48•10− 7, respectively) (Figure S9, Additional file 1). Interestingly, plants bacterized with PICF7 and then inoculated with V. dahliae showed a significant increase of Novosphingobium at 15 DAB compared with all the other treatments (ANCOMB p < 0.05) (Figure S9, Additional file 1).
With regard to the fungal dataset the most abundant phyla were Ascomycota, Basidiomycota and Glomeromycota, accounting for at least 97% of the total sequences. The comparison among treatments revealed that plants inoculated with V. dahliae showed a decrease in Glomeromycota, although this difference was only statistically significant versus the PIC73 treatment (ANCOMB p = 0.003) (Figure S7B, Additional file 1). Considering each sampling time, significant changes were mostly found in minor phyla (relative abundance < 0.1%) (Figure S8B, Additional file 1; Additional file 5). The same trend was observed at the genus level (Fig. 2B and Figure S10, Additional file 1) (Additional file 6), except for the major genera (relative abundance > 1%) Rhizoctonia considering all the sampling times. It was present in the PICF7 treated plants at 15 and 30 DAB and absent at 50 DAB, while it showed the reverse tendency in PICF7_Vd samples (ANCOMB p < 0.05) (Figure S10, Additional file 1).
Bacterial (A) and fungal (B) taxonomic profiles at genus level for the different treatments considered in this study: control (CON], Paenibacillus polymyxa PIC73-treated (PIC73), P. polymyxa PIC73/V. dahliae-inoculated (PIC73_Vd), Pseudomonas simiae PICF7-treated (PICF7), P. simiae PICF7/V. dahliae-inoculated (PICF7_Vd), and V. dahliae-inoculated (Vd). Only the genera with a relative abundance > 2% are shown (n = 10). Asterisks indicate the genera that showed significant differences (ANCOMB p < 0.05) by the taxonomical analysis (see main text).
The biocontrol strains PIC73 and PICF7 produced distinctive alterations in the topology of the ‘Picual’ root microbiota co-occurrence network
The introduction of the BCAs provoked a reduction of the network complexity and the increase of the distance among nodes compared to the control treatment. This shift in the network topology was more evident in the presence of PIC73. Indeed, smaller number of total links, lower average degree of nodes (avgK) and higher geodesic distance (GD) values were observed (Table 3). The network of the PIC73 treatment showed the lowest average clustering coefficient (avgCC) and percentage of positive edges (PEP) values denoting decreasing of connectivity and increasing in the number of negative interactions compared with the control (Table 3; Fig. 3). These changes were not observed after PICF7 inoculation, whose network otherwise showed the highest modularity (Table 3). Interestingly, the keystones detected in the PIC73 network were not observed in the other networks, while the PICF7 network presented one module hub identical to that of the control network (Verrucomicrobia/Aterococcus), and absence of connectors (Table 4). The networks of plants bacterized with BCAs showed fungi among their keystone. Thus, the genus Clitopilus (Basidiomycota) was a connector in the PIC73 network, while an unidentified Ascomycota genus acted as module hub in the PICF7 network (Table 4).
Verticillium dahliae reduces the complexity of the ‘Picual’ root microbiota co-occurrence network
Similarly to the introduction of the BCAs, the network of the V. dahliae-inoculated plants showed less complexity compared with the control treatment. Moreover, this network presented the lowest number of total nodes and links among all networks analysed. It also showed higher modularity, more distance among modules [i.e., less interaction/edges among them], and major compartmentalization compared with the control one (Table 3). Finally, a huge abundance of Proteobacteria representatives were observed as keystone species for the Vd network (Table 4).
Co-occurrence networks respond differently to the V. dahliae inoculation depending on the previously-introduced BCA
The inoculation of V. dahliae in PIC73-treated plants produced an increase in modularity (Table 3) compared with the pre-existing situation (i.e., the PIC73 network). Furthermore, the PIC73_Vd network was the only one showing Chloroflexi (genus Ktedonobacter) and Plantomycetes (genus Bythopirellula) as keystone taxa (Table 4). Conversely, the PICF7_Vd network displayed a decrease of modularity and an important increase of total links compared with the situation observed prior to the inoculation with the pathogen (i.e., the PICF7 network, Table 3). Both networks showed a decrease in the distance among modules (GD) and an increase of connectivity (avgCC), more pronounced in the case of the PICF7_Vd treatment. Besides, these two networks presented an increase of PEP, similarly to the scenario observed in the Vd network. Furthermore, they presented two identical keystones (Proteobacteria/Bradyrhizobiaceae and Actinobacteria/Jatrophihabitans) (Table 4). The topology of these networks shares some similarities to that of the control (e.g., all showed one of the major modules with an overwhelming presence of positive interactions; Fig. 3). The detailed analysis of these modules revealed very similar compositions. They shared 14 bacterial taxa, mostly belonging to the genera Actinoplanes, Chryseolina and Terrimonas. Moreover, representatives of Actinoplanes and Chryseolina were module hubs in the three networks (Table 4).
Co-occurrence networks of the microbial communities unveiled for each of the treatments considered in this study: control (CON), Verticillium dahliae-inoculated (Vd), Paenibacillus polymyxa-treated (PIC73), P. polymyxa/V. dahliae-inoculated (PIC73_Vd), Pseudomonas simiae-treated (PICF7), and P. simiae/V. dahliae-inoculated (PICF7_Vd) plants. The modular layout of the networks is shown, with the nodes coloured according to their phylum. The green and red lines (links) indicate positive and negative interactions, respectively. Rhombi and arrowheads represent module hubs and connectors, respectively.
Discussion
The plant and its associated microbiome can be regarded as a meta-organism, the so-called “holobiont” [24]. Millions of microbes live in close association with plants, forming a complex community that influences plant growth and health through its collective metabolic activities and host interactions [31]. In this context, the impact of applying BCAs should be evaluated considering not only their separated effects on the pathogen and/or the host but rather on the whole system, which includes the resident microbiota. This knowledge will be instrumental for environmental risk assessment of BCA formulations [24, 32]. The first relevant result of our study was that neither the inoculation with the BCAs nor the subsequent inoculation with V. dahliae significantly modified the structure of the microbial communities, as revealed by the analysis of the alpha and beta diversities. This outcome is in accordance with our previous findings on root microbial communities of ‘Picual’ inoculated with V. dahliae [34] or banana bacterized with PICF7 [32]. Indeed, in both scenarios (i.e., introduction of a soil-borne pathogen or a beneficial rhizobacteria) no significant changes in the structure of the belowground microbiota were observed.
Concerning the ‘Picual’ root microbiota composition significant changes were detected mostly in taxa with minor relative abundance, except for few interesting differences found in major taxa deserving discussion. At phylum level, a decrease in the relative abundance of Verrucomicrobia and Acidobacteria was observed after inoculation with PICF7. Previous studies have shown that the abundance of these taxa changes with the availability of labile carbon originated from rhizodeposits, exudates and mucigel [46, 47]. Our results might suggest that introduction of PICF7 modifies the production of plant exudates, thereby affecting the abundance of components of these phyla in ‘Picual’ roots. Recently, the ability of an inoculant to modify the rate and composition of root exudates as an indirect biocontrol mechanism has been described [48]. Another significant change in our study was the decrease of Glomeromycota in plants inoculated only with V. dahliae, confirming our previous results [34]. This may suggest a strong competition for space and nutrients between pathogenic microorganisms and arbuscular mycorrhizal fungi (Glomeromycota) in roots, as reported in peanut (Arachis hypogaea L.) [49], pea (Pisum sativum L.) [50] and olive [51]. At the genus level, a reduction in the relative abundance of Dyella was observed in plants inoculated only with the pathogen, in accordance with studies reporting a decrease in the relative abundance of this bacterium in rice infected with Burkholderia glumae [52] and in wheat inoculated with Rhizoctonia solani [53]. It is tempting to speculate that Dyella may play a role as indicator of stress for the olive root microbial community upon V. dahliae inoculation. At the first sampling time [15 DAB], and regardless of whether or not the pathogen was present, plants inoculated with PICF7 showed an increase of Streptacidiphilus, a genus of acidophilic Actinobacteria that are well-known for their antifungal activity [54]. A recent study has reported the antibacterial and antifungal ability of eleven species of this genus through a comparative genome analysis [55]. At the same sampling time, plants inoculated with PICF7 and subsequently inoculated with V. dahliae showed a significant increase of Novosphingobium. Some species of this genus are known for their metabolic versatility and involvement in cell-cell signalling [56]. Indeed, several species of this genus are able to produce chemical signals to their surroundings thereby activating population-wide responses leading to the coordination of gene activation or repression in response to environmental cues [56, 57]. Plants of this treatment also showed a decrease of Rhizoctonia at 15 DAB, compared with plants only bacterized with PICF7, although the relative abundance of this genus gradually increased at later sampling times. This could suggest antagonism between this fungus and V. dahliae, as previously reported in antagonism tests [58]. Interestingly, the opposite trend was observed in plants bacterized with PICF7 [i.e., an increase of Rhizoctonia at 15 DAB followed by a decrease later on]. Altogether, these results seem to suggest the ability of PICF7 to recruit taxa either aiding to directly antagonize V. dahliae [i.e., antifungal activity] or able to activate a response in the holobiont due to an emerging stress (i.e., presence of the pathogen).
Relating to the impacts of strain PIC73, it is worth mentioning the increase in relative abundances of Rhizobium and Flavobacterium at 15 DAB. Both genera are well-known PGP rhizobacteria [59,60,61]. Thus, it can be argued that the presence of PIC73 facilitates the recruitment of beneficial rhizobacteria able to stimulate the plant’s growth by mechanisms such as [micro]nutrients mobilization and suppression of pathogens. Indeed, some species of Flavobacterium showed biocontrol activity against Phytophthora capsici in pepper [61] and Clavibacter michiganensis in tomato [62]. It is tempting to speculate that one of the underlying biocontrol mechanisms exerted by these BCAs could be the consequence [side effect] of subtle modifications on the taxonomic profile of the olive root microbiota. In both cases the recruitment of V. dahliae antagonists and/or beneficial microorganisms seemed to be crucial, although each of the BCA facilitated/mediated the “enrolment” of different taxa. It is worth mentioning that strain PIC73 displays a broad antagonist activity [7]. The in situ antibiosis exerted by this rhizobacteria may be thus relevant not only because of the direct inhibition of V. dahliae, but also due to changes in the resident microbiota to better cope with the pathogen. In contrast, and without excluding the involvement of antibiosis and modifications of the indigenous microbiota, niche competition and induction of host defence responses seem to play a more decisive role in VWO biocontrol exerted by strain PICF7 [20, 21].
The ASVs corresponding to each of the introduced BCA were not consistently found in BCA-treated samples. Nevertheless, ASV00685 (i.e., strain PICF7) was detected in some samples of PIC73-treated and V. dahliae-inoculated plants. This outcome is not totally unexpected, since PICF7 is a natural inhabitant of ‘Picual’ roots and was originally isolated form nursery-produced plants of this cultivar [12]. It is plausible to think that both BCAs experienced a rapid decline over time under our experimental conditions, hindering their detection by the sequencing approach conducted. This result agrees with previous studies reporting a transient establishment and subsequent decline of microbial inoculants. For instance, Pseudomonas jessenii and Serratia plymuthica experienced a sharp decrease of their relative abundances in the lettuce rhizosphere two weeks after the inoculation of these BCAs [63]; two strains of P. fluorescence almost disappeared in the rhizosphere of cucumber seven days after bacterization [30]; or PICF7 showed a rapid decrease in its relative abundance two days after being inoculated in banana roots [32]. Furthermore, considering that alpha and beta diversities did not significantly change and that most of the relevant taxonomical changes occurred at 15 DAB, the impact caused by the introduction of the two BCAs on the indigenous microbiota can be regarded as minor and just taking place during a short period of time after bacterization.
The most outstanding impact after BCA treatment and/or V. dahliae inoculation was found in the interactions among components of the belowground ‘Picual’ microbiota, unveiled through co-occurrence network analysis. The network of the plants inoculated only with V. dahliae showed a severe decrease in complexity [i.e., reduction of total nodes, links and avgK] compared with the control. Our previous findings were also supported by the fact that GD increased, showing that the native microbial population mitigated the pathogen’s deleterious impact by reducing the number of interactions between modules [34]. The analysis of the PIC73 and PICF7 networks revealed that their effects on the ‘Picual’ root microbiota differed notably. On the one hand, bacterization with PIC73 provoked a diminution of PEP. Recent studies related negative and positive connections with competition and cooperation, respectively [64,65,66]. This result suggests that the presence of PIC73 increased the number of competitive interactions among the olive root microbiota members compared to the control. This modification could enhance the ability of the microbial community to cope with the V. dahliae invasion. Indeed, the study of the rhizo-microbiome of eggplants has demonstrated that a network with more negative interactions better resisted to Ralstonia solanacearum infection, compared with a network with higher PEP [67]. On the other hand, the network of PICF7-bacterized plants presented a notable increase of modularity and no connectors, compared with the control. Modularity minimizes the effects of local perturbations on the system as a whole by confining perturbations and damage at a local level [65]. Thus, we can argue that PICF7 inoculation increased the compartmentalization [modules] among members of the microbial community as a strategy to maintain its stability, making it able to better cope with the pathogen. Therefore, the hypothesis to-be-tested in this study has been confirmed: the bacterization with PICF7 or PIC73 produces distinctive alterations in the ‘Picual’ root microbial community. Particularly, each of the BCAs showed the ability to recruit different microbial taxa to help the ‘Picual’ belowground microbiota to confront V. dahliae [see above]. But more importantly, they generated different topologies of the co-occurrence networks. Interestingly enough, and even though modifications largely differed, changes in the networks [together with minor alterations in the microbiota composition] did not mean that biocontrol was compromised. This suggests that both BCAs assist the olive holobiont to cope with the pathogen altering the root microbiota in characteristic but equally effective ways. However, additional biocontrol mechanisms cannot be ruled out as mentioned above. After challenging PIC73-treated plants with the pathogen, the resulting PIC73_Vd network increased the modularity and shifted the keystones taxonomy. Thus, bacteria belonging to low abundant phyla (i.e., Chloroflexi, Planctomyces) acted as module hubs and connectors, unlike the situation observed in control and PIC73 networks where taxa of high abundant phyla (i.e., Proteobacteria, Bacteroidetes, Verrucomicrobia) were keystones. This suggests that low abundant taxa may play an important role in maintaining the structure and function of the network to face an incoming perturbation (i.e., V. dahliae). It has been proposed that dominant and minor species might temporally “switch” their roles to deal with sudden environmental stimuli [68]. Thus, network analyses can provide key information to determine the role that minor representatives within the microbial community may play in response to pathogen invasion. Moreover, in our case, it may offer clues on the biocontrol mechanism involved. Indeed, the suggestion that PIC73 may confront V. dahliae mainly through antibiosis (see above) could be supported by the higher abundance of Chloroflexi and Planctomyces, genera which are well-known to resist a broad spectrum of antibiotics [67,68,69,70,71]. Furthermore, the increase in modularity may be related to a restructuring of the community to minimize the effects of the perturbation caused not only by the inoculation of the pathogen but also by the previous effects caused by PIC73. Conversely, the PICF7_Vd network showed a significant decrease of modularity and increase of complexity, suggestive of a network with many highly connected taxa [nodes and modules] thereby providing more stability [72]. The increase of complexity in this network suggests an increase of interactions/cooperation within the microbial community, probably among the beneficial taxa recruited by this BCA to cope with the presence of the pathogen [see above]. The presence of a Bradyrhizobiaceae representative as a connector may support this hypothesis. It is interesting to note that all the networks inoculated with V. dahliae presented high PEP, suggesting more cooperation among community members [64, 65]. This could be a likely consequence of the stress caused by the presence of the pathogen. Increase of positive interaction in the rhizosphere microbiome of Vicia faba under saline stress has been reported as well [73].
Conclusion
The two examined BCAs did not decisively alter the ‘Picual’ root microbiota at structural or compositional levels. More precisely, most of the changes observed were limited to minor taxa (Fig. 4) and at early time after bacterization. This outcome may have important practical consequences regarding future applications of these BCAs. For instance, the low/null environmental impact (i.e. minor or transient effects on the root-associated microbial diversity) may facilitate their release in olive orchards as a sustainable approach within a VWO integrated management strategy. Furthermore, these BCAs either alone or in combination with other beneficial rhizobacteria (i.e. as part of synthetic communities) could also be useful in breeding programs for VWO resistance, as well as during the nursery propagation stage to produce plants more effective to confront future attacks of the pathogen under field conditions and from the holobiont perspective. However, while the structure and composition of the olive bewlowground microbiota was not significantly altered, relevant changes in the topology of the co-occurrence networks were found after bacterization. The presence of PIC73 increased the competitive interactions within the ‘Picual’ microbiota, while PICF7 inoculation provoked an increase in compartmentalization of the network [Fig. 4]. These results, together with the few taxonomic changes observed in major taxa, demonstrate the differential impact caused by the BCAs, and may help to better understand their distinct biocontrol strategies against V. dahliae. This work confirms the importance of co-occurrence network analysis to detect the actual impacts (beyond potential changes in structure and composition) caused by external factors/perturbations on microbial communities. Moreover, this methodological approach would be instrumental to analyse the eligibility of new BCA inoculants.
Schematic summary of the major results of this study. (In the upper table, phyla and genera that showed significant changes in relative abundance, at “Treatment” level, after bacterization/inoculation compared to the control treatment are indicated. Changes in major (M) and minor (m) abundant taxa are indicated. Relevant changes in co-occurrence network topologies due to the different treatments are also shown schematically. In the network diagrams, red and green edges represent negative and positive interactions among modules (coloured circles), respectively. The insets within each network scheme refer to the network to which comparisons were made. Different colours of the modules represent differences in keystone taxa. In the bottom table, decrease (red arrows) or increase (green arrows) of the indicated network parameters are shown compared to the network reported in the insets of each scheme)
Availability of data and material
The datasets generated and analysed during the current study are available in the NCBI Sequence Read Archive (SRA) under the BioProject number PRJNA856429.
Change history
27 October 2023
The Funding declaration of this article has been corrected following original publication.
References
Bizos G, Papatheodorou, Efimia M, Chatzistathis T, Ntalli N, Aschonitis VG, Nikolaos M. The role of microbial inoculants on plant protection, growth stimulation, and crop productivity of the olive tree [Olea europea L]. Plants. 2020;9743:1–16.
Ben Amira M, Lopez D, Triki Mohamed A, Khouaja A, Chaar H, Fumanal B, et al. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol Control. 2017;110:70–8.
Gómez-Lama Cabanás CGL, Schilirò E, Valverde-Corredor A, Mercado-Blanco J. The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces systemic defense responses in aerial tissues upon colonization of olive roots. Front Microbiol. 2014;5:1–14.
López-Escudero FJ, Mercado-Blanco J. Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil. 2011;3441:1–50.
Keykhasaber M, Thomma BPHJ, Hiemstra JA. Verticillium wilt caused by Verticillium dahliae in woody plants with emphasis on olive and shade trees. Eur J Plant Pathol. 2018;1501:21–37.
Montes-Osuna N, Mercado-Blanco J. Verticillium wilt of olive and its control: What did we learn during the last decade?Plants. 2020;11[9]:735 – 65.
Gómez-Lama Cabanás C, Ruano-Rosa D, Legarda G, Pizarro-Tobías P, Valverde-Corredor A, Triviño JC, et al. Bacillales members from the olive rhizosphere are effective biological control agents against the defoliating pathotype of Verticillium dahliae. Agric. 2018;87:1–23.
Deketelaere S, Tyvaert L, França SC, Höfte M. Desirable traits of a good biocontrol agent against Verticillium wilt. Front Microbiol. 2017;8:1–23.
Mulero-Aparicio A, Agustí-Brisach C, Varo Á, López-Escudero FJ, Trapero A. A non-pathogenic strain of Fusarium oxysporum as a potential biocontrol agent against Verticillium wilt of olive. Biol Control. 2019;139:104045.
Markakis EA, Tjamos SE, Antoniou PP, Paplomatas EJ, Tjamos EC. Biological control of Verticillium wilt of olive by Paenibacillus alvei, strain K165. BioControl. 2016;61[3]:293–303.
Carrero-Carrón I, Trapero-Casas JL, Olivares-García C, Monte E, Hermosa R, Jiménez-Díaz RM. Trichoderma asperellum is effective for biocontrol of Verticillium wilt in olive caused by the defoliating pathotype of Verticillium dahliae. Crop Prot. 2016;88:45–52.
Mercado-Blanco J, Rodríguez-Jurado D, Hervás A, Jiménez-Diaz RM. Suppression of Verticillium wilt in olive planting stocks by root-associated fluorescent Pseudomonas spp. Biol Control. 2004;302:474–86.
Gómez-Lama Cabanás C, Legarda G, Ruano-Rosa D, Pizarro-Tobías P, Valverde-Corredor A, Niqui JL, et al. Indigenous Pseudomonas spp. strains from the olive [Olea europaea L.] rhizosphere as effective biocontrol agents against Verticillium dahliae: from the host roots to the bacterial genomes. Front Microbiol. 2018;9:1–23.
Martínez-García PM, Ruano-Rosa D, Schilirò E, Prieto P, Ramos C, Rodríguez-Palenzuela P, et al. Complete genome sequence of Pseudomonas fluorescens strain PICF7, an indigenous root endophyte from olive [Olea europaea L.] and effective biocontrol agent against Verticillium dahliae. Stand Genomic Sci. 2015;10:1–7.
Montes-Osuna N, Gómez-Lama Cabanás C, Valverde-Corredor A, Berendsen RL, Prieto P, Mercado-Blanco J. Assessing the involvement of selected phenotypes of Pseudomonas simiae PICF7 in olive root colonization and biological control of Verticillium dahliae. Plants. 2021;102:1–25.
Mercado-Blanco J, Alós E, Rey MD, Prieto P. Pseudomonas fluorescens PICF7 displays an endophytic lifestyle in cultivated cereals and enhances yield in barley. FEMS Microbiol Ecol. 2016;928:1–13.
Maldonado-González MM, Bakker PAHM, Prieto P, Mercado-Blanco J. Arabidopsis thaliana as a tool to identify traits involved in Verticillium dahliae biocontrol by the olive root endophyte Pseudomonas fluorescens PICF7. Front Microbiol. 2015;6:1–12.
Gómez-Lama Cabanás C, Fernández-González AJ, Cardoni M, Valverde-Corredor A, López-Cepero J, Fernández-López M, et al. The banana root endophytome: differences between mother plants and suckers and evaluation of selected bacteria to control Fusarium oxysporum f.sp. Cubense. J Fungi. 2021;73:1–26.
Desrut A, Thibault F, Mercado-Blanco J, Coutos-Thévenot P, Vriet C. Transcriptional regulation of plant sugar transporter genes by beneficial rhizobacteria. J Plant Interact. 2021;161:443–51.
Schilirò E, Ferrara M, Nigro F, Mercado-Blanco J. Genetic responses induced in olive roots upon colonization by the biocontrol endophytic bacterium Pseudomonas fluorescens PICF7.PLoS One. 2012;7[11] 48646.
Gómez-Lama Cabanás C, Sesmero R, Valverde-Corredor A, Javier López-Escudero F, Mercado-Blanco J. A split-root system to assess biocontrol effectiveness and defense-related genetic responses in above-ground tissues during the tripartite interaction Verticillium dahliae-olive-Pseudomonas fluorescens PICF7 in roots.Plant Soil. 2017;417[1–2]:433–52.
Rybakova D, Cernava T, Köberl M, Liebminger S, Etemadi M, Berg G. Endophytes-assisted biocontrol: novel insights in ecology and the mode of action of Paenibacillus. Plant Soil. 2016;405(1–2):125–40.
Raza W, Yang W, Shen QR. Paenibacillus polymyxa: antibiotics, hydrolytic enzymes and hazard assessment. J Plant Pathol. 2008;903:419–30.
Berg G, Kusstatscher P, Abdelfattah A, Cernava T, Smalla K. Microbiome modulation—toward a better understanding of plant microbiome response to microbial inoculants.Front Microbiol. 2021;12[65010].
Mawarda PC, Le Roux X, Dirk van Elsas J, Salles JF. Deliberate introduction of invisible invaders: A critical appraisal of the impact of microbial inoculants on soil microbial communities.Soil Biol Biochem. 2020;148[107874].
Mallon CA, Le Roux X, Van Doorn GS, Dini-Andreote F, Poly F, Salles JF. The impact of failure: unsuccessful bacterial invasions steer the soil microbial community away from the invader’s niche. ISME J. 2018;123:728–41.
Scherwinski K, Grosch R, Berg G. Effect of bacterial antagonists on lettuce: active biocontrol of Rhizoctonia solani and negligible, short-term effects on non-target microorganisms. FEMS Microbiol Ecol. 2008;641:106–16.
Schwieger F, Tebbe CC. Effect of field inoculation with Sinorhizobium meliloti L33 on the composition of bacterial communities in rhizospheres of a target plant [Medicago sativa] and a non-target plant [Chenopodium album] - linking of 16S rRNA gene-based single-strand conforma. Appl Environ Microbiol. 2000;668:3556–65.
Johansen A, Olsson S. Using phospholipid fatty acid technique to study short-term effects of the biological control agent Pseudomonas fluorescens DR54 on the microbial microbiota in barley rhizosphere. Microb Ecol. 2005;492:272–81.
Yin D, Wang N, Xia F, Li Q, Wang W. Impact of biocontrol agents Pseudomonas fluorescens 2P24 and CPF10 on the bacterial community in the cucumber rhizosphere. Eur J Soil Biol. 2013;59:36–42.
Schmidt R, Köberl M, Mostafa A, Ramadan EM, Monschein M, Jensen KB, et al. Effects of bacterial inoculants on the indigenous microbiome and secondary metabolites of chamomile plants. Front Microbiol. 2014;5:1–11.
Gómez-Lama Cabanás C, Wentzien NM, Zorrilla-Fontanesi Y, Valverde-Corredor A, Fernández-González AJ, Fernández-López M, et al. Impacts of the biocontrol strain Pseudomonas simiae PICF7 on the banana holobiont: alteration of root microbial co-occurrence networks and effect on host defense responses. Front Microbiol. 2022;13:1–16.
Agler MT, Ruhe J, Kroll S, Morhenn C, Kim ST, Weigel D, et al. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016;141:1–31.
Fernández-González AJ, Cardoni M, Gómez-Lama Cabanás C, Valverde-Corredor A, Villadas PJ, Fernández-López M, et al. Linking belowground microbial network changes to different tolerance level towards Verticillium wilt of olive. Microbiome. 2020;81:1–19.
Cardoni M, Mercado-Blanco J, Villar R. Functional traits of olive varieties and their relationship with the tolerance level towards verticillium wilt.Plants. 2021;10[6].
Prieto P, Mercado-Blanco J. Endophytic colonization of olive roots by the biocontrol strain Pseudomonas fluorescens PICF7. FEMS. Microbiol Ecol. 2008;642:297–306.
Collado-Romero M, Mercado-Blanco J, Olivares-García C, Valverde-Corredor A, Jiménez-Díaz RM. Molecular variability within and among Verticillium dahliae vegetative compatibility groups determined by fluorescent amplified fragment length polymorphism and polymerase chain reaction markers. Phytopathology. 2006;965:485–95.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;137:581–3.
Wentzien NM. Micro4all workflow. 2021. https://nuriamw.github.io/micro4all/tutorial/package_workflow.html
Callahan B. RDP taxonomic training data formatted for DADA2 [RDP trainset 18/release 11.5]. 2020. https://zenodo.org/record/4310151#.YyBhoXZByUk
UNITE Community. UNITE mothur release. UNITE Community. 2017. https://plutof.ut.ee/#/doi/10.15156/BIO/587478
Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;101:57–9.
Fox J, Weisberg S, An R. Companion to Applied Regression. 3rd Edn. Thousand OAks, CA:Sage Publication Inc.; 2018.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;261:139–40.
Lin H, Peddada S, Das. Analysis of compositions of microbiomes with bias correction. Nat Commun. 2020;111:1–11.
Merino-Martín L, Stokes A, Gweon H, Moragues-saitua L, Staunton S, Plassard C, et al. Interacting effects of land use type, soil microbes and plant traits on aggregate stability. Soil Biol Biochem. 2021;154:1–19.
Grün AL, Emmerling C. Long-term effects of environmentally relevant concentrations of silver nanoparticles on major soil bacterial phyla of a loamy soil. Environ Sci Eur. 2018;301:1–13.
Matilla MA, Ramos JL, Bakker PAHM, Doornbos R, Badri DV, Vivanco JM, et al. Pseudomonas putida KT2440 causes induced systemic resistance and changes in Arabidopsis root exudation. Environ Microbiol Rep. 2010;23:381–88.
Li X, Ding C, Zhang T, Wang X. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol Biochem. 2014;72:11–8.
Bødker L, Kjøller R, Kristensen K. Interactions between indigenous arbuscular mycorrhizal fungi and Aphanomyces euteiches in field-grown pea. Mycorrhiza. 2002;12:7–12.
Porras-Soriano A, Marcilla-Goldaracena I, Soriano-Martín ML, Porras-Piedra A. Development and resistance to Verticillium dahliae of olive plantlets inoculated with mycorrhizal fungi during the nursery period. J Agric Sci. 2006;144:151–7.
Kim N, Kim JJ, Kim I, Mannaa M, Park J, Kim J, et al. Type VI secretion systems of plant-pathogenic Burkholderia glumae BGR1 play a functionally distinct role in interspecies interactions and virulence. Mol Plant Pathol. 2020;218:1055–69.
Yin C, Hulbert SH, Schroeder KL, Mavrodi O, Mavrodi D, Dhingra A, et al. Role of bacterial communities in the natural suppression of Rhizoctonia solani bare patch disease of wheat [Triticum aestivum L]. Appl Environ Microbiol. 2013;7923:7428–38.
Cho SH, Han JH, Ko HY, Kim SB. Streptacidiphilus anmyonensis sp. nov., Streptacidiphilus rugosus sp. nov. and Streptacidiphilus melanogenes sp. nov., acidophilic actinobacteria isolated from Pinus soils. Int J Syst Evol Microbiol. 2008;587:1566–70.
Malik A, Kim YR, Kim SB. Genome mining of the genus Streptacidiphilus for biosynthetic and biodegradation potential. Genes [Basel]. 2020;1110:1–31.
Gan HM, Buckley L, Szegedi E, Hudson AO, Savka MA. Identification of an rsh gene from a Novosphingobium sp. necessary for quorum-sensing signal accumulation. J Bacteriol. 2009;1918:2551–60.
Gan HM, Hudson AO, Yamin A, Rahman A, Chan KG, Savka MA. Comparative genomic analysis of six bacteria belonging to the genus Novosphingobium: insights into marine adaptation, cell-cell signaling and bioremediation. BMC Genomics. 2013;14:431–45.
Demirci E, Dane E, Eken C. In vitro antagonistic activity of fungi isolated from sclerotia on potato tubers against Rhizoctonia solani in vitro. Turkish J Biol. 2011;354:457–62.
Walitang DI, Kim K, Madhaiyan M, Kim YK, Kang Y, Sa T. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of rice. BMC Microbiol. 2017;171:1–13.
Castro-Sowinski S. Microbial Models: From Environmental to Industrial Sustainability. Castro-Sowinski S, editor. Microbial Models: From Environmental to Industrial Sustainability. Uttar Pradesh, India: Springer; 2016.
Sang MK, Kim KD. The volatile-producing Flavobacterium johnsoniae strain GSE09 shows biocontrol activity against Phytophthora capsici in pepper. J Appl Microbiol. 2012;1132:383–98.
Kolton M, Frenkel O, Elad Y, Cytryn E. Potential role of flavobacterial gliding-motility and type IX secretion system complex in root colonization and plant defense. Mol Plant-Microbe Interact. 2014;279:1005–13.
Schreiter S, Sandmann M, Smalla K, Grosch R. Soil type dependent rhizosphere competence and biocontrol of two bacterial inoculant strains and their effects on the rhizosphere microbial community of field-grown lettuce. PLoS ONE. 2014;98:1–11.
Zhang Y, Zhao Z, Dai M, Jiao N, Herndl GJ. Drivers shaping the diversity and biogeography of total and active bacterial communities in the South China Sea. Mol Ecol. 2014;239:2260–74.
Ding J, Zhang Y, Deng Y, Cong J, Lu H, Sun X, et al. Integrated metagenomics and network analysis of soil microbial community of the forest timberline. Sci Rep. 2015;5:1–10.
Li M, Wei Z, Wang J, Jousset A, Friman VP, Xu Y, et al. Facilitation promotes invasions in plant-associated microbial communities. Ecol Lett. 2019;221:149–58.
Jiang G, Zhang Y, Gan G, Li W, Wan W, Jiang Y, et al. Exploring rhizo-microbiome transplants as a tool for protective plant-microbiome manipulation. ISME Commun. 2022;21:1–10.
Tao J, Meng D, Qin C, Liu X, Liang Y, Xiao Y, et al. Integrated network analysis reveals the importance of microbial interactions for maize growth. Appl Microbiol Biotechnol. 2018;1028:3805–18.
Feng Y, Hu J, Chen Y, Xu J, Yang B, Jiang J. Ecological effects of antibiotics on aquaculture ecosystems based on microbial community in sediments. Ocean Coast Manag. 2022;224:1061–73.
Zhang Q, Wu J, Yu YY, He YJ, Huang Y, Fan NS, et al. Microbial and genetic responses of anammox process to the successive exposure of different antibiotics. Chem Eng J. 2021;420:127576.
Zhu B, Chen Q, Chen S, Zhu YG. Does organically produced lettuce harbor higher abundance of antibiotic resistance genes than conventionally produced? Environ Int. 2017;98:152–9.
Yuan MM, Guo X, Wu L, Zhang Y, Xiao N, Ning D, et al. Climate warming enhances microbial network complexity and stability. Nat Clim Chang. 2021;114:343–8.
Benidire L, El Khalloufi F, Oufdou K, Barakat M, Tulumello J, Ortet P, et al. Phytobeneficial bacteria improve saline stress tolerance in Vicia faba and modulate microbial interaction network. Sci Total Environ. 2020;729:139020.
Acknowledgements
We thank to Carmen Gómez-Lama Cabanás for her assistance with inocula preparations.
Funding
This work was supported by the Spanish Ministerio de Ciencia, Innovación y Universidades/Agencia Estatal de Investigación (grant PID2019-106283RB-I00), with the support of EU ERDF funds.
Author information
Authors and Affiliations
Contributions
J.M.B. and M.F.L. conceived and designed the study. M.C. and J.M.B. wrote the manuscript. M.C. and A.V.C. run the greenhouse experiment and carried out the sampling. M.C. performed nucleic acids extractions. M.C. and A.J.F.G. performed the bioinformatics and statistical analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cardoni, M., Fernández-González, A.J., Valverde-Corredor, A. et al. Co-occurrence network analysis unveils the actual differential impact on the olive root microbiota by two Verticillium wilt biocontrol rhizobacteria. Environmental Microbiome 18, 21 (2023). https://doi.org/10.1186/s40793-023-00480-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40793-023-00480-2