Abstract
Background
APC and MUTYH are both well-known colorectal polyposis causative genes. However, 30–50% of colorectal adenomatous polyposis cases are classified as colonic adenomatous polyposis of unknown etiology and lack identifiable pathogenic variants. Although guidelines recommend total proctocolectomy for colonic adenomatous polyposis of unknown etiology with over 100 adenomas, evidence is lacking. This study presents a unique case of localized colonic adenomatous polyposis of unknown etiology with multiple adenocarcinomas, treated with hemicolectomy and regional lymph node dissection.
Case presentation
The patient was a 72-year-old woman whose colonoscopy revealed numerous polyps and two adenocarcinomas localized in the right side of the colon, with no lesions in the left side. The patient had no family history of polyposis or colorectal cancer. No extracolonic lesions, enlarged lymph nodes, or distant metastases were found. Considering the patient’s age and lesion localization, laparoscopic right hemicolectomy with regional lymph node dissection was performed. Histopathological diagnosis revealed three adenocarcinoma lesions with no lymph node metastasis. The most advanced pathological stage was T2N0M0 Stage I (UICC 8th edition). The patient was alive 5 years postoperatively, without recurrence of cancer or polyposis in the remaining colon and rectum. To diagnose hereditary colorectal cancer/polyposis, a germline multigene panel testing for APC, EPCAM, MBD4, MLH1, MLH3, MSH2, MSH3, MSH6, MUTYH, NTHL1, PMS2, POLD1, POLE, and TP53 was performed using DNA extracted from blood samples: however, no pathogenic variant was detected. Therefore, the patient was diagnosed with colonic adenomatous polyposis of unknown etiology.
Conclusions
In this rare case, colonic adenomatous polyposis of unknown etiology, with numerous adenomatous polyps and multiple adenocarcinomas localized in the right side of the colon, was successfully treated with right hemicolectomy and regional lymph node dissection. Despite genetic analysis, no causative germline variants were identified. Segmental colectomy according to the distribution of polyps might be a curative approach.
Similar content being viewed by others
Background
The most common cause of colorectal adenomatous polyposis is hereditary adenomatous polyposis, typically resulting from germline pathogenic variants in the APC or MUTYH genes. However, the causative germline variants cannot be identified in many cases of colorectal adenomatous polyposis, accounting for as many as 30–50% of polyposis cases [1, 2]. In such cases, the condition is classified as colonic adenomatous polyposis of unknown etiology (CPUE). The National Comprehensive Cancer Network guidelines recommend that the management of CPUE cases wherein cumulative lifetime adenomas exceed 100 should be equivalent to that of familial adenomatous polyposis (FAP), with total proctocolectomy considered the standard treatment [3]. However, no evidence is available to date to support this treatment approach, and the inclusion of various genetic diseases within the CPUE category makes it difficult to establish a uniform treatment strategy [4].
In this report, we present a case of CPUE with numerous adenomas and multiple adenocarcinoma lesions confined to the right side of the colon, wherein right hemicolectomy was performed, and long-term survival was achieved.
Case presentation
A 72-year-old woman was referred to our hospital owing to numerous polyps in the right side of the colon detected on colonoscopy performed for the examination of fecal occult blood. The patient had several comorbidities, including type 2 diabetes, hypertension, and dyslipidemia, with no history of smoking or alcohol consumption or of treatment for childhood or adult cancers. The patient’s father and brother had lung and gastric cancers, respectively; however, the patient had no family history of colorectal polyposis or cancer.
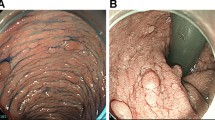
Colonoscopy revealed > 100 polyps, with a normal mucosal background confined from the cecum to the splenic flexure of the transverse colon (Fig. 1a, b). A clinical diagnosis of sparse FAP was made based on guidelines [5, 6]. No polyp lesions were observed in the descending colon, sigmoid colon, or rectum. Biopsy results confirmed that the polyps were adenomas, with two moderately differentiated tubular adenocarcinomas detected in the ascending and transverse colon. The lesion in the transverse colon was suspected of submucosal invasion (Fig. 1c, d). Computed tomography revealed no evidence of enlarged lymph nodes, distant metastases, or other organ lesions. Upper gastrointestinal endoscopy and ocular fundoscopy revealed no evidence of extracolonic lesions.
Laparoscopic right hemicolectomy with regional lymph node dissection was performed (Fig. 2), and the patient was discharged on postoperative day 7 without any postoperative complications. Histopathological examination revealed a moderately differentiated adenocarcinoma in the transverse colon with invasion of the muscularis propria and two lesions of highly differentiated intraepithelial adenocarcinoma in the cecum and ascending colon. No lymphatic or venous invasion or lymph node metastasis was observed. Approximately 800 adenomas were diffusely distributed from the ascending colon to the splenic flexure of the transverse colon against a background of normal mucosa, some densely fused, with adenocarcinomas detected in such areas. The pathological stage of the most advanced one was T2N0M0 Stage I according to the eighth edition of the Tumor Node Metastasis Classification of the International Union Against Cancer [7]. Most polyps were tubular adenomas; however, some showed serrated changes. The resection margins showed no neoplastic or adenomatous atypia. The tumors were microsatellite stable. At 5 years postoperatively, the patient remains cancer-free, with no recurrence of cancer or polyposis in the remaining colon or rectum, although few sporadic adenomatous polyps developed in the sigmoid colon.
To identify causal genetic factors, DNA sequencing of blood samples was performed using an original multigene panel for APC, EPCAM, MBD4, MLH1, MLH3, MSH2, MSH3, MSH6, MUTYH, NTHL1, PMS2, POLD1, POLE, and TP53. The exons and 5-bp flanking regions of these genes were sequenced and evaluated. For APC and mismatch repair genes (MLH1, MSH2, MSH6, and PMS2), splicing abnormalities were also analyzed in mRNA. The results revealed a heterozygous variant in MSH3 (c.606G > A, p.Cln202 =), which was interpreted as likely benign in ClinVar [8], and no difference in MSH3 expression was observed between the normal mucosa, adenomas, and carcinomas using tissue immunostaining (Fig. 3) of formalin-fixed, paraffin-embedded specimens with a rabbit monoclonal anti-MSH3 antibody (ab111107; Abcam, Cambridge, UK), performed as described previously [9]. Because serrated lesions were observed in some polyps on hematoxylin and eosin staining, the possibility of serrated polyposis was considered. RNA sequencing of RNF43 using the blood samples was also performed; however, no variant was found. Based on the above findings, the final diagnosis was CPUE.
Discussion
Herein, we report a rare case of CPUE with localized lesions, including multiple carcinomas, in the right side of the colon. In contrast to the recommended total proctocolectomy for CPUE cases with over 100 adenomatous polyps, we opted for a right hemicolectomy with regional lymph node dissection, resulting in long-term survival. Despite a comprehensive search for germline variants in genes associated with polyposis diseases, including APC and MUTYH, no causative germline variant was identified.
While a definitive consensus on the trend in the lesion distribution in CPUE remains elusive, several reports have suggested that lesions are more frequently found on the right side in CPUE cases with a small number of lesions [10]. However, CPUE cases with > 100 lesions distributed in a local area of the colon or rectum have not been reported, other than the present case. With respect to FAP cases with APC pathogenic variants, a search of the PubMed database revealed two prior reports of typical FAP cases involving localized lesions. In one case, a complication of ulcerative colitis was reported, and the right side of the colon was covered by innumerable small adenomatous polyps; conversely, ulcerative colitis was observed on the left side of the colon [11]. This patient underwent total abdominal colectomy as a curative treatment for both FAP and ulcerative colitis. In the other case, numerous right-sided polyps were detected, sparing the left side of the colon [12]. In this case, laparoscopic subtotal colectomy was performed, while laparoscopic pancreatoduodenectomy was conducted simultaneously for ampullary cancer. In our case, the patient had > 100 colon adenomas and was diagnosed with CPUE after germline multigene panel testing. Although the condition in our case may have been caused by a variant in a gene that was not included in our multigene panel, to the best of our knowledge, this is the first reported case of localized polyposis lesions in CPUE with > 100 lesions.
According to guidelines [3], the management of CPUE cases with > 100 adenomatous polyps should follow that of FAP; as such, the standard treatment is total proctocolectomy. However, no strong evidence is available to support this treatment approach. Total proctocolectomy is associated with a decreased quality of life [13]. Given that our patient was older, the detrimental impact on quality of life was expected to be even greater. Accounting for the localization of the polyps and adenocarcinomas, we opted to perform segmental resection and regional lymph node dissection of the diseased colon, assuming strict endoscopic surveillance after the surgery. In attenuated FAP cases, endoscopic polypectomy followed by endoscopic surveillance is considered a standard treatment [14]. Therefore, we deemed that our strategy, with strict endoscopic surveillance, would be a feasible approach in our case. Postoperatively, periodic surveillance with colonoscopy for > 5 years revealed no evidence of polyposis or cancer recurrence in the remaining colon or rectum. Even in cases of CPUE with > 100 lesions, curative resection might be achieved by resecting the diseased bowel (with lymph node dissection in cases with adenocarcinomas) instead of performing total proctocolectomy when the lesion is localized, as in the present case.
In addition to FAP and MUTYH-associated polyposis, colorectal adenomatous polyposis can be caused by other hereditary diseases, including polymerase proofreading-associated polyposis [15], NTLH1 tumor syndrome [16], and MBD4-associated neoplasia syndrome [17]. Pathogenic biallelic germline variants in one of the four mismatch repair genes (MLH1, MSH2, MSH6, or PMS2) can cause colorectal adenomatous polyposis in childhood [18]. In our case, we performed germline multigene panel testing to detect genes associated with these diseases with blood samples; however, no pathogenic variants suggestive of these diseases were found in the germline. Pathogenic biallelic germline variants in MSH3 are also associated with colorectal polyposis, namely, MSH3-associated polyposis [19]. In the present case, we identified a heterozygous variant in MSH3. Although it was expected to be silent, we tried to annotate the variant; in the tissue immunostaining, the expression of MSH3 was the same in the normal mucosa, adenomas, and carcinomas, suggesting that the MSH3 variant in our case was unlikely to have contributed to the development of polyposis or carcinogenesis. Germline loss-of-function variants in AXIN2 are also reported to be associated with colonic polyposis and colorectal cancers [20]. We added the mRNA sequence of AXIN2 using blood samples, and the function of AXIN2 was deemed to be normal. In addition to germline variants related to adenomatous polyposis, loss-of-function of RNF43 can cause serrated polyposis syndrome [21]. In the present case, serrated changes were observed in part of the polyps. However, no obvious variants in the RNF43 gene sequence were detected. Overall, no pathogenic variants were detected in the present case, and we diagnosed the patient with CPUE. The sporadicity and localization of the lesions indicated the possibility of the involvement of somatic variants, such as APC mosaicism [22]. In our case, the variant frequency threshold was lowered to 0.5% for APC to investigate the possibility of mosaic, but no variants were detected as possible causes from blood samples. Mosaicism might be detected by genetic searches in adenomas and surrounding normal mucosa. In addition to genetic factors, colorectal polyposis has been associated with cancer treatment in childhood or adulthood [23, 24]; however, no such history was found in our patient.
Herein, we reported the first CPUE case with numerous adenomas and multiple adenocarcinomas, all located in the right side of the colon. The diagnosis of CPUE was made after a germline multigene panel testing was conducted on a blood sample to detect polyposis-associated germline variants. However, no pathogenic variant was detected. The sporadicity of the case and localization of the lesions raised the possibility of the involvement of somatic variants. Given that CPUE cases may encompass some undiagnosed hereditary diseases, it is generally accepted that the standard treatment for CPUE cases with numerous adenomas is total proctocolectomy. However, in this specific case, considering the patient’s general condition and distribution of the lesions, we opted to perform right hemicolectomy and regional lymph node dissection as a curative treatment. This approach resulted in a long-term recurrence-free survival for the patient. Therefore, in cases with localized lesions, segmental colectomy might be an option for curative treatment.
Conclusions
We present a rare case of CPUE involving numerous adenomatous polyposis and multiple carcinomas localized in the right side of the colon. The patient’s condition was successfully treated with right hemicolectomy and regional lymph node dissection, and long-term recurrence-free survival was achieved. Genetic analysis using blood samples did not reveal a causative germline variant related to polyposis. Segmental colectomy might be a curative approach in CPUE cases with localized lesions.
Availability of data and materials
The study data can be made available upon any reasonable request.
Abbreviations
- FAP:
-
Familial adenomatous polyposis
- CPUE:
-
Colonic adenomatous polyposis of unknown etiology
References
Grover S, Kastrinos F, Steyerberg EW, Cook EF, Dewanwala A, Burbidge LA, et al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA. 2012;308:485–92.
Horpaopan S, Spier I, Zink AM, Altmüller J, Holzapfel S, Laner A, et al. Genome-wide CNV analysis in 221 unrelated patients and targeted high-throughput sequencing reveal novel causative candidate genes for colorectal adenomatous polyposis. Int J Cancer. 2015;136:E578–89.
Weiss JM, Gupta S, Burke CA, Axell L, Chen LM, Chung DC, et al. NCCN guidelines® insights: genetic/familial high-risk assessment: colorectal, version 1.2021. J Natl Compr Canc Netw. 2021;19:1122–32.
Yuan Z, Yang M, Yuan Y. The progress of colorectal polyposis syndrome in Chinese population. Clin Colon Rectal Surg. 2023;36:391–9.
Tomita N, Ishida H, Tanakaya K, Yamaguchi T, Kumamoto K, Tanaka T, et al. Japanese Society for cancer of the colon and rectum (JSCCR) guidelines 2020 for the clinical practice of hereditary colorectal cancer. Int J Clin Oncol. 2021;26:1353–419.
Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom cancer genetics group (UKCGG). Gut. 2020;69:411–44.
Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Hoboken: Wiley; 2017. p. 272.
Landrum MJ, Chitipiralla S, Brown GR, Chen C, Gu B, Hart J, et al. ClinVar: improvements to accessing data. Nucleic Acids Res. 2020;48:D835–44.
Munakata K, Koi M, Kitajima T, Tseng-Rogenski S, Uemura M, Matsuno H, et al. Inflammation-associated microsatellite alterations caused by MSH3 dysfunction are prevalent in ulcerative colitis and increase with neoplastic advancement. Clin Transl Gastroenterol. 2019;10: e00105.
Feldman D, Rodgers-Fouche L, Hicks S, Chung DC. Clinical features and long-term outcomes of patients with colonic oligopolyposis of unknown etiology. World J Gastroenterol. 2022;28:6950–61.
Samadder NJ, Gornick M, Everett J, Greenson JK, Gruber SB. Inflammatory bowel disease and familial adenomatous polyposis. J Crohns Colitis. 2013;7:e103–7.
Khaled YS, Ammori MB, Sharif HI, Ammori BJ. Simultaneous laparoscopic subtotal colectomy and pancreaticoduodenectomy for colonic FAP and ampullary cancer. Surg Laparosc Endosc Percutan Tech. 2012;22:e79-82.
Melnitchouk N, Saadat LV, Bleday R, Goldberg JE. A decision analysis for rectal-sparing familial adenomatous polyposis: total colectomy with ileorectal anastomosis versus proctocolectomy with IPAA. Dis Colon Rectum. 2019;62:27–32.
Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–62.
The CORGI Consortium, The WGS500 Consortium, Palles C, Cazier JB, Howarth KM, Domingo E, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45:136–44.
Weren RDA, Ligtenberg MJL, Kets CM, De Voer RM, Verwiel ETP, Spruijt L, et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet. 2015;47:668–71.
Blombery P, Ryland GL, Fox LC, Stark Z, Wall M, Jarmolowicz A, et al. Methyl-CpG binding domain 4, DNA glycosylase (MBD4)-associated neoplasia syndrome associated with a homozygous missense variant in MBD4: expansion of an emerging phenotype. Br J Haematol. 2022;198:196–9.
Wimmer K, Kratz CP, Vasen HFA, Caron O, Colas C, Entz-Werle N, et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium ‘care for CMMRD’ (C4CMMRD). J Med Genet. 2014;51:355–65.
Adam R, Spier I, Zhao B, Kloth M, Marquez J, Hinrichsen I, et al. Exome sequencing identifies biallelic MSH3 germline mutations as a recessive subtype of colorectal adenomatous polyposis. Am J Hum Genet. 2016;99:337–51.
Chan JM, Clendenning M, Joseland S, Georgeson P, Mahmood K, Walker R, et al. Rare germline variants in the AXIN2 gene in families with colonic polyposis and colorectal cancer. Fam Cancer. 2022;21:399–413.
Stanich PP, Pearlman R. Hereditary or not? Understanding serrated polyposis syndrome. Curr Treat Options Gastro. 2019;17:692–701.
Hes FJ, Nielsen M, Bik EC, Konvalinka D, Wijnen JT, Bakker E, et al. Somatic APC mosaicism: an underestimated cause of polyposis coli. Gut. 2007;57:71–6.
Yurgelun MB, Hornick JL, Curry VK, Ukaegbu CI, Brown EK, Hiller E, et al. Therapy-associated polyposis as a late sequela of cancer treatment. Clin Gastroenterol Hepatol. 2014;12:1046–50.
Biller LH, Ukaegbu C, Dhingra TG, Burke CA, Chertock Y, Chittenden A, et al. A multi-institutional cohort of therapy-associated polyposis in childhood and young adulthood cancer survivors. Cancer Prev Res (Phila). 2020;13:291–8.
Acknowledgements
The genetic study in this report was supported by the Dial Study of the Japan Agency for Medical Research and Development.
Funding
The authors received no funding for this study. The Dial Study was supported by Japan AMED under Grant reference JP18kk0205004 and by JSPS KAKENHI under Grant reference JP18K07339 and JP22K07266.
Author information
Authors and Affiliations
Contributions
SA, AI, and YK reported the case and wrote the manuscript. TK, YO, YN, and TS were involved in patient care, including surgery. HK, MH, YM, AT, and MM helped draft the manuscript. HF, GY, and KA provided data and worked on the manuscript. KI and KF critically revised the manuscript. All authors have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of this work and will ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Dial Study was approved by the Institutional Review Board of Osaka General Medical Center (No. 2021-030). The study was conducted in accordance with the principles of the Declaration of Helsinki. Before genetic testing, the patient underwent genetic counseling from clinical geneticists. Written informed consent was obtained from the patient involved in the study.
Consent for publication
Written informed consent was obtained from the patient for participation in the Dial Study and the publication of this case report, including the accompanying interests.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aoyama, S., Inoue, A., Kagawa, Y. et al. Curative resection via right hemicolectomy and regional lymph node dissection for colonic adenomatous polyposis of unknown etiology with adenocarcinomas localized in the right side of the colon: a case report. surg case rep 10, 93 (2024). https://doi.org/10.1186/s40792-024-01890-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-024-01890-1