Abstract
Background
Undifferentiated embryonal sarcoma of the liver (UESL) primarily occurs in children; it is rarely seen in adults and appears to have a poor prognosis. However, in recent years, some cases indicated that long-term survival was possible due to a combination of multiple surgeries, chemotherapy, and liver transplantation.
Case presentation
A 33-year-old female patient presented with a complaint of epigastric pain, for which she underwent a medical examination. Computed tomography (CT) and magnetic resonance imaging showed a cystic tumor in the right hepatic lobe, approximately 10 cm in size. During observation, the abdominal pain worsened, and a contrast-enhanced CT revealed that the tumor’s peripheral solid components increased in size and volume, suggesting a malignant tumor threatening hepatic rupture. Subsequently, transcatheter arterial embolization of the anterior and posterior segmental branches of the hepatic artery was performed, followed by right trisectionectomy. Histopathological and immunohistochemical examinations of the lesion revealed UESL. Two months after the surgery, we initiated sarcoma-directed chemotherapy with doxorubicin because of multiple metastases to the liver. After initiating the chemotherapy, she received another regimen using gemcitabine/docetaxel, eribulin, trabectedin, ifosfamide/mesna, pazopanib, and cisplatin. During the chemotherapy, she underwent palliative surgery twice due to the progressive disease. She lived for 49 months after the initial operation.
Conclusions
Improved long-term survival was achieved in an adult patient with UESL after multidisciplinary therapy, involving a combination of three surgical procedures and several chemotherapies.
Similar content being viewed by others
Background
Undifferentiated embryonal sarcoma of the liver (UESL) is a malignant mesenchymal tumor that occurs predominantly in juveniles aged 6–10 years [1]. Since UESL is rare in adults [1] and often asymptomatic, the diagnosis at an early stage is challenging, and the prognosis is very poor. Although the standard treatment for UESL had not yet been established, previous literature suggested that a multidisciplinary approach involving surgery, chemotherapy, and radiation therapy could improve the prognosis. Herein, we report improved long-term survival in an adult patient with UESL and reviewed related literature.
Case presentation
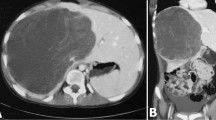
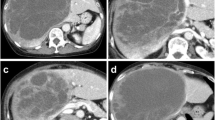
A 33-year-old woman with epigastric pain was identified to have a 10-cm cystic mass in the right lobe of her liver on imaging. Upper and lower gastrointestinal endoscopy and whole-body computed tomography (CT) scan did not identify any primary tumor, except that in the liver. Laboratory tests showed a normal serum bilirubin level, slightly decreased hemoglobin level (10.4 g/dl), and insignificantly increased values of hepatobiliary enzymes, including alkaline phosphatase (359 U/L), γ-glutamyl transpeptidase γ-(GTP) (58 U/L), lactate dehydrogenase (494 U/L), aspartate aminotransferase (35 U/L), and alanine transferase (31 U/L). Studies for hepatitis B and C viral markers were negative. Tests for tumor markers, including carcinoembryonic antigen, α-fetoprotein, cancer antigen 19–9, and protein induced by vitamin K absence-II, were within normal range. Abdominal pain got worse several months after follow-up, and the CT scan revealed that the tumor had enlarged, nearing imminent rupture (Figs. 1, 2). Immediately after the urgent transcatheter arterial embolization (TAE), she was transferred to our hospital. Right hepatic trisectionectomy was performed to treat the tumor. The resected specimen revealed a cystic tumor, weighing 1985 g and 17 × 15 × 15 cm in size, composed of dark reddish hemorrhage and grayish-white solid lesion. The tumor border was partially unclear with a deficient capsule (Fig. 3). Histopathological examination showed a tumor composed of proliferating stellate or spindle-shaped pleomorphic atypical cells on a background of myxomatous stroma and atypical cells with irregular giant nuclear or multinuclear cells. Only about 10% of viable tumor cells were observed, and most of them were found to be hemorrhagic and necrosed due to TAE. Some atypical cells were with d-Periodic acid Schiff (d-PAS-positive) cytoplasmic inclusions. Bile duct-like structures were observed in the neoplasm margin area (Fig. 4). The resection margin was too degenerated to evaluate, but atypical cells were found close to the edge. The immunohistochemical evaluation showed diffusely positive expression of vimentin; alpha 1-antichymotrypsin (α1ACT) and alpha 1-antitrypsin (α1AT); and focally positive expression of desmin, α-smooth muscle actin (α-SMA), glypican-3, and discovered on the gastrointestinal stromal tumor-1 (DOG-1) and negative expression for CAM 5.2, AE1/AE3, Hepatocyte Paraffin-1, S-100, HMB45, CD34, and c-kit (Fig. 5). Ki-67 labeling index was 40%. Based on these findings, the pathological diagnosis was UESL rather than gastrointestinal stromal tumor (GIST). The patient was discharged on postoperative day 15 as per the enhanced recovery after surgery protocols in hepatectomy [2]. Several liver metastases were observed 2 months after surgery, and sarcoma-directed chemotherapy with doxorubicin was initiated (details of chemotherapy regimens are described in Fig. 6). We changed the regimen to eribulin when there was an increase in liver metastases despite gemcitabine/docetaxel. About two years after the surgery, she felt abdominal pain again, and a CT scan was performed. One of the liver metastases grew rapidly, and an impending rupture was suspected. Palliative surgery involving partial hepatectomy and splenectomy was performed. Pathological findings showed the neoplasm with the same histopathology as the primary tumor, UESL, and Ki-67 immunohistochemistry was up to 70%. One month after the second surgery, she resumed the eribulin therapy, but the liver metastases grew gradually. The drug was changed from eribulin to trabectedin; however, 39 months after the initial surgery, one of the liver metastases increased rapidly, and a second palliative surgery with partial liver resection was performed. The ifosfamide/mesna therapy was restarted postoperatively, but the disease had progressed significantly. Forty-three months after primary operation, radiological findings revealed lung metastases. Because of neutropenia, doxorubicin and eribulin were reduced to 80% and gemcitabine/docetaxel to 75% after the second course. Trabectedin was reduced to 80% after the second course because of hepatic impairment. Pazopanib and cisplatin were used in sequence, and continued palliative care was administered; however, the patient died 49 months after the initial surgery.
a The tumor is composed of spindle-shaped cells (× 200, HE stain). b Some tumor cells contain eosinophilic globules, which are d-PAS-positive (× 400, PAS stain, black arrow). c Sarcomatous cells surround bile duct-like structures (× 400, HE stain, black arrow). d-PAS, d-periodic acid Schiff; HE, hematoxylin and eosin
a Immunohistochemical analysis reveals that the tumor is stained with vimentin; b α1ACT; c α1AT (× 200); and d focally stained with DOG-1 (× 400) and negative for CAM 5.2 (e), AE1/AE3 (f), and Hep Par-1 (g) (× 200). α1ACT alpha 1-antichymotrypsin, α1AT alpha 1-antitrypsin, DOG-1 discovered on gastrointestinal stromal tumor-1, Hep Par-1 hepatocyte paraffin-1
This figure shows the progress in chemotherapy (black bars). Gray bars indicate drug dose reductions. In the second and third operations, palliative partial hepatectomy of one rapidly growing tumor (yellow circles) is performed. The patient is administered doxorubicin (75 mg/m2/dose every 3 weeks for 3 cycles); gemcitabine (900 mg/m2/dose, days 1 and 8) and docetaxel (70 mg/m2/dose every 3 weeks for 16 cycles, day 8); eribulin (1.4 mg/m2/dose every 3 weeks for 6 cycles, days 1 and 8); trabectedin (1.2 mg/m2/dose every 3 weeks for 3 cycles); ifosfamide (2 g/m2/dose, days 1–5) and mesna (400 mg/m2/dose given 4 weeks for 15 cycles, days 1–5); pazopanib (800 mg/day dose given 4 weeks for 2 cycles); and CDDP (100 mg/m2/dose given 3 weeks for 3 cycles). GEM gemcitabine, DOC docetaxel, CDDP cisplatin
Discussion
UESL is a primary mesenchymal malignant liver neoplasm, and the concept of this neoplasm was first proposed by Stocker and Ishak in 1978 [1]. It accounts for approximately 9–13% of primary liver tumors in children [3]; however, it is extremely rare in adults, accounting for approximately 0.2% of the primary liver tumors [4]. Furthermore, UESL has a worse prognosis in adults, with a 5-year overall survival (OS) rate of 48.2% compared to 84.4% in children [5, 6]. The standard treatment strategy for UESL has not been established, and there is no drug for the treatment of UESL. However, recent studies have shown that margin-negative resection improves the OS [5, 6]. Currently, most patients receive vincristine, actinomycin-D, cyclophosphamide (VAC), or ifosfamide based on the Intergroup Rhabdomyosarcoma Study protocol [7]. To our knowledge, only 16 adult patients over the age of 18 years, including our patient, have survived for more than 48 months [3, 4, 8,9,10,11,12,13,14,15,16,17,18,19] (Table 1). With the exception of one patient who underwent liver transplantation after neoadjuvant chemotherapy [15], all the other patients received initial surgical treatment. We found that 10 patients, including our patient, who survived for more than 48 months had recurrence after the primary surgery. Of the 10 patients who relapsed, eight (80%) presented with hepatic recurrence. Although the high recurrence rate of residual liver tissue indicates that current radical resection and chemotherapy may not be sufficient to achieve complete resolution, long-term survival appeared possible even in cases with recurrence by combining surgery and chemotherapy (and/or radiation therapy). Liver transplantation for UESL has been performed to control hepatic recurrence, and there have been reports of patients who have survived for more than 10 years with the combination of chemotherapy and liver transplantation for unresectable cases [3, 20]. However, the number of such cases is small, and careful consideration and more extensive studies are required to determine the indications for combined chemotherapy and liver transplantation for adult UESL in the future.
UESL is commonly characterized by a lack of differentiation tendency, without specifically oriented differentiation, such as vessels, striated muscle, smooth muscle, fat, and nerves, and there is no specific immunohistochemical pattern [1]. In UESL, pathological findings reveal a proliferation of spindle, oval, or stellate pleomorphic immature cells with poorly defined cell borders embedded in the mucinous stroma; and near the tumor margin, entrapped bile duct-like structures surrounded by tumor cells were observed [1]. It often accompanies multinucleated giant cells and d-PAS-positive granule-containing cells [1].
In our case, immunohistochemical analysis showed diffusely positive expression of vimentin, α1ACT, and α1AT and was focally positive for desmin, α-SMA, glypican-3, and DOG-1. Local expression of myogenic markers and DOG-1 may reflect the local differentiation trend in the undifferentiated tumor. There are no prior reports on the expression of DOG-1 in UESL. DOG-1 is a member of the transmembrane protein 16 family, featured as a calcium-activated chloride channel and expressed in GIST, but the detailed functions are unknown. Recently, the details of DOG-1 have been revealed gradually by reported expression in poorly differentiated tumors [21, 22], such as sarcomatous carcinoma of the liver, in lymph node metastasis of colorectal cancer [23], and as a poor prognostic factor in breast cancer [24]. The clinical significance of DOG-1 positivity in UESL is unclear; however, it may become more evident with the accumulation of cases.
The occurrence of UESL has not been understood, but Mori et al. reported that a hamartoma-like lesion is one form of UESL and that this type of case has a good prognosis [25]. It is yet to be determined whether hamartoma becomes malignant and transforms to UESL or some UESLs present with hamartoma-like lesions. Although hamartoma-like lesions were not observed in our case, further pathological analysis and treatment development may be the key to long-term survival of patients.
Conclusions
We verified a long-term survival case of adult UESL. Long-term survival may be achieved by combining radical surgery and multi-agent chemotherapy. Furthermore, detailed immunohistological analysis of UESL may result in more effective medications and improve OS.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. Further material and information on this case report are available from the corresponding author on reasonable request.
Abbreviations
- UESL:
-
Undifferentiated embryonal sarcoma of the liver
- CT:
-
Computed tomography
- TAE:
-
Transcatheter arterial embolization
- d-PAS:
-
D-Periodic acid Schiff
- GIST:
-
Gastrointestinal stromal tumor
- DOG-1:
-
Discovered on gastrointestinal stromal tumor -1
- OS:
-
Overall survival
- α1ACT:
-
Alpha 1-antichymotrypsin
- α1AT:
-
Alpha 1-antitrypsin
- α-SMA:
-
α-Smooth muscle actin
References
Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:33648. https://doi.org/10.1002/1097-0142(197807)42:1%3c336::aid-cncr2820420151%3e3.0.co;2-v.
Fujio A, Miyagi S, Tokodai K, Nakanishi W, Nishimura R, Mitsui K, et al. Effects of a new perioperative enhanced recovery after surgery protocol in hepatectomy for hepatocellular carcinoma. Surg Today. 2020;50:615–22. https://doi.org/10.1007/s00595-019-01930-6.
Babu BI, Bigam DL, Gilmour SM, Dajani KZ, Shapiro AMJ, Kneteman NM. Liver transplantation in locally unresectable, undifferentiated embryonal cell sarcoma. Transplant Direct. 2021;7: e654. https://doi.org/10.1097/TXD.0000000000001106.
Esteban S-MG, Emilio C-GU, Emmanuel A-BF, Oscar SJ, Paulina CE, Angel MM. Undifferentiated embryonal sarcoma of the liver in adult patient: a report of two cases. Ann Hepatobiliary Pancreat Surg. 2018;22:269–73. https://doi.org/10.14701/ahbps.2018.22.3.269.
Wu Z, Wei Y, Cai Z, Zhou Y. Long-term survival outcomes of undifferentiated embryonal sarcoma of the liver: a pooled analysis of 308 patients. ANZ J Surg. 2020;90:1615–20. https://doi.org/10.1111/ans.15684.
Ziogas IA, Zamora IJ, III, Lovvorn HN, Bailey CE, Alexopoulos SP. Undifferentiated embryonal sarcoma of the liver in children versus adults: a national cancer database analysis. Cancers (Basel). 2021. Bailey CE: Alexopoulos;13. https://doi.org/10.3390/cancers13122918
Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, et al. Intergroup rhabdomyosarcoma Study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–102. https://doi.org/10.1200/JCO.2001.19.12.3091.
Grazi GL, Gallucci A, Masetti M, Jovine E, Fiorentino M, Mazziotti A, et al. Surgical therapy for undifferentiated (embryonal) sarcomas of the liver in adults. Am Surg. 1996;62:901–6.
Almogy G, Lieberman S, Gips M, Pappo O, Edden Y, Jurim O, et al. Clinical outcomes of surgical resections for primary liver sarcoma in adults: results from a single centre. Eur J Surg Oncol. 2004;30:421–7. https://doi.org/10.1016/j.ejso.2004.01.004.
Almogy G, Pappo O, Gips M, Lieberman S, Edden Y, Eid A. Improved survival with surgery and systemic chemotherapy for undifferentiated embryonal sarcoma of the liver. Case Commun Isr Med Assoc J. 2005;7:672–3.
Lepreux S, Rebouissou S, Le Bail B, Saric J, Balabaud C, Bloch B, et al. Mutation of TP53 gene is involved in carcinogenesis of hepatic undifferentiated (embryonal) sarcoma of the adult, in contrast with Wnt or telomerase pathways: an immunohistochemical study of three cases with genomic relation in two cases. J Hepatol. 2005;42:424–9. https://doi.org/10.1016/j.jhep.2004.10.021.
Lenze F, Birkfellner T, Lenz P, et al. Undifferentiated embryonal sarcoma of the liver in adults. Am Cancer Soc. 2008;112:2274–82. https://doi.org/10.1002/cncr.23431.
Tsukada A, Ishizaki Y, Nobukawa B, Kawasaki S. Embryonal sarcoma of the liver in an adult mimicking complicated hepatic cyst: MRI findings. J Magn Reson Imaging. 2010;31: 147780. https://doi.org/10.1002/jmri.22177.
Kim HH, Kim JC, Park EK, Hur YH, Koh YS, Cho CK, et al. Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult. Hepatobiliary Pancreat Dis Int. 2011;10:657–60. https://doi.org/10.1016/S1499-3872(11)60112-4.
Dhanasekaran R, Hemming A, Salazar E, Cabrera R. Rare case of adult undifferentiated (embryonal) sarcoma of the liver treated with liver transplantation: excellent long-term survival. Case Rep Hepatol. 2012;2012: 519741. https://doi.org/10.1155/2012/519741.
Noguchi K, Yokoo H, Nakanishi K, Kakisaka T, Tsuruga Y, Kamachi H, et al. A long-term survival case of adult undifferentiated embryonal sarcoma of liver. World J Surg Oncol. 2012;10:65. https://doi.org/10.1186/1477-7819-10-65.
Masuda T, Beppu T, Doi K, Miyata T, Nakagawa S, Okabe H, et al. Repeated hepatic resections and radio-frequency ablations may improve the survival of adult undifferentiated embryonal sarcoma of the liver: report of two cases. Surg Case Rep. 2015;1:55. https://doi.org/10.1186/s40792-015-0056-y.
Beksac K, Mammadov R, Ciftci T, Guner G, Akyol A, Kaynaroglu V. Undifferentiated embryonal sarcoma of the liver in an adult patient. Cureus. 2018;10: e3037. https://doi.org/10.7759/cureus.3037.
Capozza MA, Ruggiero A, Maurizi P, Mastrangelo S, Attinà G, Triarico S, et al. Undifferentiated embryonal sarcoma of the liver (UESL) in adolescents: an unexpected diagnosis. J Pediatr Hematol Oncol. 2019;41: e1324. https://doi.org/10.1097/MPH.0000000000001191.
Shi Y, Rojas Y, Zhang W, Beierle EA, Doski JJ, Goldfarb M, et al. Characteristics and outcomes in children with undifferentiated embryonal sarcoma of the liver: a report from the National Cancer Database. Pediatr Blood Cancer. 2017;64:1–8. https://doi.org/10.1002/pbc.26272.
Ikura Y, Shigaki Y, Kadota C, Hayakumo T, Iwai Y. Unexpected DOG-1 immunoreactivity in sarcomatous carcinoma of the liver: a diagnostic pitfall. Pathology. 2014;46:572–4. https://doi.org/10.1097/PAT.0000000000000154.
Hemminger J, Iwenofu OH. Discovered on gastrointestinal stromal tumors 1 (DOG1) expression in non-gastrointestinal stromal tumour (GIST) neoplasms. Histopathology. 2012;61:170–7. https://doi.org/10.1111/j.1365-2559.2011.04150.x.
Li H, Yang Q, Huo S, Du Z, Wu F, Zhao H, et al. Expression of TMEM16A in colorectal cancer and its correlation with clinical and pathological parameters. Front Oncol. 2021;11: 652262. https://doi.org/10.3389/fonc.2021.652262.
Bae JS, Park JY, Park SH, Ha SH, An AR, Noh SJ, et al. Expression of ANO1/DOG1 is associated with shorter survival and progression of breast carcinomas. Oncotarget. 2018;9:607–21. https://doi.org/10.18632/oncotarget.23078.
Mori A, Fukase K, Masuda K, Sakata N, Mizuma M, Ohtsuka H, et al. A case of adult undifferentiated embryonal sarcoma of the liver successfully treated with right trisectionectomy: a case report. Surg Case Rep. 2017;3:19. https://doi.org/10.1186/s40792-017-0295-1.
Acknowledgements
None.
Funding
This report did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
YEK and AF participated in the study design and drafted the manuscript. AF, KT, and SM treated the patient and performed data acquisition and analysis. FF carried out the pathological studies. KM and FF revised the article critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was approved by the ethics committee by Tohoku University Hospital (no. 24169).
Consent for publication
We obtained consent for publication from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Endo, Y.K., Fujio, A., Murakami, K. et al. Long-term survival of an adult patient with undifferentiated embryonal sarcoma of the liver with multidisciplinary treatment: a case report and literature review. surg case rep 8, 85 (2022). https://doi.org/10.1186/s40792-022-01436-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-022-01436-3