Abstract
Background
Meningioma is the most common type of benign primary brain tumor that is rarely associated with distant metastasis. No established treatment strategy for metastatic meningiomas exists to date. Herein, we report a case of solitary pulmonary metastasis of meningioma detected 2 years after neurosurgical resection of the primary tumor.
Case presentation
A 75-year-old male patient underwent neurosurgical resection of a convexity meningioma (World Health Organization grade II atypical meningioma), followed by postoperative radiotherapy for the residual tumor. Two postoperative years later, a solitary 10-mm pulmonary nodule in the left lower lung lobe was detected on chest computed tomography. The patient underwent video-assisted thoracoscopic left lower lobectomy for suspected pulmonary metastasis of meningioma. The pathological diagnosis was solitary pulmonary metastasis of meningioma. No sign of further recurrence was noted at 8 months postoperatively.
Conclusions
We present a rare and unique surgical case of solitary pulmonary metastasis of meningioma. Further investigation is necessary to establish the standardized treatment strategy for metastatic meningiomas.
Similar content being viewed by others
Background
Meningioma is the most common type of primary brain tumor, accounting for approximately 30% of all primary brain tumors [1]. Although meningiomas are usually associated with a benign clinical course, they can sometimes show malignant behavior, such as distant metastasis. Although only a few cases of pulmonary metastasis of meningioma have been reported to date, the lungs have been described as the most frequent site of meningioma metastasis [2]. We herein report a unique and rare surgical case of metastatic meningioma presenting with solitary pulmonary metastasis 2 years after neurosurgical resection of the primary tumor.
Case presentation
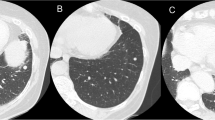
A 75-year-old male patient with a 0.5 pack-year smoking history was referred our department for further investigation of a solitary nodule in the left lower lung lobe (Fig. 1a). The patient underwent craniotomy for neurosurgical resection of the left convexity meningioma 2 years before the referral (Fig. 1b, c). The surgery resulted in incomplete resection with a residual tumor around the superior sagittal sinus. Thus, the patient subsequently underwent postoperative radiotherapy for the residual tumor. The pathological diagnosis was World Health Organization (WHO) grade II atypical meningioma [3]. The lesion showed an MIB-1 labeling index of 10% (Fig. 2a–c). Of note, chest computed tomography scan was not performed prior to or at the time of neurosurgical resection of the primary meningioma.
Medical imaging findings. a Computed tomography of the chest showing a 10-mm solitary pulmonary nodule in the left lower lobe of the lung (yellow arrow). b Two years before the referral, magnetic resonance imaging revealing a 65-mm tumor in the left parietal region (yellow arrow, axial section) and c skull infiltration of the tumor (white arrow, coronal section)
Pathological findings of the primary intracranial meningioma. a Short spindle-shaped tumor cells with acidophilic cytoplasm were densely proliferated, and the area of necrotic tumor cells were also confirmed (hematoxylin–eosin staining, original magnification 200×). b Tumor cells were observed to infiltrate the bone tissue (hematoxylin–eosin staining, original magnification 200×). c Ki-67 staining with MIB-1 antibody stained 10% of tumor cells (immunostaining with anti-MIB-1 protein polyclonal antibody, original magnification 100×)
Chest computed tomography at our department revealed a solitary 10-mm nodule in the left lower lobe of the lung. Neither intracranial recurrence nor distant metastasis other than the left lung was detected. The patient’s clinical history led us to suspect solitary pulmonary metastasis of meningioma, considering stage I primary lung cancer as a potential differential diagnosis. The patient finally underwent video-assisted thoracoscopic left lower lobectomy. Pathological investigation revealed short spindle-shaped tumor cells with clear margins that exhibited complex proliferation (Fig. 3a, b). According to the WHO classification of tumors, pulmonary metastasis of meningioma is histologically identical to primary pulmonary meningioma, which by definition does not involve lesions in the central nervous system [4]. As the patient in the current case had a neurosurgical history of left convexity meningioma, the risk of primary pulmonary meningioma was ruled out. Thus, the final pathological diagnosis was solitary pulmonary metastasis of meningioma. The patient was discharged from the hospital on the 11th postoperative day without any complication. No sign of further recurrence was noted at 8 months postoperatively.
Pathological findings of the pulmonary metastasis of meningioma. a The boundary between the tumor and surrounding tissue was clear (hematoxylin–eosin staining, original magnification 20×). b Short spindle-shaped tumor cells with a slightly acidophilic cytoplasm and exhibiting complex proliferation were detected (hematoxylin–eosin staining, original magnification 400×)
Discussion
Meningioma accounts for approximately 30% of all primary brain tumors [1], frequently affecting female patients aged between 20 and 60 years. The supratentorial region is the most common site of meningioma involvement, followed by the infratentorial region and the spinal cord [5]. According to the WHO histological classification of meningiomas, grade I indicates low-grade meningioma; grade II, intermediate grade meningioma; and grade III, high-grade meningioma [3]. Most cases (90.0%–94.3%) are classified as grade I, 4.7%–7.2% as grade II, and 1.0%–2.8% as grade III [6].
In addition to the WHO histological grade, the MIB-1 labeling index [7] has recently been reported to be associated with local recurrence and prognosis. In general, the cell-division potential is considered high when the MIB-1 labeling index is ≥ 5%, and the risk of meningioma-related mortality increases when the MIB-1 labeling index of the primary lesion was ≥ 19.2% [8].
The first choice of treatment for primary intracranial meningioma is neurosurgical resection. Radiotherapy is usually considered for patients with incompletely resected tumor, those with local recurrence, and inoperable patients [5]. Distant metastasis is rare and found in only 0.15%–0.76% of patients with primary intracranial meningioma [8]. The lung is the most common site of meningioma metastasis, followed by the bones and liver [2]. Pulmonary metastasis of meningioma accounts for 60% of the cases of metastatic meningiomas [9, 10], presenting more frequently as multiple pulmonary metastases than solitary pulmonary metastasis [2].
Metastasis of meningioma could be explained by hematogenous metastasis in most cases, but some could be explained by seeding in the central nervous system via the cerebrospinal fluid [11, 12]. Histologically, pulmonary metastasis of cranial meningioma is identical to primary pulmonary meningioma, a rare extracranial meningioma [4], with only 53 cases reported to date [13, 14]. Thus, the detection of meningioma in the central nervous system is a key for obtaining differential diagnosis. Risk factors for distant metastasis of meningioma include history of open cranial neurosurgery (i.e., inflow of tumor cells into the bloodstream or cerebrospinal fluid during the surgical procedure), venous sinus infiltration, local recurrence, and histological malignancy [8]. Our patient had multiple risk factors for distant metastasis of meningioma: a history of open cranial neurosurgery, superior sagittal sinus infiltration, and histology of WHO grade II atypical meningioma.
Consequently, no standardized treatment strategy for pulmonary metastasis of meningioma has been established. Specifically, the treatment efficacy of surgical resection of pulmonary metastasis of meningioma remains unclear. To the best of our knowledge, 70 cases of pulmonary metastasis of meningioma have been reported to date in the English and Japanese literature [5, 6, 9, 10, 15, 16]. Among these, 30 cases have been reported with information about the outcomes of surgical resection for lung metastasis of cranial meningioma (Table 1) [5, 6, 15, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. A brief summary of clinical characteristics is as follows: the median age of the patients was 57 (range: 15–75) years, and women accounted for 46.7% (14 of 30 cases) of all patients. Moreover, WHO grade I, II, and III diseases accounted for 62.1% (18 of 29), 20.7% (6 of 29), and 17.2% (5 of 29), respectively, of all cases. All pulmonary lesions were resected during the first metastasectomy in 25 patients and the first and the second metastasectomies in 5 patients. The median survival time after the first pulmonary metastasectomy was 19 (range: 1–294) months. The 1-, 2-, and 5-year overall survival rates based on the integration of the reported 30 cases were 96.7%, 80.7%, and 40.4%, respectively (Fig. 4A). Further, WHO grade III meningioma was more likely to be associated with worse overall survival than WHO grade I meningioma (P = 0.082, Fig. 4B). Nevertheless, with consideration of publication and selection bias, small sample size, and heterogeneity of clinical and pathological background of the cases, which patients can benefit from pulmonary metastasectomy for lung metastasis of cranial meningioma remains unclear. Notably, a mortality case of metastatic meningioma, whose multiple pulmonary metastases and malignant pleural effusion seemed to be associated with respiratory failure, has been reported [39]. This indicates that prevention of multiple pulmonary metastasis would be beneficial for selected patients with metastatic meningioma. In the presented case, we decided to perform lung resection for the following reasons: (1) histological diagnosis to exclude the possibility of primary lung cancer; (2) possible benefit of disease control of metastatic meningioma by surgical removal of pulmonary metastasis, and (3) patient’s tolerability of lung resection. Further studies should address the question of whether surgical resection of pulmonary metastasis of meningioma can help improve patient prognosis.
Overall survival after the first pulmonary metastasectomy for lung metastasis of cranial meningioma based on the integration of the reported cases (n = 30) [5, 6, 15, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. a The 1-, 2-, and 5-year overall survival rates based on the integration of all reported cases (n = 30) were 96.7%, 80.7%, and 40.4%, respectively. b WHO grade III meningioma was more likely to be associated with worse overall survival than WHO grade I meningioma (P = 0.082). The overall survival did not significantly differ between patients with WHO grade I and II meningioma (P = 0.67) and between patients with WHO grade II and III meningioma (P = 0.29, 0.082)
Conclusions
We report a rare and unique surgical case of solitary pulmonary metastasis of meningioma. Further investigation is necessary to establish the standardized treatment strategy for metastatic meningioma.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- WHO:
-
World Health Organization
References
Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review J Neurosurg. 2015;122:4–23.
Wang K-D, Yi-Bing Su, Zhang Y. Recurrent intracranial meningioma with multiple pulmonary metastases: a case report. Oncol Lett. 2015;10:2765–8.
Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningiomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. Lyon: International Agency for Research on Cancer; 2007. p. 164–72.
WHO Classification of Tumours Editorial Board. Thoracic tumours. In: WHO Classification of Tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2021.
Sazawa Y, Inazawa K, Masaoka T, Kanamori M. A case of lung metastasis of meningioma associated with primary lung cancer. J Jpn Surg Assoc. 2009;70:2663–8.
Kimura M, Kamiyama K, Dai Y. A case of pulmonary metastases from intracranial atypical meningioma. Haigan. 2020;60:38–42 (Article in Japanese).
Chen WC, Magill ST, Wu A, Vasudevan HN, Morin O, Aghi MK, et al. Histopathological features predictive of local control of atypical meningioma after surgery and adjuvant radiotherapy. J Neurosurg. 2018;130:443–50.
Endo T, Narisawa A, Ali HS, Murakami K, Watanabe T, Watanabe M, et al. A study of prognostic factors in 45 cases of atypical meningioma. Acta Neurochir. 2016;158:1661–7.
Tao LC. Pulmonary metastases from intracranial meningioma diagnosed by aspiration biopsy cytology. Acta Cytol. 1991;35:524–8.
Stoller JK, Kavuru M, Mehta AC, Weinstein CE, Estes ML, Gephardt GN. Intracranial meningioma metastatic to the lung. Cleve Clin J Med. 1987;54:521–7.
Ikeda M, Obase Y, Ohue Y, Naritomi M, Moriya T, Oka M. A case of multiple lung metastasis of recurrent meningothelial meningioma found at 23 years after craniotomy without local relapse. Nihon Kokyūki Gakkai shi. 2013;6:832–5 (Article in Japanese).
Chamberlain MC, Glantz MJ. Cerebrospinal fluid-disseminated meningioma. Cancer. 2005;103:1427–30.
Han D, Deng H, Liu Y. Primary pulmonary meningiomas: report of two cases and review of the literature. Pathol Res Pract. 2020;216:153232.
Chakrabarti R, Ghuman D. Diagnostic and management considerations in a patient with primary pulmonary meningioma with associated micro-solid nodules. Cureus. 2021;13(6):e15700.
Adlakha A, Rao K, Adlakha H, Perry A, Crotty TB, Scheithauer BW, et al. Meningioma metastatic to the lung. Mayo Clin Proc. 1999;74:1129–33.
Sathirareuangchai S, Kakazu K, Tauchi-Nishi P, Morris P, Sae-Ow W. Low grade intracranial meningioma presenting with pulmonary metastasis: case report and literature review. Pathol Res Pract. 2019;215:152390.
Miller DC, Ojemann RG, Proppe KH, McGinnis BD, Grillo HC. Benign metastasizing meningioma, case report. J Neurosurg. 1985;62:763–6.
LeMay DR, Bucci MN, Farhat SM. Malignant transformation of recurrent meningioma with pulmonary metastases. Surg Neurol. 1989;31:365–8.
Kodama K, Doi O, Higashiyama M, Horai T, Tateishi R, Nakagawa H. Primary and metastatic pulmonary meningioma. Cancer. 1991;67:1412–7.
Kodama K, Doi O, Higashiyama M, Yokouchi H, Kabuto T. Cell kinetics in two cases of meningioma with ultra-late pulmonary metastases. Nihon Kyobu Geka Gakkai zasshi. 1992;40:891–5 (Article in Japanese).
Pramesh CS, Saklani AP, Pantvaidya GH, Heroor AA, Naresh KN, Sharma S, et al. Benign metastasizing meningioma. Jpn J Clin Oncol. 2003;33:86–8.
Knoop M, Sola S, Hebecker R, Mann S, Bauer HG, Schmidt W. Rare pulmonary manifestation of an intracranial meningothelial meningioma. Dtsch Med Wochenschr. 2004;129:1854–7.
D’Aiuto M, Veronesi G, Pelosi G, Presicci PF, Ferraroli GM, Gasparri R, et al. Two-year survival after multiple bilateral lung metastasectomies for cranial meningioma. Ann Thorac Surg. 2005;80:1129–30.
Asioli S, Senetta R, Maldi E, D’Ambrosio E, Satolli MA, Bussolati G, et al. Benign metastatic meningioma: clinic-pathological analysis of one case metastasising to the lung and overview on the concepts of either primitive or metastatic meningiomas of the lung. Virchows Arch. 2007;450:591–4.
Gladin CR, Salsano E, Menghi F, Grisoli M, Ghielmetti F, Milanesi I, et al. Loss of heterozygosity studies in extracranial metastatic meningiomas. J Neurooncol. 2007;85:81–5.
Fulkerson DH, Horner TG, Hattab EM. Histologically benign intraventricular meningioma with concurrent pulmonary metastasis: case report and review of the literature. Clin Neurol Neurosurg. 2008;110:416–9.
Ishibashi H, Ohta S, Hirose M, Furuhashi K, Suzuki M, Nakajima M. Pulmonary metastatic meningioma 26-year after craniotomy. Kyobu Geka. 2008;61:478–81 (Article in Japanese).
Psaras T, Pantazis G, Steger V, Meyermann R, Honegger J, Beschorner R. Benign meningioma developing late lung metastases: case report and review of the literature. Clin Neuropathol. 2009;28:453–9.
Estanislau ES, Carvalho GT, Reis BL, de Freitas BW, Brandao RA, Sousa AA, et al. Malignant meningioma with extracranial metastases. Arq Neuropsiquiatr. 2009;67:730–2.
Etienne-Mastroianni B, Girard N, Ginguene C, Tronc F, Vasiljevic A, Vallee B, et al. Pulmonary metastases from malignant meningioma. Rev Mal Respir. 2010;27:764–9.
Cheng YJ, Wu JT, Chen HY, Wang KH, Tsai JW, Liliang PC, et al. Coexistence of intracranial meningioma, pulmonary meningiomas, and lung cancer. Ann Thorac Surg. 2011;91:1283–5.
Kanzaki R, Higashiyama M, Fujiwara A, Tokunaga T, Maeda J, Okami J, et al. Surgical resection of pulmonary metastases from meningioma: report of a case. Surg Today. 2011;41:995–8.
Nakayama Y, Horio H, Horiguchi S, Hato T. Pulmonary and pleural metastases from benign meningeal meningioma: a case report. Ann Thorac Cardiovasc Surg. 2014;20:410–3.
Sakamoto K, Ando K, Noma D, Sudo S, Goto H, Yamakawa Y, et al. Pulmonary metastases from an intracranial meningioma in a juvenile patient. J Japan Soc Resp Endosc. 2014;36:466–70 (Article in Japanese).
Tao CY, Wang JJ, Li H, You C. Malignant intraventricular meningioma with craniospinal dissemination and concurrent pulmonary metastasis. World J Surg Oncol. 2014;36:466–70.
Chiarelli M, De Simone M, Gerosa M, Guttadauro A, Cioffi U. An incidental pulmonary meningioma revealing an intracranial meningioma: primary or secondary lesion? Ann Thorac Surg. 2015;99:e83–4.
Frydrychowicz C, Holland H, Hantmann H, Gradistanac T, Hoffmann KT, Mueller W, et al. Two cases of atypical meningioma with pulmonary metastases: a comparative cytogenetic analysis of chromosomes 1p and 22 and a review of the literature. Neuropathology. 2015;35:175–83.
Mardani P, Safarian A, Ashari A, Pourjafar S, Anbardar MH, Azarpira N, et al. Low-grade intracranial meningioma with bilateral pulmonary metastases incidentally detected postpartum: a case report and review of the literature. J Med case Rep. 2021;15:509.
Takahashi T, Honda N, Hosono M, Oku S, Kashimada A, Osada H, et al. Aggressive multiple lung metastases from intracranial atypical meningioma. Japanese J Tomography. 2005;32:24–7.
Acknowledgements
The authors would like to thank Enago (https://www.enago.jp) for the English language review of the manuscript.
Funding
This report did not receive any funding.
Author information
Authors and Affiliations
Contributions
TU wrote the first draft of the manuscript. TU and TM performed the operation. TU, NM, HM, YT, TS, HH, and TM determined the treatment plan. MI contributed to the drafting of the pathological findings. TU and TS wrote the final version of the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was obtained for this case report.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Utsumi, T., Saito, T., Ishida, M. et al. Solitary pulmonary metastasis after meningioma surgery of the head: a case report. surg case rep 8, 26 (2022). https://doi.org/10.1186/s40792-022-01379-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-022-01379-9