Abstract
Anaplastic thyroid carcinoma (ATC) is an aggressive malignancy and characterized by spreading to regional lymph nodes and distant metastases, but we were unable to find a previous report of simultaneous metastases of transformed ATC to either the small intestine or thoracic esophagus in the English language literature. A 60-year-old man suffered from well-differentiated thyroid carcinoma and underwent total thyroidectomy. Eight years later, local recurrence of thyroid cancer showed intense fluorodeoxyglucose/positron emission tomography (FDG-PET) uptake at the paratracheal region, which was suspected as a remnant tumor of the thyroid that transformed from differentiated to ATC. At that time, the patient underwent resection of the small intestine to remove an abdominal mass and consequently developed stenosis of the thoracic esophagus caused by the esophageal tumor. Histological scrutiny of specimens from both tumors in the small intestine and thoracic esophagus demonstrated the same pattern as that of undifferentiated carcinoma. Regarding histological verification and a change in the FDP-PET uptake level, it is strongly possible that our case demonstrated coincident metastases of ATC to both the small intestine and esophagus. In conclusion and to the best of our knowledge, this report is the first to present evidence suggesting that ATC has the potential to metastasize to any organs, including the digestive tract.
Similar content being viewed by others
Background
Anaplastic thyroid carcinoma (ATC) is an extremely aggressive human malignancy with a very poor prognosis. It is usually characterized by the spread to regional lymph nodes and distant metastases to other organs, most commonly to the lungs and bones [1]. However, metastases to the digestive tract is considered quite rare [2]. To the best of our knowledge, this case is the first report of ATC with simultaneous isolated metastases to the small intestine and thoracic esophagus, which had possibly transformed through a former instance of differentiated thyroid cancer in this patient.
Case presentation
A 60-year-old man underwent total thyroidectomy with bilateral neck dissection in July 2004 for the treatment of well-differentiated papillary thyroid carcinoma (PTC) (Fig. 1). The pathological classification was pT4a (esophagus), pN1a, M0, stage IVA. In September 2006, local recurrence to the neck (left of paratracheal sites) and distant metastasis to the femur were observed by positron emission tomography/computed tomography (PET/CT) with fluorodeoxyglucose (FDG). The patient rejected our proposed treatment regimen of resection at the local site recurrence and internal radiation therapy against the distant metastasis and was thus required to start specific substance of Maruyama (SSA). Thus, only external radiation therapy to both regions was administered as a palliative therapy. A trial of suppressive therapy with levothyroxine was also initiated. Follow-up CT in May 2007 still indicated the existence of metastatic tumor at left side of trachea without changing its size. Until December 2010, there was no evidence of growth of the neck tumor or new recurrence site observed by CT scan. In July 2012, the patient (68 years old) developed persistent abdominal pain accompanied by a fever of 38 °C. At the time of admission to our hospital, an emergent CT scan revealed an intra-abdominal mass that partially involved the adjacent small bowel (Fig. 2) and the abdominal symptoms worsened; therefore, we decided to perform emergent exploratory laparotomy. Intraoperatively, we identified an intra-abdominal abscess with a 5 cm diameter adjacent to the mesenteric vessels with partial involvement of the small intestine. The mass was completely excised together with partial resection of the small intestine. Macroscopically, the tumor was mainly composed of infectious tissue and an abscess caused by perforation of a small intestinal tumor, which extended through the muscularis propria of the small intestine and connected to the abscess cavity (Fig. 3). Histologically, the tumor was diagnosed as undifferentiated carcinoma. The postoperative course was uneventful, and the patient was discharged on postoperative day 12. Following the pathological results of the small intestinal tumor, we started to apply chemotherapy using FOLFIRI regimen [3] until March 2013.
During chemotherapy, a follow-up PET and CT scan in October 2012 detected progression of lymph node metastasis at the cervical paratracheal site suspected as a recurrence of the former thyroid cancer. Maximum standardized uptake value (SUVmax) of fluorodeoxyglucose-positron emission tomography (FDG-PET) at the paratracheal lesion increased from 6.0 in 2006 to 28.8 in 2012 (Fig. 4). This result suggested that the remnant PTC, which was pathologically determined as a composition of differentiated cancer cells in 2006, might have transformed to poorly differentiated or undifferentiated carcinoma. The serum thyroglobulin level and thyroid function tests (thyroid-stimulating hormone, free thyroxine) were within normal ranges. Simultaneously, FDG uptake of the thoracic esophagus at the middle mediastinum was observed positively, comparing with the find at the prior PET scan in 2006 that showed no accumulation of FDG around the thoracic esophagus (Fig. 5). Despite the deterioration of the PET results in 2012, the patient had no clinical symptoms of trouble in swallowing at that point.
In March 2013, the patient developed dysphagia and was readmitted to our hospital. Endoscopic examination revealed a circular occlusion caused by an esophageal tumor (Fig. 6) at the middle part of the esophagus and a biopsy confirmed the suspicion of undifferentiated carcinoma. But we could not find any mucosal degeneration of esophageal part adjacent to the prior recurrent site of cervical parathoracic lesion. A CT scan at this time clearly indicated the esophageal tumor expanding around the middle portion of the mediastinum (Fig. 7). To relieve stenosis of the esophagus, endoscopic balloon dilation and the placement of a self-expandable metal stent were performed. Although the patient was able to swallow food, he died 9 days after endoscopic treatment due to dyspnea caused by tracheal stenosis. Permission for an autopsy was denied.
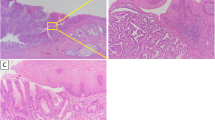
Histological scrutiny of both specimens from the small intestine (Fig. 8) and esophagus (Fig. 9) revealed that the tumors were similarly composed of large anaplastic polygonal cells that were discohesive and tended to dissociate from each other with a prominent neutrophilic infiltrate. There were no patterns specific of adenocarcinoma, squamous cell carcinoma, or PTC. The antibodies used in this study for immunohistochemical analysis included pankeratin (clone AE1/AE3), cytokeratin 7 (CK7), cytokeratin 20 (CK20), high molecular weight keratin (HMW-CK, clone 34Ebeta12), transcription termination factor 1 (TTF-1, clone 8G7G3/1), p53 (clone DO-7), and caudal type homeobox 2 (CDX-2, clone DAX-CDX-2), which were all obtained from DakoCytomation (Glostrup, Denmark), and thyroglobulin (polyclonal; Nichirei Biosciences, Inc., Tokyo, Japan). Cells from both tumors were strongly positive for pankeratin and p53 and negative for CK7, CK20, HMW-CK, CDX-2, TTF-1, and thyroglobulin (Table 1).
The pathological findings of the esophageal tumor were almost identical to those of the small intestinal tumor, which were both diagnosed as ATC. Considering the clinical progression of disease in this patient, we concluded that both tumors were derived from the same origin—a transformation of the former differentiated thyroid carcinoma treated 8 years before.
Discussion
ATC is a rapidly growing neoplasm associated with a poor prognosis and mean survival time of 4 to 7 months [4, 5]. Approximately 20–50 % of patients with early tumor dissemination are positive for distant metastases and 90 % for adjacent tissue invasion on presentation [6]. The most common site of metastases is the lung and bone with occasional involvement of the heart, adrenals, pleura, kidneys, and pancreas [7]. A case of ATC with isolated metastasis to the small bowel is very rare, as only two cases have been reported in the PubMed database to date [8, 9]. Moreover, isolated metastasis of ATC to the thoracic esophagus is exceedingly rare, despite close proximity to the cervical esophagus. Because we found no previous reports of simultaneous isolated metastases of ATC to both the esophagus and small intestine in the literature, we presume that this is the first case report.
In some clinical situations, it is difficult to determine whether a tumor developed primarily at the site of detection or metastasized from another malignancy. Often there is no specific morphological pattern associated with an undifferentiated carcinoma; thus, such histological ambiguity makes it more difficult to identify the origin of a tumor. In the case reported in this study, microscopic findings of widely invasive growth composed of an admixture of pleomorphic giant cells and spindle cells were observed for both specimens from the small intestine and esophagus, but characteristic structures of adenocarcinoma, squamous cell carcinoma, and PTC were absent. In this case, immunohistochemical findings of positive for p53 expression and negative TTF-1 and thyroglobulin expression suggest that the tumor did not have typical patterns of either papillary or follicular thyroid carcinoma. The negative results for CK20 and CDX-2 suggested that the tumor did not likely originate from the gastrointestinal tract. Moreover, the findings of negative HMW-CK expression for both tumors indicated that these undifferentiated cells were not derived from squamous cell carcinoma of the esophagus.
Approximately 20 % of patients with ATC have a history of differentiated thyroid cancer [10], and transformation from differentiated to anaplastic cancer was previously described in a patient who was followed up with serial biopsy [11]. Moreover, mutations in the p53 tumor suppression protein associated with late de-differentiating events in an anaplastic tumor rather than a precursor from a well-differentiated tumor have been reported [12]. A recent study showed that thyroid cancer dedifferentiation is characterized by upregulation of the glucose transporter GLUT1 and a high FDG uptake [13]. Compared with other imaging modalities, PET may improve disease detection and better facilitate the management of patients with ATC [14]. The American Thyroid Association recommends FDG-PET/CT for the evaluation of metastatic disease and suggests that this modality may be useful to distinguish ATC from PTC according to the SUVmax level because of the higher FDG uptake in the former [15]. In the present case, the patient underwent treatment for advanced PTC 8 years before presentation, after which local recurrence with bone metastasis from the original PTC was identified. The latest FGD-PET showed intense uptake in a region of local recurrence of pre-existing differentiated thyroid cancer. The finding of increased activity, as indicated by a SUVmax value from 6 to 29, clearly suggests a possible transformation from differentiated thyroid cancer to ATC. The uptake of PET around the tumors of the intestine and esophagus also exhibited the same level as that of a dedifferentiated lesion.
Conclusions
Technically, it is very difficult to confirm whether the tumor developed whether primarily or secondarily from another malignancy, if both tumors show the same pathological character. Unfortunately, we could not get permission for an autopsy and were compelled to make a final diagnosis from clinical evidence. But in conclusion, based on the histological verification and the degree of FDP uptake on PET, it is very likely that in our case, coincident metastases of ATC to both the small intestine and thoracic esophagus occurred. This case is unique because there were no previous reports on simultaneous metastases to these two organs from transformed ATC. Therefore, this report presents the first evidence suggesting that ATC has the potential to metastasize to any organs, including the digestive tract.
Consent
Written informed consent was obtained from the patient’s family for this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- ATC:
-
anaplastic thyroid carcinoma
- CDX-2:
-
caudal type homeobox 2
- CK:
-
cytokeratin
- FDG-PET:
-
fluorodeoxyglucose-positron emission tomography
- HMW-CK:
-
high molecular weight cytokeratin
- PTC:
-
papillary thyroid carcinoma
- TTF-1:
-
transcription terminal factor 1
References
Carcangiu ML, Steeper T, Zampi G, Rosai J. Anaplastic thyroid carcinoma. A study of 70 cases. Am J Clin Pathol. 1985;83(2):135–58.
Yazaki N, Naitoh T, Miura K, Ogawa H, Kinouchi M, Tanaka N, et al. A case report of anaplastic thyroid carcinoma metastasized to the small intestine causing intussusception. Jpn J Gastroenterol Surg. 2011;44(11):1426–33.
Zaanan A, Gauthier M, Malka D, Locher C, Gornet JM, Thirot-Bidault A, et al. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer. 2011;117(7):1422–8.
Nel CJ, van Heerden JA, Goellner JR, Gharib H, McConahey WM, Taylor WF, et al. Anaplastic carcinoma of the thyroid: a clinicopathologic study of 82 cases. Mayo Clin Proc. 1985;60(1):51–8.
Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66(2):321–30.
O’Neill JP, Shaha AR. Anaplastic thyroid cancer. Oral Oncol. 2013;49(7):702–6.
Silverberg SG, Hutter RV, Foote Jr FW. Fatal carcinoma of the thyroid: histology, metastases, and causes of death. Cancer. 1970;25(4):792–802.
Phillips DL, Benner KG, Keeffe EB, Traweek ST. Isolated metastasis to small bowel from anaplastic thyroid carcinoma. With a review of extra-abdominal malignancies that spread to the bowel. J Clin Gastroenterol. 1987;9(5):563–7.
Ricciardelli L, Rapicano G, Pinto A, Napolitano G, Feleppa C, Martino G, et al. Small bowel inussusception caused by metastasis from anaplastic thyroid carcinoma: case report and literature review. Ann Ital Chir. 2006;77(1):63–7.
McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130(6):1028–34.
Moore Jr JH, Bacharach B, Choi HY. Anaplastic transformation of metastatic follicular carcinoma of the thyroid. J Surg Oncol. 1985;29(4):216–21.
Moretti F, Farsetti A, Soddu S, Misiti S, Crescenzi M, Filetti S, et al. p53 re-expression inhibits proliferation and restores differentiation of human thyroid anaplastic carcinoma cells. Oncogene. 1997;14(6):729–40.
Grabellus F, Nagarajah J, Bockisch A, Schmid KW, Sheu SY. Glucose transporter 1 expression, tumor proliferation, and iodine/glucose uptake in thyroid cancer with emphasis on poorly differentiated thyroid carcinoma. Clin Nucl Med. 2012;37(2):121–7.
Bogsrud TV, Karantanis D, Nathan MA, Mullan BP, Wiseman GA, Kasperbauer JL, et al. 18F-FDG PET in the management of patients with anaplastic thyroid carcinoma. Thyroid. 2008;18(7):713–9.
Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–39.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
MK have made substantial contributions and is the corresponding author. HI participated in the acquisition of clinical data and images of the patient. TI carried out the histological verification and gave pathology advice. NY is a doctor of otorhinolaryngology and was involved in the first surgical treatment, which was a total thyroidectomy and an additional neck dissection. TK, a radiologist, carried out scrutiny using PET/CT and evaluated the date and image in this article. AT has been involved in revising the manuscript for important intellectual content. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kobayashi, M., Itabashi, H., Ikeda, T. et al. Simultaneous occurrence of distant metastases to the small intestine and the thoracic esophagus from anaplastic thyroid carcinoma: a case report. surg case rep 1, 63 (2015). https://doi.org/10.1186/s40792-015-0066-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-015-0066-9