Abstract
Introduction
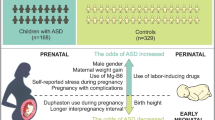
The constellation of pre and perinatal predictors are introduced as predictor for autism spectrum disorders (ASD), however, the information about the direction and strength of these predictors are lacking in Western, Iran. The current study aimed to determine the pre and perinatal predictors of ASD among children in this region.
Methods
This case-control study was conducted in Hamadan, Western Iran during January to March 2022. The study included 100 children with ASD who referred to the autism center as case group. Hundred children without ASD from registration system of health service centers were selected as control group and were matched (1:1) to cases by age and place of residency. A structured questionnaire about pre and perinatal predictors of ASD was developed by an expert panel. The questionnaire was administered by interviewing the mothers of children.
Results
Boy gender (OR: 3.51, 95% CI: 1.74–7.10, p-value < 0.001), small for gestational age (SGA) (3.92, 1.64–9.39, 0.002), maternal diabetes (3.51, 1.03–24.95, 0.04) and family history of mental disorders (3.64, 1.18–11.27, 0.04) were identified as significant predictors in a multivariable analysis.
Conclusion
Our study emphasizes on the importance of screening and monitoring for ASD in the boys, those with history of SGA, from mothers with history of diabetes and with family history of mental disorders. Proposing the replication of findings emphasizes the necessity of conducting studies with larger sample sizes.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder whose specific manifestations include social and communication problems related to restrictive and repetitive behaviors, interests, or activities [1, 2].
The exact etiology of ASD is not yet known and a set of genetic and environmental factors are effective in its occurrence, However, studies show that genetic factors account for only 35–40% of the factors that contribute to the development of ASD, and the remaining are likely other factors such as the pre- perinatal, and postnatal factors [3, 4].
The prevalence rate of ASD has reached more than 1–2% worldwide [5] and since ASD is a neurodevelopmental disorder and the perinatal period is a critical time for neural development. Complications observed in children, which may act as indicators, can enable early diagnosis and the possibility of intervention in children with autism [6], because, early diagnosis especially at 12–18 months, and intervention along with medical services are very useful for these children, and it causes the recovery of most children with ASD before school age [7].
Some of the studies reported that ASD is associated with a family history of psychiatric disorders, hypertension, diabetes, younger maternal age, preeclampsia, labor complications, low birth weight (LBW), small for gestational age (SGA), male gender, and newborn complications [8]. However, the most reliable predictors associated with ASD vary with the child’s age, and the presence of some of these predictors becomes more problematic as the child ages [9].
A case-control study conducted in Iran reported that ASD was significantly associated with third-degree relative’s consanguinity, short birth length, short head circumference, respiratory distress syndrome at birth, respiratory assistance at birth, birth hypoxia, and low 1-minute Apgar score. However, the number of cases in this study was low (41 child) and was not included all aspects of perinatal problems.
Consequently, it is important to identify predictors that allow early detection and intervention, which may prevent the development of other traits and/or behaviors associated with ASD [3]. To date, studies have been conducted in Iran regarding perinatal predictors of ASD among children were limit. Therefore, this study aimed to determine pre- and perinatal Predictors of ASD in the west of Iran.
Methods
Study Design
The STROBE reporting guidelines were used in the design of this study. The Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1400.800) approved the protocol of the study. All children 3–14 ages with ASD, whose contact information was available in the Autism Research Center, were included in the study with the informed consent of a parent and/or legal guardian.
Participants
This case-control study was performed on 200 subjects from January 2, 2022, to March 17, 2022. The inclusion criteria for the control group were mothers who had a child of 3–14 years without ASD and their health records were registered in comprehensive health service centers in Hamadan city.
The inclusion criteria for the cases group were mothers who had a child of 3–14 years with ASD and they referred to the autism Center to receive rehabilitation services for their child. The diagnosis of children with ASD is based on the criteria of DSM-V and modified Autism Diagnostic Interview-Revised (ADI-R) and the final diagnosis is made by a psychiatrist. Proposed DSM-5 autism spectrum criteria includes three severity classifications include Level 1 (‘‘Requiring support’’), Level 2 (‘‘Requiring sub- stantial support’’), and Level 3 (‘‘Requiring very sub- stantial support’’) [10]. We included levels 2 and 3 in this study. All mothers completed the questionnaire with consent. There were a total of 100 children between 3 and 14 years with ASD in Hamadan city by the census. Therefore, the inclusion criteria included all children with ASD between 3 and 14 years of age in Hamadan city were included in this study. Exclusion criteria included lack of access to parents of children with ASD.
Healthy children aged 3 to 14 years with records in health service centers were considered as a control group for each case. In this study, 100 children were in the case group and 100 children were in the control group. Cases were selected using a census sampling method, and the control group was chosen through random sampling at health service centers. The case and control groups are matched based on the age of the child and their place of residence.
Questionnaire
A researcher-made questionnaire was used including parents’ age, child’s gender, mother’s occupation, fetal manifestations, history of premature labor, post-term, small for gestational age (SGA), low birth weight (LBW), diabetes, preeclampsia, childbirth, fetal presentation type, mother’s job, mother’s education, father’s education, and family history of mental disorders (anxiety Disorders, depression, bipolar Disorder, Post-Traumatic Stress Disorder (PTSD), schizophrenia, disruptive behavior and dissocial disorders, neurodevelopmental disorders). This questionnaire was developed by 10 experts in this field.
Statistical analysis
Descriptive categorical and continuous predictors were expressed as number (%) and mean (standard deviation) according to the study groups, respectively and were tested using chi-square and independent t-test as appropriate. The effect of the studied predictors on ASD was evaluated using univariate and multivariable binary logistic regression. The results of logistic analysis were expressed using odds ratio and 95% confidence interval. The statistical significance was p-value < 0.05. All of statistical analysis was conducted using Stata version 14. (Stata Corp., College Station, TX, USA).
Result
We compared 100 autistic child and 100 healthy children in the case and control groups. The baseline characteristics of the case and control groups are presented in Table 1. Two groups were homogenous in regards to birth order, weight, mother and father’s age, delivery type, presentation, history of preterm, post-term, preeclampsia, mother’s education, and Family history of mental disorders (P > 0.05). The proportion of males to females was significantly higher in the case group (P = 0.003). The case group had significantly higher SGA (68.29% vs. 31.71%), LBW (68.75% vs. 31.25%), maternal diabetes (80% vs. 20%), and housekeeper job in mothers (55.70% vs. 44.30%) (P < 0.05).
The results of the crude multivariable analyses are presented in Table 2. After adjusting for confounding variables the results showing significant predictors of ASD score were boy gender [OR: 3.51, 95% CI: 1.74, 7.1 (p0.001)], SGA [OR: 3.92, 95% CI: 1.64, 9.39 (p0.002)], maternal diabetes [OR: 3.51, 95% CI: 1.03, 24.95 (p = 0.046)] and family history of mental disorders [OR: 3.64, 95% CI: 1.18, 11.27 (p = 0.046)].
Discussion
The findings of the present study reported that the perinatal predictors of ASD were male gender, SGA, maternal diabetes, and family history of mental disorders.
ASD diagnosis is delayed due to the definition of this disorder as well as the complexity of symptoms in children. Due to the lack of specific biological markers for ASD, the diagnosis is based on clinical manifestations by health professionals [3]. The findings reported that the perinatal predictors of ASD were boy gender, SGA, maternal diabetes, and family history of mental disorders.
The most common definition of SGA is birth weight in the tenth percentile of the population [11]. The association between SGA and ASD may reflect neurodevelopmental problems that occur during pregnancy. Pathophysiology that limits fetal growth may also disrupt neurologic development during the prenatal period. The main cause of SGA is placental insufficiency, a condition in which the fetus does not reach its full growth potential due to a lack of nutrients and oxygen transport [12]. A meta-analysis study by Jenabi et al. showed that SGA is a risk factor for ASD (1.17:95% CI, 1.09–1.24) [13]. In our study, based on OR adjusted model, SGA increased 3.92 the risk of ASD. Therefore, this study confirmed the increase in the risk.
The study by wan et al. reported that maternal diabetes increased risk of the ASD in offspring (RR: 1.48) [14]. A meta-analysis showed that maternal diabetes based on cohort studies was 1.48 (RR: 1.48; 1.25–1.75) and based on case-control studies was 1.72 (RR: 1.72; 1.24–2.41) after adjusting the confounder variables [15]. Our study is in line with the above studies.
Xie et al. (2019) reported that a family history of neurological disorders was related to ASD risk [16]. Another study showed that a family history of psychiatric disorders was the risk factor for ASD (ORA: 2.2; 1.1–4.4) [17]. Our studies confirmed the findings of these studies.
LBW is a term used to describe a neonate born weighing 5.5 pounds (2500 g) or less. LBW can occur when a neonate is born too early (premature). These neonates may be at risk of developing serious health problems. Smoking, exposure to tobacco smoke, drinking alcohol, and taking certain medications during pregnancy can increase the risk of having an LBW neonate [18]. Ma et al. in a study showed that LBW may increase the risk of ASD. They showed that there was an increased risk of ASD concerning LBW (OR = 1.63, 95% CI = 1.48–1.81) [11]. The present study showed that LBW was more in the case group compared with the control group. Although this correlation was not significant in the model adjusted.
The male gender was associated with an increased risk of ASD (RR = 1.47, 95% CI: 1.39, 1.55) [19]. Although the primary cause of the high prevalence of ASD in boys is unknown, examination of hypothesized molecular and cellular factors proposed that fetal testosterone and/or other steroid hormones are involved in a regulatory pathway involving the RORA gene, and sex-differentiated function of astrocytes and microglia may participate in sex-differential risk mechanisms. Although each of these observations helps to close gaps in our understanding of the interactions between sex-different biology and ASD risk pathways, each sex-difference factor associated with ASD has been implicated in relative isolation.
Our study confirmed it and presented that the male gender increased the 3.51 risk of ASD after adjusting confounder variables [20, 21].
Some of the studies reported preeclampsia as a risk factor for ASD [3, 22, 23]. The present did not report it. This could be due to the small sample size and racial differences. Therefore, we suggest that this study be conducted with larger and prospective sample size or international studies. In observational studies, we may not have included all demographic and unmeasured parental characteristics in the study. In this study, we could not include all aspects of perinatal problems in the questionnaire due to incomplete information. Therefore, the data gathered was not comprehensive, which may affect the study’s conclusions. In addition, the confidence intervals for some variables mentioned in the study were excessively wide due to low sample size. However, this limitations could lead to bias in the findings.
Conclusion
The present study’s findings revealed that perinatal predictors of ASD included male gender, SGA, maternal diabetes, and a family history of mental disorders. Large studies are needed to replicate and confirm the findings of our study.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Cogley C, O’Reilly H, Bramham J, Downes M. A systematic review of the risk factors for autism spectrum disorder in children born preterm. Child Psychiatry Hum Dev. 2021;52(5):841–55.
Bashirian S, Soltanian AR, Seyedi M, Khazaei S, Jenabi E, Razjouyan K, et al. The psychometric properties of the Iranian version of Autism Diagnostic interview-revised (ADI-R) in children with autism spectrum disorder. Advances in Autism; 2021.
Perales-Marín A, Peraita-Costa I, Cervera-Boada P, Tellez de Meneses M, Llopis-González A, Marí-Bauset S, et al. Perinatal and obstetric predictors for Autism Spectrum Disorder. J Autism Dev Disord. 2021;51(11):3908–16.
Styles M, Alsharshani D, Samara M, Alsharshani M, Khattab A, Qoronfleh MW, et al. Risk factors, diagnosis, prognosis and treatment of autism. Front Biosci. 2020;25(9):1682–717.
Jenabi E, Ahmadi M, Maleki A. Is fetal nuchal cord associated with autism spectrum disorder? A meta-analysis. Clin Experimental Pediatr. 2022;65(3):131.
Froehlich-Santino W, Tobon AL, Cleveland S, Torres A, Phillips J, Cohen B, et al. Prenatal and perinatal risk factors in a twin study of autism spectrum disorders. J Psychiatr Res. 2014;54:100–8.
Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries. 2018;67(6):1.
Jenabi E, Bashirian S, Salehi AM, Khazaei S. Not breastfeeding and risk of autism spectrum disorders among children: a meta-analysis. Clin Experimental Pediatr. 2022.
Emerson E, Kiernan C, Alborz A, Reeves D, Mason H, Swarbrick R, et al. Predicting the persistence of severe self-injurious behavior. Res Dev Disabil. 2001;22(1):67–75.
American Psychatric Association. DSM-5 Development: Autistic disorder. 2012 [ https://www.cdc.gov/ncbddd/autism/hcp-dsm.html.
McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynecol. 2009;23(6):779–93.
Brook CG, Clayton P, Brown R. Brook’s clinical pediatric endocrinology. Wiley; 2009.
Jenabi E, Bashirian S, Asali Z, Seyedi M. Association between small for gestational age and risk of autism spectrum disorders: a meta-analysis. Clin Experimental Pediatr. 2021;64(10):538.
Wan H, Zhang C, Li H, Luan S, Liu C. Association of maternal diabetes with autism spectrum disorders in offspring: a systemic review and meta-analysis. Medicine. 2018;97(2).
Carpita B, Muti D, Dell’Osso L. Oxidative stress, maternal diabetes, and autism spectrum disorders. Oxidative medicine and cellular longevity. 2018;2018.
Xie S, Karlsson H, Dalman C, Widman L, Rai D, Gardner RM, et al. Family history of mental and neurological disorders and risk of autism. JAMA Netw open. 2019;2(3):e190154–e.
Guisso DR, Saadeh FS, Saab D, El Deek J, Chamseddine S, El Hassan HA, et al. Association of autism with maternal infections, perinatal and other risk factors: a case-control study. J Autism Dev Disord. 2018;48(6):2010–21.
Hoffman BL, Schorge JO, Bradshaw KD, Halvorson LM, Schaffer JI, Corton MM. Williams gynecology: cGraw-Hill Education; 2016.
Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine. 2017;96:18.
Werling DM. The role of sex-differential biology in risk for autism spectrum disorder. Biology sex Differences. 2016;7(1):1–18.
Werling DM, Parikshak NN, Geschwind DH. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat Commun. 2016;7(1):1–11.
Jenabi E, Karami M, Khazaei S, Bashirian S. The association between preeclampsia and autism spectrum disorders among children: a meta-analysis. Korean J Pediatr. 2019;62(4):126.
Dachew BA, Mamun A, Maravilla JC, Alati R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. Br J Psychiatry. 2018;212(3):142–7.
Acknowledgements
Not applicable.
Funding
This study was financially supported by Hamadan University of Medical Sciences, Iran with Code 140010288896.
Author information
Authors and Affiliations
Contributions
Conception or design: EJ, AS. Acquisition, analysis, or interpretation of data: MS, HJ, SK. Drafting the work or revising: EA, AS. Final approval of the manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of the Hamadan University of Medical Sciences approved the protocol of this study (IR.UMSHA.REC.1400.800). Informed assent and written informed consent was obtained from all participants and their care givers.
Consent for publication
Written consent for publication obtained from children’s parents.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jenabi, E., Salehi, A.M., Ayubi, E. et al. Pre and perinatal predictors on autism spectrum disorders: a case-control study in the west of Iran. matern health, neonatol and perinatol 10, 13 (2024). https://doi.org/10.1186/s40748-024-00183-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40748-024-00183-7