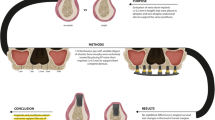
Abstract
Purpose
Short implants often have the disadvantage of reduced primary stability. The present study was conducted to evaluate the feasibility and safety of a new expandable short dental implant system intended to increase primary stability.
Methods
As a “proof of concept”, a prospective clinical cohort study was designed to investigate intraoperative handling, primary and secondary implant stability (resonance frequency analysis), crestal bone changes, implant survival and implant success, of an innovative short expandable screw implant. From 2014 until 2015, 9 patients (7–9-mm vertical bone height) with 30 implants (length 5–7 mm, diameter 3.75–4.1 mm) were recruited consecutively.
Results
All 30 implants in the 9 patients (age 44 to 80 years) could be inserted and expanded without intraoperative problems. Over the 3-year follow-up period, the implant success rate was 28/30 (93.3%). The mean implant stability quotients (ISQ) were as follows: primary stability, 69.7 ± 10.3 ISQ units, and secondary stability, 69.8 ± 10.2 ISQ units (p = 0.780), both without significant differences between the maxilla and mandible (p ≥ 0.780). The mean crestal bone changes after loading were (each measured from the baseline) as follows: in the first year, 1.0 ± 0.9 mm in the maxilla and 0.7 ± 0.4 mm in the mandible, and in the second year, 1.3 ± 0.8 mm and 1.0 ± 0.7 mm, respectively.
Conclusions
Compared to other prospective studies, in this indication, the success rate is acceptable. Implant stability shows high initial and secondary stability values. The system might present an extension of functional rehabilitation to the group of elderly patients with limited vertical bone height. Further long-term investigations should directly compare this compressive implant with standard short implants.
Similar content being viewed by others
Introduction
Endosseous implants have been established over several decades. The evaluation of treatment results under biomechanical, physiological, psychological, social and economic aspects has been well documented [1]. Furthermore, patient-based outcomes reveal a predictable gain in oral health-related quality of life [2].
Especially in patients with limited vertical bone height, process of treatment is extensive. Prior to implantation, augmentation procedures are required [3]. Depending on gender, vascularisation and bone mineralisation up to 25% of the primary volume are resorbed due to remodeling of augmented alveolar ridges [4]. Recently, short dental implants have evolved into a promising and reliable treatment option in the orofacial rehabilitation of atrophic mandibles and maxillae, namely as an alternative to vertical ridge augmentation [5,6,7,8]. The prognosis of short implants and patient satisfaction is high [9,10,11,12].
The definition of short implants in the literature is not uniform. In this present study, we considered short implants with 5–8-mm length [5, 7, 13]. Other authors set the cut-off at 6 mm [8, 9, 11, 14, 15]. According to the recent consensus paper of the 11th European Consensus Conference (EuCC), dental implants are referred to as “short” if their intrabony length measures ≤ 8 mm and considered as “ultra-short” with lengths < 6 mm [16].
Biomechanical studies show that the crestal bone is strained under axial and extra-axial loading [17]. While bone quality, implant design and position, prosthetic devices and material characteristics contribute to the character of stress distribution, the role of implant length seems to be of underpart [17, 18]. Nevertheless, implant length is crucial in D4 bone quality [19], and the crown-to-implant length itself influences stress distribution under extra-axial loading in the crestal bone [20] and in the abutment screw [21]. According to Petrie and Williams [22], the influence of increased implant diameter on stress reduction in the crestal bone is more efficient than increased implant length. Möhlhenrich and co-authors [23] confirmed these findings that the diameter of an implant has greater influence on primary stability than implant length. Based on in vitro analysis, they concluded additionally that especially in patients with poor bone quality, a variation of implant dimensions is expected to lead to a significant increase of primary stability. Furthermore, stress distribution on short implants is affected by the bone-to-implant contact ratio [24]. Consequently, several options to increase the implant surface of short implants are elaborated, which consecutively enhance the implant stability: thread number, thread shape, thread depth, implant diameter, implant design and surface topography [25,26,27].
It is known that achievement of primary stability is one precondition for osseointegration and treatment success. There are few reports of immediate [14] and early (6 weeks) functional loading of short implants [28]. This is related to good bone quality, implant design or implant site preparation (e.g. under-drilling). However, under-drilling of the crestal aspect may lead to decreased bone-to-implant contact [29]. It is desirable to reduce the periimplant stress on the crestal bone while providing sufficient primary stability for all bone densities.
Therefore, optimisation of the macro- and microdesign of short dental implants to improve the success rate and long-term stability is preferable. In fact, elderly patients with general comorbidity should benefit from the overall short treatment time [28, 30]. As previously shown, the oral health-related quality of life is compromised during the healing period after implant insertion [31], especially when augmentation procedures are required [11]. For several reasons, the overall treatment time should be reduced in patients with atrophic alveolar ridges.
The purpose of the present study was to clinically analyse the feasibility and safety of a new short dental implant system with an expandable compressive design in the apical region. We hypothesised that the innovative expandable macrodesign of this implant provides a reliable implant success rate and ensures high implant stability in vivo.
Material and methods
Study population and measures
The study was designed as a prospective monocentric longitudinal cohort study according to the STROBE criteria. The participants of this study were recruited at the university hospital of Martin Luther University Halle-Wittenberg, Department of Oral and Plastic Maxillofacial Surgery, implantological consultation from 2014 (June) until 2015 (June). Inclusion and exclusion criteria of adult patients interested in implantological treatment are summarised in Table 1. Written informed consent was obtained from all individual participants included in this study.
As a “proof of concept”, the pilot study was designed to investigate the intraoperative handling and to evaluate the feasibility and safety of a new short implant system. Therefore, sample size calculation was not performed. The primary outcome variable was implant success rate, which was calculated considering known success criteria (implant in function, no sign of infection or pain, no mobility, no radiolucent area around the implant) [32, 33]. The implant survival was calculated according to the Kaplan-Meyer method. Secondary measures were implant stability (initial and secondary) and periimplant crestal bone changes. Implant stability was measured by resonance frequency analysis (RFA; Osstell AB, Göteborg, Sweden).
Primary stability was measured immediately after implant insertion and completed expansion (see below), and secondary stability after the submerged healing period (3 months in the mandible, 6 months in the maxilla; Table 2) during the re-entry operation just before the healing abutments were inserted. Implant stability quotient (ISQ) values were obtained using the Smartpegs (type 17 and 35). According to each measurement, implant stability was classified as low with ISQ values < 60, medium with ISQ values 60–70, and high with ISQ values > 70 [34].
Digital radiograms (orthopantomogram, standard periapical radiograms) were taken prior to surgery, after implant insertion and re-entry, and at yearly follow-up examinations for crestal and periapical bone evaluation (see below).
Implants
In this study, a short expandable titanium screw implant (PYRAMIDION dental implant, DenTack Implants Ltd., Kfar-Saba, Israel) was used, which leads to dynamic condensing of the apical bone. The implants had the following dimensions and special characteristics: 5, 6 and 7 mm in length, 3.75 and 4.1 mm in diameter and an internal (7-mm length) or external (5- and 6-mm length) hexagon platform. The apical expansion is performed after implant insertion using a special expansion tool and a ratchet torque, resulting in a pyramid shape (Fig. 1a–f). The implant expansion process using the expansion tool is visualised in the movie clip (Additional file 1).
Simulation of the expansion process. At the end of the expansion process, a minimal snap back is realised. (MP4 9407 kb)
a Closed short expandable dental implant (4.1 × 7 mm). The implant-abutment connection is characterised by an internal hexagon for rotation stability, combining the advantages of conical and parallel surfaces to reduce microgaps and micromovement [68]. The microthread concept and platform switching concept are implemented in the implant shoulder to reduce periimplant bone strain [53]. b Manual fixation of the expansion tool. Take note of the distance between both yellow rings. c Completion of the expansion process using the ratchet. Take note of the contact between both yellow rings. d Opened short expandable dental implant (4.1 × 7 mm). The expanded implant provides an increased bone-to-implant interface (pyramid shape) in the apical portion [54]. e Cross-section view of the implant apex. The apical expansion process is characterised by the unfolding of four wings, which are connected by four foils. D1: diameter of the closed implant. D2: diameter of the opened implant. f Top view of the expanded implant. The expanded implant (4.1-mm diameter) displays an apical diameter of 4.7 mm and length of the edge (base) of 4.4 mm
Surgical and prosthetic protocol
Planning of the implantological treatment followed usual clinical and radiological examination and, concerning the position and number of implants, the recommended categories from the German consensus conference [35]. The drilling sequence, condensing preparation (where necessary) and manual implant insertion as well as expansion are described in detail in Table 2. Participants were instructed not to wear their denture 1 week after surgery. Afterwards, the conventional dentures were relined with soft material (Visco-gel, Dentsply, Salzburg, Austria). In this study, conventional periods of submerged healing were chosen: 3 months in the mandible and 6 months in the maxilla. During the re-entry surgery, a minimum of 2-mm keratinised periimplant soft tissue mucosa was considered.
All prosthetic treatments were provided at the University School of Dental Medicine, Department of Prosthetic Dentistry. At the earliest, 2 weeks after surgical re-entry, prosthetic treatment was started. All treatment steps were performed as described in detail in Table 3. The abutment screws were fixed with a torque of 15 N cm. Wherever possible, adjacent implants were primarily splinted (crowns, bar) and extra-axial loading during dynamic occlusion was avoided. In other cases, eccentric group guidance was achieved. To reduce overloading in the periimplant bone and implant-abutment connection, the occlusal surface was designed smaller [20, 21, 30, 36]. Patients were instructed about optimal oral hygiene, and the use of a dental water jet was recommended.
All treatments were provided by two experienced maxillofacial surgeons (WR, CH) and two experienced prosthodontists (RS, JH) to minimise bias.
Follow-up investigation
The first clinical follow-up was arranged at the latest 4 weeks after prosthetic treatment was completed. Further follow-ups were scheduled quarterly in the first year and later every 6 months. Patients were screened clinically and radiologically (yearly) for biological and technical complications. The authors applied the abovementioned success criteria according to Buser et al. [32]. Crestal bone changes were evaluated on digital radiograms (SIDEXIS imaging software, Sirona, Bensheim, Germany). The distance between the implant shoulder and first bone-implant contact at the mesial and distal aspect of each implant was measured (implant length as reference) by the first author (WR), and the mean values per implant were calculated [37] 1 and 2 years after loading.
Data gathering and statistics
All patients were pseudonymised, parameters were attached to a databank and analysed statistically (Additional file 2). Statistical analyses were performed using statistics software (IBM SPSS statistics, version 20, Chicago, IL, USA). The descriptive statistics presented the frequency and distribution of several occurrences as well as combinations of certain features. Analytical statistics were performed depending on the scale: paired and independent t tests for differences of mean values. The implant survival was calculated according to the Kaplan-Meyer method. The level of significance was set at 5%.
Results
The first results of this longitudinal study include data from 9 patients with an average age of 57 years (range from 44 to 80) in whom 30 implants were inserted (maxilla n = 15, mandible n = 15). All 30 implants in the 9 patients could be inserted without intraoperative problems. Based on intraoperative and radiological findings, the bone quality was assessed as follows: D1 in n = 2, D2 in n = 3, D3 in n = 2 and D4 in n = 2 cases. The employed implant dimensions were as follows: 4.1 × 5 mm (n = 2), 4.1 × 6 mm (n = 1), 4.1 × 7 mm (n = 10) and 3.75 × 7 mm (n = 17). The expansion process could successfully be performed in every case. The healing period was uneventful. Patients were rehabilitated with fixed dentures in 5 cases and with removable dentures in 4 cases. Basic clinical characteristics are summarised in detail in Table 4.
Over the 3-year follow-up period, the overall cumulative implant success rate in these patients was 28/30 (93.3%). Two implants were lost in the posterior maxilla. The two affected patients had highly atrophic posterior maxillae (Cawood et Howell IV–V) [38] and a bone quality of D3–D4 (Table 4). The male patient was a smoker and suffered from a squamous cell carcinoma of mouth floor. In both cases, the manufactured removable denture was successfully relined and no technical complications were observed to date.
The Kaplan-Meyer analysis of implant survival for both jaws is visualised in Fig. 2 (log rank test, p = 0.173): 1-year survival 96.7% and 2-year survival 93.3%. The 3-year follow-up has not yet been completed by all patients (Table 4).
Cumulative implant survival over the follow-up period. The Kaplan-Meyer diagram visualises the analysis of implant survival in the maxilla and in the mandible (log rank test, p = 0.173) over the follow-up period up to 37 months (Table 4)
Measurements of implant stability by resonance frequency analysis (RFA) displayed the following ISQ values: primary stability 69.7 ± 10.3 95% CI (65.9; 73.6) ISQ units and secondary stability 69.8 ± 10.2 95% CI (65.8; 73.5) ISQ units (Fig. 3a, b). The differences were not statistically significant (p = 0.780; paired t test). In detail, the ISQ values for primary stability displayed in the maxilla 66.9 ± 8.9 95% CI (61.9; 71.8), and in the mandible 72.5 ± 11.1 95% CI (66.4; 78.7). The differences were not statistically significant (p = 0.134; independent t test). According to the measurement of secondary implant stability, we observed comparable ISQ values in the maxilla 66.4 ± 10.0 95% CI (60.9; 71.9) and higher ISQ values in the mandible 73.0 ± 9.7 95% CI (67.6; 78.4). The differences were as well not statistically significant (p = 0.780; independent t test).
a Primary implant stability. The histogram visualises the distribution of the implant stability quotients (ISQ) for both jaws measured by resonance frequency analysis (Osstell AB, Göteborg, Sweden). b Secondary implant stability. The histogram shows the distribution of the implant stability quotients (ISQ) of osseointegrated implants. According to the measurement implant stability was classified as low with ISQ values < 60, medium with ISQ values 60–70, and high with values ISQ > 70 [34]
Over the follow-up period, the mean crestal bone changes after loading were as follows (each compared to the baseline): in the first year, 1.0 ± 0.9 mm 95% CI (0.5; 1.5) in the maxilla and 0.7 ± 0.4 mm 95% CI (0.5; 1.0) in the mandible (p = 0.011; independent t test), and in the second year, 1.3 ± 0.8 mm 95% CI (0.8; 1.7) in the maxilla and 1.0 ± 0.7 mm 95% CI (0.6; 1.4) in the mandible (p = 0.644; independent t test). Clinical and radiological investigations did not reveal any inflammatory signs or radiolucency in the periapical region for all inserted implants.
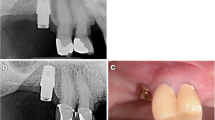
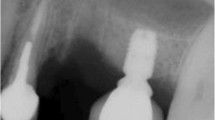
A representative case of a rehabilitated female patient is visualised in Fig. 4a–h and Fig. 5a–d (radiograms).
Discussion
Recent literature has shown that short implants have achieved growing acceptance in the field of oral implantology [9, 10, 39, 40]. Since the last years, concern has decreased about the length of endosseous implants; it should be noted that all extraoral screw implants are short implants [41, 42]. Nevertheless, there are local physiological and biomechanical differences regarding long-term stability.
The survival rate of short dental implants was found to increase from 80 to > 90% over time [39]. This is also confirmed in recent studies. For short dental implants supporting single crowns and fixed bridges especially in the mandible, a 2-year success rate of 97% [43] and a 5-year outcome of 92.2% [10] are reported. Otherwise, the success rate of 100% in the maxilla (3-year outcome) [15] must be critically questioned in view of our findings.
Only a few reports in the literature have addressed expandable dental implants [44,45,46,47]. In 2001, Jo and co-authors reported about a 40-month prospective survival of an expandable standard-length implant (10–16 mm) for immediate loading. They found a 3-year survival rate of 96.1% in the maxilla and of 94.8% in the mandible [46]. Huré and co-authors [47] performed a biomechanical and histologic canine study on early loaded expandable implants of 10- and 11.5-mm lengths. Six years later (2010), in orthopaedic surgery, an expandable implant was introduced [48]. Similar with the present study, these authors addressed implants under difficult regional bony conditions.
The purpose of our study was to evaluate the intraoperative handling, safety and feasibility of a new expandable dental implant system in a heterogeneous study cohort. We found in the present pilot study an overall 3-year implant success rate of 93.3%, which is comparable with recent literature [39]. To the best of our knowledge (PubMed), the present clinical study is the first published investigation about the usage of an expandable short dental implant system. Therefore, directly comparable data from other clinical studies are missing.
Several investigators analysed the preferred indications of short dental implants in the posterior mandible or maxilla and outlined the cost efficiency compared to additional vertical augmentations. In the present trial, we used a new short implant in both jaws and nearly all possible indication categories were represented, which proves the broad versatility (Table 4).
In our study, two implant failures occurred early in the prosthetic period and under loading. In a former systematic review, 11 studies reported more short implant failures before loading, while 7 studies reported more implant failures after loading [39]. Regarding the implant success rate in the present study, it must be considered that the lost implants were associated with difficult surgical conditions. Besides biological failures, in this study cohort, no technical complications were observed. In accordance with earlier comparative studies, it is evident that when using short implants, there is a lower risk of complications compared to augmentation [4, 7, 8] and nerve lateralisation [40].
Why design modifications? This is a matter of reduction of the healing period [46], the gain of stability under difficult conditions and increased bone-to-implant contact [27, 49,50,51] and the fact that most complications of short implants occur in the preprosthetic period [39]. It is also a question of long-term crestal bone stability. Earlier biomechanical finite-element studies confirmed that apical expansion results in favourable stress reduction in the crestal bone of nearly 10% [52]. It is assumable that additionally to the microthread and platform-switching concept [53], the periimplant bone strain could be reduced by apical expansion. This issue requires separate consideration in further studies. The employed implant design (especially its 7-mm length) combines several favourable biomechanical features, which were considered in this study (Fig. 1a, d).
According to Gehrke and co-authors, and in relation to the present study, the apical implant design influences the implant stability and bone-to-implant contact [54]. The expansion procedure presents an additional bicortical anchorage [17] in the oro-vestibular direction. In hard bone, this might be a disadvantage and lead to asymmetrical expansion. Manufacturers’ recommendations for hard bone should be strongly considered.
Regarding resonance frequency analysis, the values are related to bone quality and quantity as well as the exposed implant height above the alveolar crest, which depends on the type of implant and insertion technique [55,56,57]. Our results (primary stability in the maxilla 66.9 ± 8.9 ISQ units and in the mandible 72.5 ± 11.1 ISQ units; secondary stability in the maxilla 66.4 ± 10.0 ISQ units and in the mandible 73.0 ± 9.7 ISQ units) are comparable with the results from Becker and co-authors (standard-length implants): primary stability 72.1 ISQ units and secondary stability 72.6 ISQ units [58]. These values are marginally lower than those of short implants inserted only in the posterior mandible (79.0 ISQ units) [12]. Other authors measured in the posterior maxilla 68.2 ISQ units (6-mm implants) [15]. Altogether, our mean results (Fig. 2a, b) represent high stability values [34]. Huré and co-authors [47] measured in their animal study the following stability values (expandable implant of ≥ 10-mm length): for primary stability, 53.6 ± 3.0 ISQ units, and for secondary stability (3 months after insertion), 59 ± 4.5 ISQ units. The evaluation of stability values during the osseointegration period was not possible in our trial due to submerged healing. The question, whether the level of implant stability achieved at insertion can be maintained during the early healing period, remains. This should be analysed separately for all bone types in front of the known lowest stability values at 3–4 weeks after placement for all bone types [59,60,61] and the recent attempts of immediate [14] or early (6 weeks) functional loading of other short implant systems [28]. In relation to the results by McCullough and Klokkevold [62], who found that the macrothread design appears to play a positive role in implant stability in the early healing period, this can also be assumed for the employed implant system. Additionally, with regard to the results by Marković and co-authors [61], a critical stability drop down due to bone remodeling after bone condensing (implant site preparation and/or using expandable implants) should not be suspected; the opposite can be expected. The authors analysed the implant stability (4.1 × 10-mm screw implant) in the posterior maxilla in vivo depending on the implant site preparation (bone condensing vs. bone drilling) and confirmed that, after bone condensing, significantly higher implant stability results were achieved, immediately after implant insertion as well as during the whole observation period of 6 weeks. Especially in the third week in both groups, the following results were measured: 66.7 ± 1.64 vs. 57.1 ± 1.45 (p < 0.001). [61]. In the present study, we measured in the posterior maxilla 66.3 ± 10.4 ISQ units for primary stability and 66.9 ± 12.0 ISQ units for secondary stability, respectively.
Contrary to conventional hollow-screw implants (only marginal gap), a problem of the expandable implant is the presence of gaps down to the apical region. Former microbial assessment of different implant-abutment interfaces displayed that none of the marginal connections had the capacity to prevent microbial leakage [63,64,65]. Therefore, an apical microleakage (comparable to distractable implants and endodontically treated teeth) might be a disadvantage of the evolved implant system [66, 67]. However, according to the manufacturer’s information, a microbiological study revealed no microbial leakage through the expanded implants. Over the follow-up period, we equally did not observe any inflammatory signs in the apical region, neither clinically nor radiologically (Figs. 4b and 5c–d). Nevertheless, this aspect should be analysed under mechanical loading in vitro. Based on an earlier animal histologic study [47], as well as a clinical up-to-40-month study [46], which referred to comparable apically expandable implants, authors did not report any periapical inflammatory complications. To eliminate the potential risk of deep intrabony microleakage, it is questionable whether equal biomechanical stability values can be achieved only by the macrothread design avoiding any deep microgaps.
In the present study, the crestal bone changes under loading in the first year exceeded that of the second year. Moreover, the differences between the maxilla and mandible in each year were not statistically significant, which only partially agrees with previous findings in the literature [7, 58]. Besides microbiological conditions, there are several biomechanical aspects which influence maintenance of periimplant crestal bone. Conical and parallel surfaces of the implant-abutment connection (internal hexagon) provide rotational stability and reduce microgaps and micromovement [68]. Another important factor is the thickness of the implant shoulder [69], which might be a weak point in the design of a short implant due to elastic deformity under extra-axial loading. This fact might be the reason for non-inflammatory periimplant crestal bone loss. We addressed this aspect by splitting adjacent implants wherever possible [50, 51]. According to Brenner and co-authors [30] as well as Pommer and co-authors [50], the following prosthodontic factors are to be considered to avoid screw loosening, component fracture, loss of marginal bone or even loss of osseointegration: crown-to-implant ratio (extra-axial loading), cantilever length, status of opposing dentition, splinting of adjacent implants, occlusal surface relief and dimensions.
Comparable studies displayed at 24 months a crestal bone loss of 0.5–0.6 mm [15]. Other authors reported at 2, 3 and 5 years a mean loss of 0.57, 0.55 and 0.53 mm, respectively, in the mandible (without significant change after 1 year) [10]. On the other hand, randomised controlled trials demonstrated 1 year after loading periimplant marginal bone loss of 0.7 mm [70] and 1.1 mm [7] in the mandible which is the same value measured in the present study.
Within the limitations of a pilot study design, low number of implants, single-arm study and short-term follow-up, the results show a basic improvement of functional rehabilitation especially for elderly patients with compromised general and local conditions for implantation. Controversial questions [5, 71] remain on whether (a) short implants are suitable for irradiated patients and (b) there is a need for expandable short implants in the D1 bone. Furthermore, potential bias should be eliminated in future studies by a randomised controlled trial.
Conclusion
Initial results of the ongoing study confirm the feasibility and safety of the employed system. The implant type seems to be useful for all bone qualities and shows high initial and secondary biomechanical stability in the maxilla and mandible. Long-term follow-up will be needed in validating these initial results in a larger 3-year clinical trial. Crestal bone changes should be evaluated in a larger study cohort. The novel system might extend the spectrum in functional rehabilitation.
Abbreviations
- D1, D2, D3, D4:
-
Bone quality (density)
- FDI:
-
World Dental Federation
- ISQ:
-
Implant stability quotient
- RFA:
-
Resonance frequency analysis
References
Schwarz F, Terheyden H. Significance of dental implants for health care. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2011;54(9):1097–101. (German)
Gates WD, Cooper LF, Sanders AE, Reside GJ, De Kok IJ. The effect of implant-supported removable partial dentures on oral health quality of life. Clin Oral Impl Res. 2014;25:207–13.
Fretwurst T, Nack C, Al-Ghrairi M, Raguse JD, Stricker A, Schmelzeisen R, Nelson K, Nahles S. Long-term retrospective evaluation of the peri-implant bone level in onlay grafted patients with iliac bone from the anterior superior iliac crest. J Craniomaxillofac Surg. 2015;43(6):956–60.
Verhoeven JW, Rujiter J, Cune MS, Terlou M, Zoon M. Onlay grafts in combination with endosseous implants in severe mandibular atrophy: one year results of a prospective, quantitative radiological study. Clin Oral Implants Res. 2000;11(6):583–94.
Nkenke E. Short implants. Do they replace reconstruction of the alveolar crest? MKG-Chirurg. 2013;6:221–7. (German)
Al-Hashedi AA, Ali TB, Yunus N. Short dental implants: an emerging concept in implant treatment. Quintessence Int. 2014;45(6):499–514.
Esposito M, Barausse C, Pistilli R, Sammartino G, Grandi G, Felice P. Short implants versus bone augmentation for placing long implants in atrophic maxillae: one-year post-loading results of a pilot randomised controlled trial. Eur J Oral Implantol. 2015;8(3):257–68.
Schincaglia GP, Thoma DS, Haas R, Tutak M, Garcia A, Taylor TD, Hämmerle CH. Randomised controlled multicenter study comparing short dental implants (6 mm) versus longer dental implants (11-15 mm) in combination with sinus floor elevation procedures. Part 2: clinical and radiographic outcomes at 1 year of loading. J Clin Periodontol. 2015;42(1):1042–51.
Srinivasan M, Vazquez L, Rieder P, Moraguez O, Bernard JP, Belser UC. Survival rates of short (6 mm) micro-rough surface implants: a review of literature and meta-analysis. Clin Oral Implants Res. 2014;25(5):539–45.
Slotte C, Grønningsaeter A, Halmøy AM, Ohrnell LO, Mordenfeld A, Isaksson S, Johansson LA. Four-milimeter-long posterior-mandible implants: 5-year outcomes of a prospective multicenter study. Clin Implant Dent Relat Res. 2015;17(Suppl 2):e385–95.
Thoma DS, Haas R, Tutak M, Garcia A, Schincaglia GP, Hämmerle CH. Randomised controlled multicenter study comparing short dental implants (6 mm) versus longer dental implants (11-15 mm) in combination with sinus floor elevation procedures. Part 1: demographic and patient-reported outcomes at 1 year of loading. J Clin Periodontol. 2015;42(1):72–80.
Hentschel A, Herrmann J, Glauche I, Vollmer A, Schlegel KA, Lutz R. Survival and patient satisfaction of short implants during the first 2 years of function: a retrospective cohort study with 694 implants in 416 patients. Clin Oral Impl Res. 2016;27:591–6.
Fan T, Li Y, Deng WW, Wu T, Zhang W. Short implants (5-8 mm) versus longer implants (>8 mm) with sinus lifting in atrophic posterior maxilla: a meta-analysis of RCTs. Clin Implant Dent Relat Res. 2017;19(1):207–15.
Anitua E, Flores J, Flores C, Alkhraisat MH. Long-term outcomes of immediate loading of short implants: a controlled retrospective cohort study. Int J Oral Maxillofac Implants. 2016;31(6):1360–6.
Bechara S, Kubilius R, Veronesi G, Pires JT, Shibli JA, Mangano FG. Short (6-mm) dental implants versus sinus floor elevation and placement of longer (≥10 mm) dental implants: a randomized controlled trial with a 3-year follow-up. Clin Oral Implants Res. 2016. doi:10.1111/cir.12923.
Zöller J, Neugebauer J. Update on short, angulated and diameter-reduced implants. Consensus paper of the 11th European Consensus Conference (EuCC) 2016 in Cologne. https://www.bdizedi.org/. Accessed 01 Oct 2017 (German).
Pierrisnard L, Renouard F, Renault P, Barquins M. Influence of implant length and bicortical anchorage on implant stress distribution. Clin Implant Dent Relat Res. 2003;5(4):254–62.
Geng JP, Tan KB, Liu GR. Application of finite element analysis in implant dentistry: a review of the literature. J Prosthet Dent. 2001;85(6):585–98.
Baggi L, Capelloni I, Di Girolamo M, Maceri F, Vairo G. The influence of implant diameter and length on stress distribution of osseointegrated implants related to crestal bone geometry: a three-dimensional finite element analysis. J Prosthet Dent. 2008;100(6):422–31.
De Moraes SL, Verri FR, Santiago JF Jr, Almeida DA, de Mello CC, Pellizzer EP. A 3-D finite element study of the influence of crown-implant ratio on stress distribution. Braz Dent J. 2013;24(6):635–41.
Moraes SL, Pellizzer EP, Verri FR, Santiago JF Jr, Silva JV. Three-dimensional finite element analysis on stress distribution in retention screws of different crown-implant ratios. Comput Methods Biomech Biomed Engin. 2015;18(7):689–96.
Petrie CS, Williams JL. Comparative evaluation of implant designs: influence of diameter, length, and taper on strains in the alveolar crest. A three-dimensional finite-element analysis. Clin Oral Implants Res. 2005;16(4):486–94.
Möhlhenrich SC, Heussen N, Elvers D, Steiner T, Hölzle F, Modabber A. Compensating for poor primary implant stability in different bone densities by varying implant geometry: a laboratory study. Int J Oral Maxillofac Surg. 2015;44:1514–20.
Yazicioglu D, Bayram B, Oguz Y, Cinar D, Uckan S. Stress distribution on short implants at maxillary posterior alveolar bone model with different bone-to-implant contact ratio: finite elemnt analysis. J Oral IMplantol. 2016;42(1):26–33.
Sivan-Gildor A, Machtei EE, Gabay E, Frankenthal S, Levin L, Suzuki M, Coelho PG, Zigdon-Giladi H. Novel implant design improves implant survival in multirooted extraction sites: a preclinical pilot study. J Periodontol. 2014;85:1458–63.
Jain N, Gulati NJM, Garg M, Pathak C. Short implants: new horizon in implant dentistry. J Clin Diagn Res. 2016;10(9):ZE14–7.
Quaranta A, D’Isidoro O, Bambini F, Putignano A. Potential bone to implant contact area of short versus standard implants: an in vitro micro-computed tomography analysis. Implant Dent. 2016;25(1):97–102.
Makowiecki A, Botzenhart U, Seeliger J, Heinemann F, Biocev P, Dominiak M. A comparative study of the effectiveness of early and delayed loading of short tissue-level dental implants with hydrophilic surfaces placed in the posterior section of the mandible—a preliminary study. Ann Anat. 2017;212:61–8.
Cohen O, Ormianer Z, Tal H, Rothamel D, Weinreb M, Moses O. Differences in crestal bone-to-implant contact following an under-drilling compared to an over-drilling protocol. A study in the rabbit tibia. Clin Oral Investig. 2016;20(9):2475–80.
Brenner M, Brandt J, Lauer HC. Prothetische Versorgung auf kurzen Implantaten. Zahnmedizin up2date. 2014;2:123–42. (German)
Eitner S, Wichmann M, Schlegel KA, Kollmannberger JE, Nickenig HJ. Oral health-related quality of life and implant therapy: an evaluation of preoperative, intermediate, and post-treatment assessments of patients and physicians. J Craniomaxillofac Surg. 2012;40(1):20–3.
Buser D, Weber HP, Lang NP. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin Oral Impl Res. 1990;1:33–40.
Karoussis IK, Brägger U, Salvi GE, Bürgin W, Lang NP. Effect of implant design on survival and success rates of titanium oral implants: a 10-year prospective cohort study of the ITI dental implant system. Clin Oral Implants Res. 2004;15(1):8–17.
Sennerby L. 20 years of experience with resonance frequency analysis. Implantologie. 2013;21(1):21–33. (German)
Terheyden H. Indikationsklassen für Implantatversorgung zur Regelversorgung. Consensus conference implantology. 2014. https://www.konsensuskonferenz-implantologie.eu/. Accessed 05 May 2014 (German).
Gross MD. Occlusion in implant dentistry. A review of the literature of prosthetic determinants and current consepts. Aust Dent J. 2008;53(Suppl1):S60–8.
Gomez-Roman G, Schulte W, d’Hoedt B, Axman-Krcmar D. The Frialit-2 implant system: five-year clinical experience in single-tooth and immediately postextraction applications. Int J Oral Maxillofac Implants. 1997;12(3):299–309.
Cowood JI, Howell RA. A classification of the edentulous jaws. Int J Oral Maxillofac Surg. 1988;17:232.
Karthikeyan I, Desai SR, Singh R. Short implants: a systematic review. J Indian Soc Periodontol. 2012;16(3):302–12.
Dursun E, Keceli HG, Uysal S, Güngör H, Muhtarogullari M, Tözüm TF. Management of limited vertical bone height in the posterior mandible: short dental implants versus nerve lateralization with standard length implants. J Craniofac Surg. 2016;27(3):578–85.
Tjellström A, Lindström J, Nylén O, Albrektsson T, Brånemark PI, Birgersson B, Nero H, Sylvén C. The bone-anchored auricular episthesis. Laryngoscope. 1981;91(5):811–5.
Choi KJ, Sajisevi MB, McClennen J, Kaylie DM. Image-guided placement of osseointegrated implants for challenging auricular, orbital, and rhinectomy defects. Ann Otol Rhinol Laryngol. 2016;125(10):801–7.
Malmstrom H, Gupta B, Ghanem A, Cacciato R, Ren Y, Romanos GE. Success rate of short dental implants supporting single crowns and fixed bridges. Clin Oral Implants Res. 2016;27(9):1093–8.
Garfield RE. An expandable implant fixture. Dent Implantol Updat. 1998;9(5):37–40.
Nowzari H, Chee W, Tuan A, Abou-Rass M, Landesman HM. Clinical and microbiological aspects of the Sargon immediate load implant. Compend Contin Edu Dent. 1998;19(7):686–9.
Jo HY, Hobo PK, Hobo S. Freestanding and multiunit immediate loading of the expandable implant: an up-to-40-month prospective survival study. J Prosthet Dent. 2001;85(2):148–55.
Huré G, Aguado E, Grizon F, Baslé MF, Chappard D. Some biomechanical and histologic characteristics of early-loaded locking pin and expandable implants: a pilot histologic canine study. Clin Implant Dent Relat Res. 2004;6(1):33–9.
Folman Y, Ron N, Shabat S, Romano G, Galasso O. The fixation expandable stem hemiarthoroplasty for displaced femoral neck fracture: technical features and pilot study. Arch Orthop Trauma Surg. 2010;130(4):527–31.
Xiao JR, Li DH, Chen YX, Chen SJ, Guan SM, Kong L. Evaluation of fixation of expandable implants in the mandibles of ovarectomized sheep. J Oral Maxillofac Surg. 2013;71:682–8.
Pommer B, Hingsammer L, Haas R, Mailath-Pokorny G, Busenlechner D, Watzek G, Fürhauser R. Denture-related biomechanical factors for fixed partial dentures retained on short dental implants. Int J Prosthodont. 2015;28(4):412–4.
Kim Y, Oh TJ, Misch CE, Wang HL. Occlusal considerations in implant therapy: clinical guidelines with biomechanical rationale. Clin Oral Implants Res. 2005;16(1):26–35.
Xiao JR, Li YF, Guan SM, Song L, Xu LX, Kong L. The biomechanical analysis of simulation implants in function under osteoporotic jawbone by comparing cylindrical, apical tapered, neck tapered, and expandable type implants: a 3-dimensional finite element analysis. J Oral Maxillofac Surg. 2011;69:e273–81.
Yun HJ, Park JC, Yun JH, Jung UW, Kim CS, Choi SH, Cho KS. A short-term clinical study of marginal bone level change around microthreded and platform-switched implants. J Periodontal Implant Sci. 2011;41(5):211–7.
Gehrke SA, Pérez-Albacete Martinez C, Piattelli A, Shibli JA, Markovic A, Calvo Guirado JL. The influence of three different apical implant designs at stability and osseointegration process: experimental study in rabbits. Clin Oral Implants Res. 2017;28(3):355–61.
Herrero-Climent M, Santos-Garcia R, Jaramillo-Santos R, Romero-Ruiz MM, Fernandez-Palacin Lazaro-Calvo P, Bullon P, Rios-Santos JV. Assesment of Osstell ISQ’s reliability for implant stability measurement: a cross-sectional clinical study. Medicina Oral Pathologia Oral y Cirugia buccal. 2013;18:e877–82.
Gupta RK, Padmanabhan TV. Resonance frequence analysis. Indian J Dent Res. 2011;22(4):567–73.
Kang IH, Kim CW, Lim YJ, Kim MJ. A comparative study on the initial stability of different implants placed above the bone level using resonance frequence analysis. J Adv Prosthodont. 2011;3(4):190–9.
Becker W, Becker BE, Hujoel P, Abu Raz Z, Goldstein M, Smidt A. Prospective clinical trial evaluating a new implant system for implant survival, implant stability and radiographic bone changes. Clin Implant Dent Relat Res. 2013;15(1):15–21.
Barewal RM, Oates TW, Meredith N, Cochrane DL. Resonance frequency measurement of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int J Oral Maxillofac Implants. 2003;18(5):641–51.
Huwiler MA, Pjetursson BE, Bosshardt DD, Salvi GE, Lang NP. Resonance frequency analysis in relation to jawbone characteristics and during early healing of implant instillation. Clin Oral Implants Res. 2007;18(3):275–80.
Marković A, Calasan D, Colić S, Stojčev-Stajčć L, Janjić B, Mišić T. Implant stability in posterior maxilla: bone-condensing versus bone-drilling: a clinical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(5):557–63.
McCullough JJ, Klokkevold PR. The effect of implant macro-thread design on implant stability in the early post-operative period: a randomized, controlled pilot study. Clin Oral Implants Res. 2016. doi:10.1111/cir.12945.
Teixeira W, Ribeiro RF, Sato S, Pedrazzi V. Microleakage into and from two-stage implants: an in vitro comparative study. Int J Oral Maxillofac Implants. 2011;6(1):56–62.
Canullo L, Penarrocha-Oltra D, Soldini C, Mazzocco F, Penarrocha M, Covani U. Microbial assessment of the implant-abutment interface in different connectioins: cross-sectional study after 5 years of functional loading. Clin Oral Implants Res. 2015;26(4):426–34.
Mishra SK, Chowdhary R, Kumari S. Microleakage at different implant abutment interface: a systematic review. J Clin Diagn Res. 2017;11(6):ZE10–5.
Feichtinger M, Gaggl A, SChultes G, Kärcher H. Evaluation of distraction implants for prosthetic treatment after vertical alveolar ridge distraction: a clinical investigation. Int J Prosthodont. 2003;16(1):19–24.
Pradeep PR, Kasti KJ, Ananthakrishna S, Raghu TN, Vikram R. Evaluation of different dentin adhesive systems and its effect on apical microleakage: an in vitro study. J Int Oral Health. 2015;7(5):44–8.
Streckbein P, Streckbein RG, Wilbrand JF, Malik CY, Schaaf H, Howaldt HP, Flach M. Non-linear 3D evaluation of different oral implant-abutment connections. J Dent Res. 2012;91(12):1184–9.
Pellizzer EP, de Mello CC, Santiago Junior JF, de Souza Batista VE, de Faria Almeida DA, Verri FR. Analysis of the biomechanical behavior of short implants: the photo-elasticity method. Mater Sci Eng C Mater Biol Appl. 2015;55:187–92.
Felice P, Pistilli R, Barausse C, Bruno V, Trullenque-Eriksson A, Esposito M. Short implants as an alternative to crestal sinus lift: a 1-year multicentre randomised controlled trial. Eur J Oral Implantol. 2015;8(4):375–84.
Schwartz SR. Short implants: are they a viable option in implant dentistry? Dent Clin N Am. 2015;59(2):317–28.
Granström G. Osseointegration in irradiated cancer patients. An analysis with respect to implant failures. J Oral Maxillofac Surg. 2005;63:579–85.
Grötz KA, Schmidt BLJ, Walther C, Al-Nawas B. In which bisphosphonate patients am I allowed to place implants? A systematic review. Z Zahnärztl Impl. 2010;26(2):153–61. (German)
Acknowledgements
The authors are grateful to the DenTack Company for providing some of the implants and components used in this study. The courtesy of Oz Vachtenberg is acknowledged for providing Fig. 1a–f and the movie clip. There are no financial supports or conflicts of interest associated with this publication that could have influenced its outcome. We thank the Osstell Company, which supplied the RFA device and Smartpegs used in this study. We acknowledge American Journal Experts (AJE) for providing English language editing (certificate verification key: 06DA-C0B2-2672-4103-A251). In addition, the authors thank the dental technicians from the dental laboratory Rübeling and Klar, Berlin, Germany, for their kind support in manufacturing all dental prostheses. Furthermore, we thank all involved members of the surgical and prosthodontic teams.
Funding
No funding received.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article and its additional files.
Author information
Authors and Affiliations
Contributions
WR was responsible for the preparation of the study protocol, surgical treatment, data acquisition, statistics and preparation of the manuscript. RS was responsible for prosthetic treatment. CH was responsible for surgical treatment, radiological analysis and statistics. JH was responsible for preparation of the study protocol, prosthetic treatment and interpretation of the data. BA was responsible for interpretation of the data and approval of the final manuscript version to be submitted. AWE was responsible for the approval of the study protocol and interpretation of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
WR is a senior physician at the University Hospital Halle, Martin Luther University Halle-Wittenberg, Department of Oral and Plastic Maxillofacial Surgery, Halle/Saale, Germany.
RS is a clinical research associate at the University School of Dental Medicine, Department of Prosthetic Dentistry, Martin Luther University Halle-Wittenberg, Halle/Saale, Germany.
CH is a consultant at the University Hospital Halle, Martin Luther University Halle-Wittenberg, Department of Oral and Plastic Maxillofacial Surgery, Halle/Saale, Germany.
JH is an assistant professor at the University School of Dental Medicine, Department of Prosthetic Dentistry, Martin Luther University Halle-Wittenberg, Halle/Saale, Germany.
BA is professor and the head of the department at the University Hospital Halle, Martin Luther University Halle-Wittenberg, Department of Oral and Plastic Maxillofacial Surgery, Halle/Saale, Germany.
AWE is a senior assistant professor at the University Hospital Halle, Martin Luther University Halle-Wittenberg, Department of Oral and Plastic Maxillofacial Surgery, Halle/Saale, Germany.
Ethics approval and consent to participate
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee (date of issue 21 August 2014, registration number 2014-60) and with the 1964 Helsinki declaration and its later amendments.
Informed consent was obtained from all individual participants included in this study.
Consent for publication
Not applicable
Competing interests
Waldemar Reich, Ramona Schweyen, Christian Heinzelmann, Jeremias Hey and Alexander Walter Eckert declare no competing interest.
Bilal Al-Nawas is giving lectures for Straumann, Camlog, Dentsply, Nobel biocare, Geistlich.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 2:
Dataset presenting relevant raw data. (SAV 3 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Reich, W., Schweyen, R., Heinzelmann, C. et al. Novel expandable short dental implants in situations with reduced vertical bone height—technical note and first results. Int J Implant Dent 3, 46 (2017). https://doi.org/10.1186/s40729-017-0107-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40729-017-0107-1