Abstract
Purpose of review
Chronic kidney disease affects approximately 3 million Canadians. Ongoing investment in high quality kidney research is needed to improve the care of patients with kidney disease. The barriers to translating such research are discussed in this review.
Sources of information
Personal knowledge, research funding body websites, and published reports.
Findings
In this review, we discuss the meaning of the term translational research and present some of the programs aimed at ensuring efficient translation of scientific discoveries with a discussion of the barriers to translation. We highlight some successes and barriers to kidney research translation using recent examples of research in Canadian nephrology. We present the following examples of kidney research: (1) research aimed at identifying the causative genes for inherited kidney diseases; (2) recent discoveries in cell-based therapies for kidney disease; (3) an examination of the impact of acute kidney injury in renal transplant patients; and (4) the development of a kidney failure risk equation to improve prognosis accuracy.
Limitations
This review focuses on research conducted by the authors.
Implications
The process of research translation is prolonged and challenging and therefore requires resources, patience, and careful planning. With increased awareness and understanding of the barriers to research translation, researchers and funding bodies can work together to increase the rate at which important research findings reach clinical practice and improve the care of patients with kidney disease.
Abrégé
Objectif de l’étude
La néphropathie chronique touche environ 3 millions de Canadiens. Un investissement soutenu dans la recherche de haute qualité en néphrologie est nécessaire à l’amélioration des soins aux patients. Dans cette étude, nous abordons les obstacles à l’application de ces recherches.
Sources d’information
Les connaissances personnelles, les sites Web d’organismes de financement de la recherche, et les rapports publiés.
Résultats
Dans cette étude, nous traitons de la signification du terme « recherche translationnelle » et présentons certains des programmes visant à assurer une circulation efficace des découvertes scientifiques, en abordant les obstacles à la circulation et à l’application. Nous présentons des réussites de circulation de la recherche en néphrologie, de même que certains obstacles, en recourant à des exemples récents de recherche canadienne en néphrologie. Nous citons les exemples suivants de recherche en néphrologie: (1) la recherche visant à déterminer les gènes responsables des néphropathies héréditaires; (2) les découvertes récentes en matière de thérapies cellulaires pour les néphropathies; (3) l’examen des conséquences d’une insuffisance rénale aiguë chez les patients ayant subi une transplantation rénale; et (4) l’élaboration d’une équation concernant le risque d’insuffisance rénale afin d’améliorer la précision du pronostic.
Limites de l’étude
L’étude se concentre sur les recherches effectuées par les auteurs.
Conséquences
Le processus de circulation et d’application de la recherche est long et ardu, si bien qu’il requiert des ressources, de la patience et une planification étroite. Grâce à une compréhension et une sensibilisation accrues des obstacles à la circulation de la recherche, les chercheurs et les organismes de financement peuvent travailler ensemble afin d’accroître le rythme avec lequel les importantes conclusions de recherches atteignent la pratique clinique et améliorent les soins aux patients atteints de néphropathies.
Similar content being viewed by others
What was known before
There is a rising awareness of the importance of research translation in the research community. Funding bodies are recognizing that increased resources are needed to improve the success rate of translating research findings into clinical practice.
What this adds
Through specific examples of Canadian nephrology research, the process of research translation is presented, with a particular emphasis on the barriers to translation.
Lay summary
What is translational research? Often this term is synonymous with “bench-to-bedside”, meaning to find a way to transform laboratory research into new medicines or treatments for patients. Going one step further, translational research also involves making sure that health care providers are consistently providing recommended treatments to patients. This review discusses the meaning of translational research within the context of kidney research. We also highlight the barriers that prevent the movement of research findings from the laboratory into clinical practice. Funding bodies and agencies have given more resources to translational research and have established specific programs to this end. This will hopefully help overcome the barriers of research translation. Here we discuss, Canadian research, funded by Canadian taxpayers and donors and performed in the context of a Canadian national training program, with an emphasis on the translational aspects of our work. Included are looks at a basic science approach to identifying inherited diseases with kidney involvement, cell-based therapies for kidney disease, an examination of the factors involved in deciding a treatment plan following acute kidney injury, and consideration of risk-based care for patients with chronic kidney disease. Where do we go next? How do we ensure the findings of current kidney research are extended into clinical practice? The road ahead looks promising as funding agencies stress a balanced approach for both basic science and clinical research, and a focus on programs directed at translating research findings.
Introduction
Translational research has become a popular term in the research community, and may have different meanings depending on the specific situation. When using the term translational research, many are referring to the concept of “bench-to-bedside” where knowledge from basic science is used to produce new drugs and treatment options for patients. For others, translational research refers to translating research into clinical practice, also known as knowledge translation, meaning: “the new treatments and research knowledge actually reach the patients or populations for whom they are intended and are implemented correctly [1]”.
The Institute of Medicine’s Clinical Research Roundtable originally described the model of translational research as a two-phase process progressing in the first phase from basic science to clinical research. They named this first phase T1. The second phase, also known as T2, was defined as the progression from clinical science to everyday clinical practice and health decision-making [1],[2]. In 2011, Drolet et al. further expanded on this model of translational research and developed what they called the Biomedical Research Translation Continuum, with three “translational chasms”. The first translational chasm (known as TI) represents the transition from basic science research to a proposed human or clinical application. Translational chasm 2 (T2) represents the transition from the proposed human application to a proven clinical application, i.e. evaluation of safety and efficacy using clinical trials. Finally, translational chasm 3 (T3) is the adoption into clinical practice or essentially knowledge translation [3]. The recent recognition of the importance of T3 has led to a growing area of practice-based research. This research can be used to determine if research findings are being adopted by clinicians and having an impact on public health. If practice-based research demonstrates that clinicians are not changing practice, then there has been a block at T3, and one must determine why the block is occurring.
Moving laboratory discoveries into the clinical realm followed by the ultimate goal of a change in clinical practice or public policy can prove to be extremely challenging with many barriers along the way. A study that reviewed articles published in leading basic science journals found that approximately 25% of articles that reported promising findings resulted in the publication of a randomized controlled trial, and less than 10% of the promising findings were introduced into clinical practice over a period of 20 years [4]. It can frequently take several years to bring discoveries made in the laboratory to the general public. In fact, studies that examined the average time from publication of a research finding to adoption into clinical practice have found that the process takes on average 17 years, a figure that has not changed much over the past century [5]. Also, a study conducted in the United States that assessed health care delivery and utilization by telephone survey and chart review found that only 55% of patients receive recommended care [6]. Taken together, these statistics illustrate that promising research findings rarely make it into clinical practice, and when they do, there is a significant lag time between discovery and implementation.
So why does the translation of research prove to be so difficult? Sung and colleagues refer to the barriers of translation as “translational blocks”, which can include financial constraints, regulatory agency burden, fragmented infrastructure, or a lack of qualified investigators [2]. If not anticipated and without the appropriate resources in place, these barriers will all result in a lack of research translation.
Despite the concept of translational research being introduced over 30 years ago [7], the subject has only become a major focus over the past decade. This is illustrated by a sharp rise in publications on the subject, with the number of publications increasing from only 5 in 1994 to 110 in 2007 [8]. Along with a large number of publications in the literature, research funding bodies and training programs have recognized the need in recent years to make translational research a priority.
The Canadian Institutes of Health Research (CIHR) has made translating knowledge from the research setting to the public a key component of its mandate, with funding and educational programs specifically dedicated to knowledge translation [9]. The CIHR defines knowledge translation as: “a dynamic and iterative process that includes synthesis, dissemination, exchange and ethically sound application of knowledge to improve the health of Canadians, provide more effective health services and products and strengthen the health care system”. A large driver of the emphasis on knowledge translation by the CIHR is the increased focus on research governance and accountability. The CIHR considers it a priority that taxpayers’ dollars invested into health research result in some sort of impact on patients [10].
The National Institutes of Health (NIH) established a National Center for Advancing Translational Sciences (NCATS) in 2011. Within the NCATS is the Clinical and Translational Science Award (CTSA) program, which supports a national consortium of more than 60 medical research institutions. Funding of NCATS has increased on an annual basis, and the requested budget for 2014 is over $665,000,000 [11]. The European Commission has also followed suit, allocating most of its 6 billion Euro health research budget for 2007–2013 to pan-European translational research projects. There are also a growing number of programs across Europe that offer training in translational research [12].
A kidney specific, national research program within Canada known as the Kidney Research Scientist Core Education and National Training (KRESCENT) program has also made knowledge translation a top priority [13]. The KRESCENT program was founded in 2004 and is headed by Dr. Kevin Burns, a nephrologist and basic scientist with the University of Ottawa and Dr. Adeera Levin, a nephrologist and clinical research scientist with the University of British Columbia. KRESCENT is a multi-partner collaboration founded by the Canadian Society of Nephrology, CIHR and the Kidney Foundation of Canada. The program emphasizes translational research by funding research fellowships to individuals from various disciplines. Members of the program range from dietitians to PhD scientists to nephrologists. The program meets on a bi-annual basis and members share their diverse research. The concept of “bench-to- bedside” is a focal point of the discussion [14]. Fellows within the KRESCENT program complete their research training equipped with the skills for research translation. This skill set is provided formally by guest speakers at the bi-annual meetings, assignments and grant writing seminars; and informally by the program heads and collaboration with other fellows in the program.
There is clearly rising awareness and funding for research translation. We present in this review four examples of recently published and ongoing Canadian translational research pertinent to nephrology. The research presented spans the continuum of basic science, clinical application, and public health policy, illustrating the breadth of work being done by the Canadian renal community. More importantly, these examples demonstrate that translation of research is not just a unidirectional process, but rather provides opportunities to move bidirectionally between the bench and the bedside to ask and answer important questions relevant to the kidney. The examples presented also highlight some of the key barriers to successful research translation.
Genetic disorders with renal involvement and cilia
Investigation of fundamental biological processes has provided critical insights into our understanding of how organs behave in health and disease. Specifically, interrogation of the phenotypic manifestations of gene mutations has informed our understanding of multiple diseases. In this regard, the kidney is no exception, as many genetic disorders have some level of renal involvement [15]–[17]. Whether these disorders solely affect the kidneys or multiple tissues within the body with accompanying renal dysfunction, often there are high rates of renal morbidity. Among these genetic disorders are the cystic kidney diseases polycystic kidney disease (PKD) and nephronophthisis (NPHP) [17],[18]. There are monogenic diseases that cause solely PKD or NPHP, but they can also occur as part of a more broad disorder such as Bardet-Biedl, Joubert, Senior–Løken, and Orofaciodigital syndromes where end stage renal disease (ESRD) is quite common [16]–[18]. The above listed diseases are among ciliopathies, where the molecular pathology is rooted in the cellular organelle, the cilium. Cilia are microtubule-based organelles that protrude from the surface of the cell with gated cytoplasm and membrane [17]–[19]. Primary (or non-motile) cilia are concentrated in several signaling pathways and serve as major sensors for the extracellular environment. In kidneys, primary cilia are present on tubule epithelial cells and are mechanosensory as well as chemosensory, in that they sense urine flow and the presence of signaling molecules [20]. Dysfunction in cilia, either from loss or alteration of a cilia-related gene, can lead to the above diseases, often resulting in over-proliferation of renal tubule cells and ultimately, cystogenesis [18],[20].
For many ciliopathies, researchers involved in basic scientific studies will have first characterized the causative gene. One example, is with IFTA-1 (intraflagellar transport subcomplex A – 1), studied in the model organism Caenorhabditis elegans [21]. In C. elegans, the IFTA-1 protein was shown to be a component of the IFT complex, a strong indication that mutation of the human ortholog WDR35 would likely result in typical ciliopathy phenotypes. Subsequently, two studies showed that mutations in IFTA-1/WDR35 (WD repeat protein 35) were causative in Sensenbrenner and short-rib polydactyly syndromes, both of which include cystic kidneys [18],[22],[23]. Previous characterization of a gene in model organisms can assist in narrowing in on causative mutations, as it did for IFTA-1/WDR35.
Another type of connection between basic and clinical science can come in the form of a comprehensive study of a novel gene. The discovery that TMEM237 (transmembrane protein 237 or JBTS14, Joubert syndrome 14) harbored causative mutations in ciliopathy patients provides such an example [24]. Common characteristics of Joubert syndrome include the molar tooth sign, hypotonia, ataxia, hyperpnea, and cystic kidneys [24]. This extensive study not only included data from the sequencing of Canadian and Austrian patients, there were also functional studies of the TMEM237 performed in C. elegans, Danio rerio and cultured mammalian cells. Together, these data provided a broad look into the function of this gene and the consequences of its loss on human health. One immediate impact from this discovery for patients and their families is genetic testing for patients to assist in diagnosis and for family members to identify disease mutation carriers. Two of the three equally contributing laboratories are from Canada.
Though the discovery of candidate and novel ciliopathy genes affects a relatively small number of patients directly, there is the potential for a broader impact. Immediate impacts include: enhanced genetic counseling for families of a diagnosed proband, faster or more accurate diagnosis through sequencing, and – though controversial – the potential for pre-implantation genetic screening [18]. Though genetic testing can identify a particular disease (or identify carriers of disease-causing mutations), the possibility of negative consequences exists, such as inability to obtain insurance coverage or potential negative psychological impact [25]. For example, six laboratories currently offer genetic testing for TMEM237/JBTS14, which is discussed above [26]. Another benefit derived from the deeper comprehension of the molecular pathology of each disease is the enriched understanding of human biological processes that may lead to targeted therapies.
Though loss or mutation of ciliary components leads to many disorders, cilia may also play a role in acute kidney injury. It has been shown in transplant patients as well as in mice that upon kidney injury, cilia become significantly shorter [27]–[31]. After the injury, during the repair phase, cilia were longer than in controls. The mechanism for this length control remains elusive and no role has been found for cilia or cilia-related signaling in kidney injury or subsequent recovery. Despite this, it is entirely possible that cilia do play a role in kidney injury and recovery because they function as the environmental sensor of the cell, sensing the surrounding conditions [30]. Changes in cilia length have been observed for many of the monogenic ciliopathies (and/or the corresponding model organisms) [18],[20],[32]. Because ciliopathy genes and kidney injury both result in alteration of cilia length, it is possible that common variants in these genes may alter ability and/or rate of recovery from kidney injury as well as potentially aiding in prediction of a patient’s prognosis. Likewise, if the role of cilia in recovery from kidney injury can be elucidated, there is the potential that it will aid in the discovery of targeted therapeutics. Therefore, research into monogenic kidney disorders may not only impact patients with these diseases, but also has the potential to enhance treatment of other disorders (Table 1).
While elucidating the role of cilia in kidney disease is an exciting and fascinating topic, this research is still far from the end goal of changing clinical practice. This research is still primarily at the basic science level and will need to overcome the barrier of transitioning to a proposed human or clinical application (T1). Presently, the primary human or clinical application of this research is genetic screening or counseling, which has its own barriers to widespread adoption into clinical practice due to concerns about cost and consequences for the patient, such as the ability to obtain insurance coverage.
Cell-based therapies for kidney disease
As discussed above, the study of fundamental biologic processes can provide important insights that ultimately inform prognostic and/or therapeutic applications of these discoveries. In most cases, as knowledge is translated towards models that more closely reflect human health and disease, further concept refinement is often required. An example of this refinement process is illustrated by the challenges that investigators have faced in translating exciting discoveries made more than 20 years ago suggesting that cell-based therapies have the potential to attenuate or even repair chronic organ injury. In the 1980’s and 1990’s, a number of groups described the presence of bone marrow-derived cell populations with the ability to regenerate and/or repair injured organs [33]–[35]. Recognizing the therapeutic potential of these fundamental discoveries, investigators began testing whether such cells could be grown in the lab and used as either a preventative or regenerative treatment for chronic injury of the kidney and other organs.
As these cells were originally thought to work by replacing lost or damaged cells in injured tissues [33],[36]–[40], initial studies focused on complex and often highly invasive infusion strategies to maximize delivery of cells to the injured organ, such as direct injections into feeding arteries [37],[41],[42]. While such studies demonstrated often dramatic protective effects of cell infusion, enthusiasm for such strategies as a clinical treatment was tempered by the invasive nature of the therapy [43]. Around the same time, concerns were also being raised regarding the safety of infusing bone marrow-derived cells, with some studies suggesting the potential for exacerbation of tissue injury [44],[45], and others even reporting uncontrolled growth of such cells following infusion [46]. Finally, other reports suggested that diseases such as diabetes and chronic kidney disease (CKD) could impair the tissue protective activity of these cells [47]–[54], rendering an autologous cell treatment strategy for CKD less attractive.
In an effort to overcome these barriers, a number of investigators made a key discovery. In most cases, infused bone marrow-derived cells are not retained in significant numbers in the injured organ despite exerting potent protective effects [55]–[57]. For example, Dr. Yuen and colleagues demonstrated that treatment with early outgrowth cells (EOCs), a type of bone marrow-derived cell that can be grown in large numbers in the lab, was able to dramatically reduce progression of experimental diabetic and non-diabetic CKD. Intriguingly, Dr. Yuen and his colleagues demonstrated that following infusion, EOCs homed to organs of the reticuloendothelial system, such as the liver, spleen, and bone marrow [55]–[57]. This finding suggested that infused EOCs might lodge in these organs, working by secreting soluble factors that act in an endocrine fashion to protect and repair the injured kidney. Subsequent studies revealed that EOCs and other bone marrow-derived cells do, in fact, release soluble factors that can mediate a wide variety of beneficial effects in vitro [55],[57]–[60]. The activity of these soluble factors could be maintained even with extensive dilution, suggesting that these factors could, in fact, work via an endocrine mode of action [61].
The potency of these factors also suggested that they might be used directly as a therapy for kidney disease without the need for, and associated risks of, cell infusion. In this regard, a recent proof-of-principle study confirmed that infusion of a cell-free preparation of EOC-derived factors significantly reduced renal injury and dysfunction in rats with experimental CKD, mimicking the effects of cell injection [61]. Follow-up studies have further demonstrated that EOC derived factors have additive renoprotective effects on top of renin-angiotensin system blockade [62].
Taken together, this work points to the exciting potential of cell-based treatment for kidney disease and provides an example of how the iterative process of translational research can rapidly refine and advance a novel treatment from the bench to the bedside. Moving forward, Dr. Yuen is part of a Canadian group of investigators that is currently planning a clinical trial comparing EOC-derived factors against standard of care therapy in high risk, proteinuric chronic kidney disease patients who continue to progress on renin-angiotensin system blockers. While such a trial would provide important proof-of-concept data supporting a cell-based treatment strategy for CKD, the long term goal is to identify the specific factor(s) responsible for the potent renoprotective activity of EOC therapy. Importantly, the identification of these factors would allow potential enhancement of renoprotection and would alleviate safety concerns regarding the administration of unknown proteins that do not contribute to renoprotection. These promising pre-clinical results have formed the basis for a proposed clinical trial of EOC-derived factors in patients with CKD (Table 1).
Now that this basic science research has overcome the barrier of finding a proposed human or clinical application (T1), the next step is to understand if this translates into clinical safety and effectiveness with phase 1, 2 and 3 clinical trials (T2).
Acute kidney injury
There are several studies in the general population demonstrating that acute kidney injury (AKI) requiring dialysis is associated with an increased risk of death and chronic kidney disease [63]–[66]. There is also increasing evidence that less severe AKI is associated with an increased risk of poor long-term outcomes [67]–[71]. However, the impact of AKI on outcomes in the renal transplant population is less well characterized. We are aware of two recently published studies that have examined this issue. Both studies showed that, similar to the general population, renal transplant patients who experience AKI have a heightened risk of poor long-term outcomes [72],[73]. With an association between AKI and poor outcomes among renal transplant patients now being demonstrated, how can this discovery be translated to patients and clinical practice?
Further characterization of the association would first be needed. It remains unclear if all transplant patients who develop AKI experience worse outcomes. Potentially certain patient characteristics or characteristics of the AKI event modify the association, and if so, which characteristics determine a worse prognosis. We propose to design a study that would examine the association of key patient and AKI event characteristics with graft loss and death. The characteristics examined would be easily ascertainable from large existing health care databases. The knowledge gained from this research could be used to develop a predictive model that could serve as a helpful tool for clinicians to identify transplant patients at high risk for poor long-term outcomes following an episode of AKI. Identifying high-risk renal transplant patients at hospital discharge could lead to a practice change of selected patients being followed more closely. It is our hope that identifying high risk patients would lead to improved patient outcomes.
However, there are several potential barriers to this research. The first barrier is to successfully design and conduct the study. The creation of a robust predictive model requires a large sample size, and patients with a kidney transplant are relatively small in number. The next limitation is that of the database itself. Due to the follow up time required for the study, and the resources involved with creating a prospective database across transplant centres, we will utilize already existing health care databases. Given that this will be a retrospective study, only variables readily available in the database can be included. The variables included in the model are also very important when considering the clinical application and uptake into clinical practice. Clinicians will want a predictive model that is easy to use and incorporates variables that are readily available. Successful implementation of the model into clinical practice will also require a knowledge translation plan.
It is our hope that simply identifying high-risk patients with the model and following them more closely will improve outcomes; however, this may not be the case. Following our development of the predictive model, an algorithm or management strategy for high-risk patients will need to be implemented and applied to clinical practice. Further research will then be needed to test whether a change in management of high-risk patients results in a change in clinical endpoints.
The information gained from this study could potentially be used to move from the bedside back to the bench. The ability to identify high-risk transplant patients could facilitate the conduct of basic science studies examining the mechanism by which AKI leads to death and graft loss. Mechanistic studies could potentially lead to new therapies for testing in randomized controlled trials (Table 1).
Risk based care for chronic kidney disease
An estimated 3 million Canadians suffer from CKD and are at risk for cardiovascular events and progression to kidney failure [74]. While all patients with CKD are at increased relative risk of kidney failure and all-cause mortality when compared to individuals without CKD, the risk for these adverse outcomes can vary greatly, even for individuals with the same level of estimated glomerular filtration rate (eGFR)/kidney function [75]–[77]. For interventions such as vascular access insertion, applying the same treatment approaches for patients at high risk for kidney failure to those at low risk can result in unnecessary cost and potential harm [78]. Even routine interventions such as renin-angiotensin aldosterone system (RAAS) inhibitors may have diminished benefits for preventing CKD progression in patients without albuminuria and higher risks of acute kidney injury and hyperkalemia in the same individuals when compared to other antihypertensive agents [79],[80]. In order to accurately estimate the risk of kidney failure requiring dialysis or transplant in an individual patient, we developed the kidney failure risk equation (KFRE) in 2011 [77].
The KFRE was developed and validated in 8,931 patients with CKD Stages 3–5 referred for nephrology care in the provinces of Ontario and British Columbia. We developed several equations, but our preferred KFREs (4 variable and 8 variable), were entirely laboratory based, and predicted the outcome of kidney failure in a 2 to 5 year horizon with a high degree of accuracy. These equations will be published with accompanying risk calculators for use on personal computers, tablets and on every smartphone platform. Links to download the individual applications, and the Microsoft Excel version of the risk calculator were provided as part of the original publication, and greatly accelerated the knowledge to action cycle for the KFRE [77],[81].
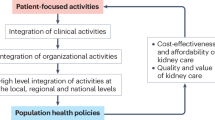
Concordantly, the KFRE has been validated externally by several independent investigators and is used routinely for the triage of nephrology consultations and dialysis access planning in advanced CKD clinics in multiple jurisdictions [82]–[84] (and unpublished observations). In particular, the province of Manitoba has adopted a centralized triage process for incoming nephrology consultations, where all consults are risk stratified by the KFRE, and those with a < 3% risk of kidney failure in the next 5 years are referred back to primary care, with instructions to re-refer if kidney function declines. The adoption of this referral strategy has resulted in a significant reduction in wait times for nephrology care (10 months to 2 months), and has thereby increased patient and provider satisfaction for high risk referrals (unpublished observations). Similar strategies in other jurisdictions have resulted in better vascular access planning and more informed treatment modality discussions in older patients with CKD [84]. Together, these global and local knowledge translation strategies have greatly increased the impact of the KFRE, and represent an example of translational research from the epidemiology bench to the health policy bedside (personal communication, Daniel Schwarz).
Future directions for knowledge translation of the KFRE include implementation in dialysis access planning pathways, cost utility analyses of referral and treatment pathways that incorporate risk, and evaluation of the KFRE against novel biomarkers for CKD progression (Table 1).
The largest barrier for this research to overcome is the widespread uptake into clinical practice (T3). The successful uptake by clinicians will require funding and strategic planning for knowledge translation. Successful widespread use of the KFRE will likely require a knowledge translation action plan followed by repeated follow up on the results of the action plan with modifications as needed. These studies will likely include decision analyses based on real data, as well as cluster randomized trials or time series analyses measuring the effects of knowledge translation.
Conclusions
This review highlights four areas of research in Canadian nephrology conducted by trainees in the KRESCENT program. The translational nature of the research highlights the success of the KRESCENT program. The diverse areas of expertise of the researchers highlight the unique opportunity for collaboration among basic and clinical scientists afforded by the KRESCENT program. In this review, important discoveries in the area of nephrology and successful examples of research translation are presented, such as the development of the KFRE influencing clinical practice and thus improving the care of renal patients locally in Manitoba. On the other hand, many barriers to research translation are illustrated in the research examples presented. For example, the research using cell-based therapies for the treatment of kidney disease highlights the great many challenges encountered and the patience and resources one requires when looking at successfully translating discoveries made in the laboratory into a clinical or human application.
It is clear that research translation is often a very difficult and lengthy process that requires ample resources and planning. The good news is that funding bodies are recognizing this and are committed to dedicating resources to the goal of ensuring that scientific discoveries eventually benefit patients in a timely fashion. Specific to the area of nephrology, the KRESCENT program has a mandate to direct its funds towards a balance of clinical and basic science research, providing a comprehensive training program with an emphasis on translational research and collaboration among investigators enrolled in the program. With enhanced funding and awareness over recent years, the success of research translation should improve over the coming years, ultimately improving the care of patients with kidney disease.
Abbreviations
- AKI:
-
Acute kidney injury
- CIHR:
-
Canadian Institutes of Health Research
- CKD:
-
Chronic kidney disease
- CTSA:
-
Clinical and Translational Science Award
- eGFR:
-
Estimated glomerular filtration rate
- EOC:
-
Early outgrowth cell
- IFT:
-
Intraflagellar transport
- IFTA:
-
Intraflagellar transport subcomplex A
- JBTS:
-
Joubert syndrome
- KFRE:
-
Kidney failure risk equation
- KRESCENT:
-
Kidney Research Scientist Core Education and National Training
- NCATS:
-
National Center for Advancing Translational Sciences
- NGS:
-
Next-Genereation Sequencing
- NIH:
-
National Institutes of Health
- NPHP:
-
Nephronophthisis
- PKD:
-
Polycystic kidney disease
- TMEM:
-
Transmembrane
- WDR:
-
WD Repeat
References
Woolf SH: The meaning of translational research and why it matters. JAMA J Am Med Assoc 2008, 299: 211–213.
Sung NS, Crowley WF Jr, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K, Larson EL, Scheinberg D, Reece EA, Slavkin H, Dobs A, Grebb J, Martinez RA, Korn A, Rimoin D: Central challenges facing the national clinical research enterprise. JAMA J Am Med Assoc 2003, 289: 1278–1287. 10.1001/jama.289.10.1278
Drolet BC, Lorenzi NM: Translational research: understanding the continuum from bench to bedside. Transl Res J Lab Clin Med 2011, 157: 1–5. 10.1016/j.trsl.2010.10.002
Contopoulos-Ioannidis DG, Ntzani E, Ioannidis JPA: Translation of highly promising basic science research into clinical applications. Am J Med 2003, 114: 477–484. 10.1016/S0002-9343(03)00013-5
Balas EA, Boren SA: Managing clinical knowledge for health care improvement. Yearb Med Informatics 2000, 2000: 65–70.
McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA: The quality of health care delivered to adults in the United States. N Engl J Med 2003, 348: 2635–2645. 10.1056/NEJMsa022615
Wolf S: Editorial: the real gap between bench and bedside. N Engl J Med 1974, 290: 802–803. 10.1056/NEJM197404042901411
Keramaris NC, Kanakaris NK, Tzioupis C, Kontakis G, Giannoudis PV: Translational research: from benchside to bedside. Injury 2008, 39: 643–650. 10.1016/j.injury.2008.01.051
Knowledge translation & commercialization publications - CIHR. [http://www.cihr-irsc.gc.ca/e/39128.html]
Tetroe J: Knowledge translation at the Canadian Institutes of Health Research: a primer. Focus Tech Brief 2007, 18: 1–8.
National Center for Advancing Translational Sciences (NCATS). [http://www.ncats.nih.gov/]
Elisabeth Pain: European programs offer translational training. Sci Careers.
Burns KD, Wolfs W, Bélanger P, McLaughlin K, Levin A: The KRESCENT Program: an initiative to match supply and demand for kidney research in Canada. Clin Investig Med Méd Clin Exp 2010, 33: E356-E367.
Kidney Research Scientist Core Education and National Training Program - The Kidney Foundation of Canada | La Fondation canadienne du rein. [http://www.krescent.ca/page.aspx?pid=444]
Eckardt K-U, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A: Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 2013, 382: 158–169. 10.1016/S0140-6736(13)60439-0
Hildebrandt F: Genetic kidney diseases. Lancet 2010, 375: 1287–1295. 10.1016/S0140-6736(10)60236-X
Baker K, Beales PL: Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C: Semin Med Genet 2009, 151C: 281–295. 10.1002/ajmg.c.30231
Arts HH, Knoers NVAM: Current insights into renal ciliopathies: what can genetics teach us? Pediatr Nephrol Berl Ger 2013, 28: 863–874. 10.1007/s00467-012-2259-9
Reiter JF, Blacque OE, Leroux MR: The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep 2012, 13: 608–618. 10.1038/embor.2012.73
Goggolidou P: Wnt and planar cell polarity signaling in cystic renal disease. Organogenesis 2013, 10:.
Blacque OE, Li C, Inglis PN, Esmail MA, Ou G, Mah AK, Baillie DL, Scholey JM, Leroux MR: The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol Biol Cell 2006, 17: 5053–5062. 10.1091/mbc.E06-06-0571
Gilissen C, Arts HH, Hoischen A, Spruijt L, Mans DA, Arts P, van Lier B, Steehouwer M, van Reeuwijk J, Kant SG, Roepman R, Knoers NVAM, Veltman JA, Brunner HG: Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am J Hum Genet 2010, 87: 418–423. 10.1016/j.ajhg.2010.08.004
Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MAM, Keighren M, Bahlo M, Bromhead CJ, Budd P, Aftimos S, Delatycki MB, Savarirayan R, Jackson IJ, Amor DJ: Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet 2011, 88: 508–515. 10.1016/j.ajhg.2011.03.015
Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, Greenberg CR, McLeod DR, Bernier FP, Chudley AE, Müller T, Shboul M, Logan CV, Loucks CM, Beaulieu CL, Bowie RV, Bell SM, Adkins J, Zuniga FI, Ross KD, Wang J, Ban MR, Becker C, Nürnberg P, Douglas S, Craft CM, et al.: TMEM237 is mutated in individuals with a joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet 2011, 89: 713–730. 10.1016/j.ajhg.2011.11.005
Clarke A, Sarangi S, Verrier-Jones K: Voicing the lifeworld: parental accounts of responsibility in genetic consultations for polycystic kidney disease. Soc Sci Med 1982 2011, 72: 1743–1751.
NCBI GTR: Genetic Testing Registry. [http://www.ncbi.nlm.nih.gov/gtr/]
Verghese E, Zhuang J, Saiti D, Ricardo SD, Deane JA: In vitro investigation of renal epithelial injury suggests that primary cilium length is regulated by hypoxia-inducible mechanisms. Cell Biol Int 2011, 35: 909–913. 10.1042/CBI20090154
Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, Deane JA: Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol JASN 2009, 20: 2147–2153. 10.1681/ASN.2008101105
Wang L, Weidenfeld R, Verghese E, Ricardo SD, Deane JA: Alterations in renal cilium length during transient complete ureteral obstruction in the mouse. J Anat 2008, 213: 79–85. 10.1111/j.1469-7580.2008.00918.x
Wang S, Dong Z: Primary cilia and kidney injury: current research status and future perspectives. Am J Physiol Renal Physiol 2013, 305: F1085-F1098. 10.1152/ajprenal.00399.2013
Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA: Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 2008, 23: 834–841.
Broekhuis JR, Leong WY, Jansen G: Regulation of cilium length and intraflagellar transport. Int Rev Cell Mol Biol 2013, 303: 101–138. 10.1016/B978-0-12-407697-6.00003-9
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275: 964–967. 10.1126/science.275.5302.964
Grigoriadis AE, Heersche JN, Aubin JE: Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol 1988, 106: 2139–2151. 10.1083/jcb.106.6.2139
Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I: Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol 1994, 161: 218–228. 10.1006/dbio.1994.1022
Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T: Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A 2000, 97: 3422–3427. 10.1073/pnas.97.7.3422
Murohara T, Ikeda H, Duan J, Shintani S, Sasaki KI, Eguchi H, Onitsuka I, Matsui K, Imaizumi T: Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 2000, 105: 1527–1536. 10.1172/JCI8296
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S: Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001, 7: 430–436. 10.1038/86498
Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T: HMG-CoA reductase inhibitor mobilizes bone marrow–derived endothelial progenitor cells. J Clin Invest 2001, 108: 399–405. 10.1172/JCI200113131
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284: 143–147. 10.1126/science.284.5411.143
Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, Van Haute I, Lootens N, Heyndrickx G, Wijns W: Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation 2005,112(9 Suppl):I178-I183.
Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, et al.: Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation 2007, 115: 3165–3172. 10.1161/CIRCULATIONAHA.106.687376
Yuen DA, Gilbert RE, Marsden PA: Bone marrow cell therapies for endothelial repair and their relevance to kidney disease. Semin Nephrol 2012, 32: 215–223. 10.1016/j.semnephrol.2012.02.008
Burger D, Gutsol A, Carter A, Allan DS, Touyz RM, Burns KD: Human cord blood CD133+ cells exacerbate ischemic acute kidney injury in mice. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 2012, 27: 3781–3789.
Madala SK, Edukulla R, Schmidt S, Davidson C, Ikegami M, Hardie WD: Bone-marrow-derived stromal cells are invasive and hyperproliferative and alter TGFα-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2014, 50: 777–786. 10.1165/rcmb.2013-0042OC
Thirabanjasak D, Tantiwongse K, Thorner PS: Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol JASN 2010, 21: 1218–1222. 10.1681/ASN.2009111156
Yuen DA, Kuliszewski MA, Liao C, Rudenko D, Leong-Poi H, Chan CT: Nocturnal hemodialysis is associated with restoration of early-outgrowth endothelial progenitor-like cell function. Clin J Am Soc Nephrol CJASN 2011, 6: 1345–1353. 10.2215/CJN.10911210
Yuen DA, Zhang Y, Thai K, Spring C, Chan L, Guo X, Advani A, Sivak JM, Gilbert RE: Angiogenic dysfunction in bone marrow-derived early outgrowth cells from diabetic animals is attenuated by SIRT1 activation. Stem Cells Transl Med 2012, 1: 921–926. 10.5966/sctm.2012-0026
Choi J-H, Kim KL, Huh W, Kim B, Byun J, Suh W, Sung J, Jeon E-S, Oh H-Y, Kim D-K: Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 2004, 24: 1246–1252. 10.1161/01.ATV.0000133488.56221.4a
Herbrig K, Pistrosch F, Oelschlaegel U, Wichmann G, Wagner A, Foerster S, Richter S, Gross P, Passauer J: Increased total number but impaired migratory activity and adhesion of endothelial progenitor cells in patients on long-term hemodialysis. Am J Kidney Dis Off J Natl Kidney Found 2004, 44: 840–849. 10.1053/j.ajkd.2004.08.001
Chan CT, Li SH, Verma S: Nocturnal hemodialysis is associated with restoration of impaired endothelial progenitor cell biology in end-stage renal disease. Am J Physiol Renal Physiol 2005, 289: F679-F684. 10.1152/ajprenal.00127.2005
Loomans CJM, de Koning EJP, Staal FJT, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld A-J: Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004, 53: 195–199. 10.2337/diabetes.53.1.195
Van Koppen A, Joles JA, Bongartz LG, van den Brandt J, Reichardt HM, Goldschmeding R, Nguyen TQ, Verhaar MC: Healthy bone marrow cells reduce progression of kidney failure better than CKD bone marrow cells in rats with established chronic kidney disease. Cell Transplant 2012, 21: 2299–2312. 10.3727/096368912X636795
Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC: Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002, 106: 2781–2786. 10.1161/01.CIR.0000039526.42991.93
Yuen DA, Connelly KA, Advani A, Liao C, Kuliszewski MA, Trogadis J, Thai K, Advani SL, Zhang Y, Kelly DJ, Leong-Poi H, Keating A, Marsden PA, Stewart DJ, Gilbert RE: Culture-modified bone marrow cells attenuate cardiac and renal injury in a chronic kidney disease rat model via a novel antifibrotic mechanism. PLoS One 2010, 5: e9543. 10.1371/journal.pone.0009543
Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S: Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 2003, 107: 2134–2139. 10.1161/01.CIR.0000062649.63838.C9
Zhang Y, Yuen DA, Advani A, Thai K, Advani SL, Kepecs D, Kabir MG, Connelly KA, Gilbert RE: Early-outgrowth bone marrow cells attenuate renal injury and dysfunction via an antioxidant effect in a mouse model of type 2 diabetes. Diabetes 2012, 61: 2114–2125. 10.2337/db11-1365
Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S: Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 2005, 39: 733–742. 10.1016/j.yjmcc.2005.07.003
Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, Astroulakis Z, Xiao Q, Hill J, Xu Q, Mayr M: Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res 2009, 104: 32–40. 10.1161/CIRCRESAHA.108.182261
Yang Z, von Ballmoos MW, Faessler D, Voelzmann J, Ortmann J, Diehm N, Kalka-Moll W, Baumgartner I, Di Santo S, Kalka C: Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis 2010, 211: 103–109. 10.1016/j.atherosclerosis.2010.02.022
Yuen DA, Connelly KA, Zhang Y, Advani SL, Thai K, Kabir G, Kepecs D, Spring C, Smith C, Batruch I, Kosanam H, Advani A, Diamandis E, Marsden PA, Gilbert RE: Early outgrowth cells release soluble endocrine antifibrotic factors that reduce progressive organ fibrosis. Stem Cells Dayt Ohio 2013, 31: 2408–2419. 10.1002/stem.1502
Yuen DA, Zhang Y, Connelly K, Advani A, Gilbert RE: Progenitor cell secretory products exert additive renoprotective effects when combined with ACE inhibitors in experimental CKD. J Am Soc Nephrol JASN 2011, 22: 765A.
Trono D, Feinberg MB, Baltimore D: HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell 1989, 59: 113–120. 10.1016/0092-8674(89)90874-X
Edis M: Interviewing: preparing for the response. Nurs Times 1989, 85: 46–48.
Morgera S, Schneider M, Neumayer HH: Long-term outcomes after acute kidney injury. Crit Care Med 2008,36(4 Suppl):S193-S197. 10.1097/CCM.0b013e318168cae2
Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG: Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA J Am Med Assoc 2009, 302: 1179–1185. 10.1001/jama.2009.1322
Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol JASN 2009, 20: 223–228. 10.1681/ASN.2007080837
Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ: Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 2008, 168: 609–616. 10.1001/archinte.168.6.609
Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM: Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 2008, 168: 987–995. 10.1001/archinte.168.9.987
Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE II, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012, 81: 477–485. 10.1038/ki.2011.405
Heung M, Chawla LS: Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens 2012, 21: 628–634. 10.1097/MNH.0b013e3283588f24
Mehrotra A, Rose C, Pannu N, Gill J, Tonelli M, Gill JS: Incidence and consequences of acute kidney injury in kidney transplant recipients. Am J Kidney Dis Off J Natl Kidney Found 2012, 59: 558–565. 10.1053/j.ajkd.2011.11.034
Nakamura M, Seki G, Iwadoh K, Nakajima I, Fuchinoue S, Fujita T, Teraoka S: Acute kidney injury as defined by the RIFLE criteria is a risk factor for kidney transplant graft failure. Clin Transplant 2012, 26: 520–528. 10.1111/j.1399-0012.2011.01546.x
Arora P, Vasa P, Brenner D, Iglar K, McFarlane P, Morrison H, Badawi A: Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ Can Med Assoc J J Assoc Medicale Can 2013, 185: E417-E423. 10.1503/cmaj.120833
Matsushita K, Van der Velde M, Astor BC, Woodward M, Levey AS, De Jong PE, Coresh J, Gansevoort RT: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010, 375: 2073–2081. 10.1016/S0140-6736(10)60674-5
Astor BC, Matsushita K, Gansevoort RT, Vder Velde M, Woodward M, Levey AS, Jong PE, De Coresh J, Astor BC, Matsushita K, Gansevoort RT, Van der Velde M, Woodward M, Levey AS, De Jong PE, Coresh J, El-Nahas M, Eckardt K-U, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, et al.: Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease: a collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011, 79: 1331–1340. 10.1038/ki.2010.550
Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA J Am Med Assoc 2011, 305: 1553–1559. 10.1001/jama.2011.451
O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, Rodriguez RA, Allon M: When to refer patients with chronic kidney disease for vascular access surgery: should age be a consideration? Kidney Int 2007, 71: 555–561. 10.1038/sj.ki.5002078
Kent DM, Jafar TH, Hayward RA, Tighiouart H, Landa M, de Jong P, de Zeeuw D, Remuzzi G, Kamper A-L, Levey AS: Progression risk, urinary protein excretion, and treatment effects of angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol JASN 2007, 18: 1959–1965. 10.1681/ASN.2006101081
Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, Teo KK, Yusuf S, Mann JFE: Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011, 123: 1098–1107. 10.1161/CIRCULATIONAHA.110.964171
Kidney failure risk equation. [http://www.qxmd.com/kidney-failure-risk-equation]
Peeters MJ, van Zuilen AD, van den Brand JAJG, Bots ML, Blankestijn PJ, Wetzels JFM: Validation of the kidney failure risk equation in European CKD patients. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 2013, 28: 1773–1779.
Drawz PE, Goswami P, Azem R, Babineau DC, Rahman M: A simple tool to predict end-stage renal disease within 1 year in elderly adults with advanced chronic kidney disease. J Am Geriatr Soc 2013, 61: 762–768. 10.1111/jgs.12223
Shirazian S, Grant C, Tangri N, Mattana J: Have we been overestimating the risk of end-stage renal disease in older adults with chronic kidney disease? J Am Geriatr Soc 2014. In press.
Acknowledgements
AOM is funded by a KRESCENT postdoctoral fellowship. DAY is supported by the KRESCENT New Investigator and Canadian Diabetes Association Clinician Scientist Award. NT is supported by the KRESCENT New Investigator Award and the MHRC Establishment Award. VLJ is funded by KRESCENT and MSFHR postdoctoral fellowships. KRESCENT is a joint initiative of the Kidney Foundation of Canada, Canadian Institute of Health Research, and the Canadian Society of Nephrology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AOM, DAY, NT and VLJ helped to draft the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Molnar, A.O., Yuen, D.A., Tangri, N. et al. Bridging the gap: a Canadian perspective on translational kidney research. Can J Kidney Health Dis 1, 18 (2014). https://doi.org/10.1186/s40697-014-0018-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40697-014-0018-5