Abstract
Background
The perimenopause is associated with considerable biopsychosocial changes. The majority of women manage to adjust to these changes and cope well with the shift from reproductive to non-reproductive life. However, some women develop burdensome physical and psychological symptoms during the perimenopause. A strong link between menopausal complaints and depressed mood has been shown in this regard. To date, the decisive factors determining whether a woman will successfully achieve a healthy transition remain unclear. Thus, the purpose of this study is to investigate a range of theory-based markers related to health in perimenopausal women.
Methods
The Swiss Perimenopause Study comprises a sample of 135 healthy perimenopausal women aged 40–56. A variety of health-related genetic, epigenetic, endocrinological, physiological, and psychosocial markers associated with the menopausal transition are investigated over a period of 13 months.
Discussion
The Swiss Perimenopause Study will contribute to a better understanding of the biopsychosocial processes associated with the perimenopause, which should help to improve the clinical care of women undergoing the menopausal transition.
Similar content being viewed by others
Background
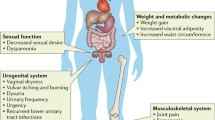
The perimenopause is related to substantial biological, psychological and social changes [1,2,3]. It describes the biological shift from reproductive to non-reproductive life, which is associated with significant alterations in the female hormonal system. The perimenopause is considered as a phase of strong fluctuations in sex hormones such as estradiol and progesterone [4, 5]. Endocrinological variations have been correlated with a higher risk of burdensome physical and psychological symptoms [6,7,8,9,10,11,12], subsumed under the term “menopausal complaints”. These complaints are generally divided into psychological, somato-vegetative, and urogenital symptoms. However, in spite of the major biopsychosocial changes and challenges, most women adjust well to the perimenopause, reporting a good quality of life and an overall positive well-being [13]. It is assumed that resilience might be a key factor in determining whether or not a woman will be negatively affected by menopausal symptoms [14]. Women with high levels of resilience are believed to more effectively and successfully adapt to the substantial changes in the perimenopause [15, 16] compared to less resilient women. In turn, women with lower levels of resilience are suggested to be more affected by menopausal complaints like vasomotor symptoms or sleep disturbances. Such symptoms may then co-occur and have been shown to interact with depressed mood [17,18,19], suggesting the perimenopause as a window of vulnerability for mood disturbances [20]. Indeed, prevalence rates of depressed mood during the perimenopause are particularly high [21]. Consequently, the investigation of depression in the course of the menopausal transition has gained importance over the last decades.
The attention paid to women’s health research has gradually increased since the 1970s [22]. One of the first longitudinal studies on the menopausal transition was the Massachusetts Women’s Health Study (MWHS) [23], followed by the Seattle Midlife Women’s Health Study (SMWHS), which collected data over 23 years [24]. In Norway, a large cohort study, the Nord-Trøndelag Health Study (HUNT) [25], began in 1984 and is considered one of the largest health studies ever performed. Further large studies on the menopausal transition, such as the Study of Women’s Health Across the Nation (SWAN) [26], the Penn Ovarian Aging Study (POAS) [4], or the Harvard Study of Moods and Cycles [27], followed in the US. Extensive research on women in midlife has also been conducted in Australia, within the Australian Longitudinal Study on Women’s Health (ALSWH) [28] or the Melbourne Women’s Midlife Health Project (MWMHP) [29]. Depressed mood was one of the focal points across these studies, and all of them highlighted the increased risk for new or recurrent depressed mood, emphasizing the prevalence peak during the menopausal transition. This previous research formed an important basis for the understanding of depressed mood in middle-aged women and the associated changes and challenges.
Previous studies frequently included women across all three menopausal stages, i.e. the pre-, the peri-, and the postmenopause. Such a selection procedure seems to be highly expedient in terms of identifying critical time points in the development of symptoms in the progression from reproductive to non-reproductive life. Moreover, this sample selection also provides the opportunity to compare the participants across the different menopausal stages. However, in terms of investigating the perimenopause as a critical window of increased biopsychosocial changes and associated complaints, and in order to specify the involved processes, it is essential to focus specifically on the perimenopausal phase.
Therefore, the Swiss Perimenopause Study employs a longitudinal design examining women in the perimenopause only and assessing a variety of genetic, epigenetic, endocrinological, physiological and psychosocial factors. As such, we aim to investigate psychosocial factors like personality traits, self-esteem, self-compassion, perceived stress, emotion regulation and coping, or social support. Furthermore, physiological parameters like blood pressure and pulse, heart rate variability, hand grip force, or body composition are assessed as measures associated with healthy aging. Body weight and body height are captured in order to take into account participants’ body mass index (BMI) when investigating biomarkers. Additionally, we measure the length of the index finger and the ring finger in order to calculate the second to fourth digit ratio (2D:4D). Recent research showed that a higher 2D:4D is associated with a higher age at menopause, while a lower 2D:4D (interpreted as suggesting a higher androgen exposure during the prenatal phase) is associated with a lower age at menopause [30]. As our workgroup has shown that looking younger than one’s actual age can be related to a variety of health outcomes [31], we take standardized facial photographs of all participants. The full list of biopsychosocial factors included in this study is shown in Tables 1 and 2. The comprehensive investigation of possible predictors of physical and psychological complaints appears particularly promising regarding the detection of underlying processes such as epigenetic variations or specific hormonal patterns.
Data concerning genetic and epigenetic markers associated with the menopausal transition are still scarce. However, valuable information might be gained by analyzing gene-environment interactions and the effect of epigenetic modifications on the menopausal transitioning [66]. For this purpose, we aim to investigate the estrogen receptor genes (ER1, ER2, GPER), the glucocorticoid receptor (NR3C1) as well as the serotonin transporter (5-HTTLPR) and the respective relationships with a resilient menopausal transition or the development of depression in the perimenopause. For example, a recent study by our workgroup found a positive relationship between the methylation of the ERα enhancer and depressed mood in women around the menopause [67]. Consequently, one goal is to investigate the autoregulation between estradiol and the estrogen receptor genes with regard to methylation patterns and subsequent effects on the participants’ mood.
Endocrinological parameters are commonly assessed in studies on the menopausal transition. Previous research in this regard showed a significant relationship between sex steroids and menopausal complaints [68,69,70]. Several studies by our workgroup showed that cortisol or alpha amylase play a key role in the reactivity of physiological stress systems [71,72,73], and inflammatory markers associated with various psychopathologies change over the menopausal transition [74]. Therefore, their assessment is included in this study. To maximize the informative value and comparability of the forthcoming results, the Swiss Perimenopause Study is designed to conduct a close endocrinological monitoring of all participants, enabling progression analyses of individual hormone profiles. Standardized methods of analysis are used, as described in the methods section.
By repeatedly assessing physiological data during the night, it may be possible to address questions about the relationship of sleep disturbances and vasomotor symptoms in women suffering from hot flushes and night sweats. To the best of our knowledge, the Swiss Perimenopause Study represents the currently largest study investigating such a wide range of health-related biopsychosocial markers in perimenopausal women.
The purpose of the Swiss Perimenopause Study is to gain a deeper understanding of the biopsychosocial factors and processes associated with 1) resilience and health in the perimenopause, and 2) the development of new or recurrent depression. One key goal of this study is to investigate biopsychosocial markers, determinants, and processes related to a resilient menopausal transitioning. In this regard, we aim to examine psychosocial variables such as optimism, emotional stability, or self-esteem as well as biological markers such as female sex steroids and gonadotropins, stress hormones or inflammatory parameters and their relationship with well-being and health. We assume that women with higher psychosocial resilience (e.g. higher optimism, higher emotional stability, higher self-esteem) and higher levels of female sex steroids will be more successful in their adaptation to changes and challenges associated with the menopausal transition. A second key goal is to investigate the biopsychosocial determinants and associations of perimenopausal depression. To achieve this, we aim to investigate specific risk factors for depressive symptoms in the perimenopause and to examine the effect of endocrinological alterations and psychosocial changes and their effect on the women’s mood. We assume that a history of depression and fluctuations of sex steroids are major risk factors for perimenopausal depression. As such, it is our endeavor to expand and develop the existing knowledge on healthy menopausal transitioning. To our knowledge, this is the first study to investigate both depression and resilience within the perimenopause.
Methods
Design
The Swiss Perimenopause Study is an exploratory, longitudinal, single-center, national study examining different facets of female menopausal development over 13 months. Data are being collected between June 2018 and January 2021. The study is divided into a screening phase and a study phase. After the application of the strict inclusion criteria, interested women were screened for about 2 months for our study purposes. Subsequently, the actual study phase began, which will last for 11 months. Relevant genetic, epigenetic, endocrinological, physiological, and psychological aspects of the menopausal transition are investigated by means of different measurement methods. The participation includes two visits to our laboratory at the Institute of Psychology, University of Zurich. The standardized lab visits include blood sampling and the assessment of different peripheral physiological (blood pressure, pulse) and anthropometric parameters (body weight, body height, bioelectrical impedance analysis (BIA), second to fourth digit ratio, grip strength, standardized facial photograph). During the months between the two lab visits, participants independently collect saliva samples at home, measure various physiological parameters with the Ava bracelet (Ava AG, Zürich, Switzerland), complete a menstrual cycle and mood diary, and answer a set of different psychosocial questionnaires. An overview of the study design can be found in Fig. 1. To date, the screening phase and t1 have been completed.
Sample
The population-based sample of 135 perimenopausal women aged 40–56 were recruited through mailing lists, social media, context-specific online forums, flyers as well as newspaper and magazine articles. For the sample size estimation of the planned project, we performed an a priori sample size estimation analysis using G*Power 3.1 for different statistical analyses such as correlation analysis, linear regression analysis, linear multiple regression analysis, or Wilcoxon signed-rank test with an α-level < .05 [75].
From June 2018 to December 2019, a total of 1121 women showed interest in participating in the Swiss Perimenopause Study by completing an online screening questionnaire. The eligibility for study participation was checked by following inclusion and exclusion criteria (Table 3).
Major reasons for exclusion included pre- or postmenopausal status (n = 717), poor subjective health (n = 97), age below 40 or above 60 years (n = 58), hormone therapy in the last 6 months (n = 34), acute or chronic somatic or mental illness (n = 22), and psychiatric or psychotropic drug use (n = 11). The sample inclusion and elimination process is displayed in Fig. 2. Participants eligible for the study were granted access to the online menstrual cycle and mood diary for the two-month screening phase. Within this phase, the perimenopausal status was ensured. The perimenopausal status was defined according to the Stages of Reproductive Aging Workshop + 10 (STRAW) criteria, representing the most frequently used classification system [76]:
-
1.
Early menopausal transition: increased variability in menstrual cycle length, defined as a persistent difference of ≤7 days in the length of 10 consecutive cycles.
-
2.
Late menopausal transition: the occurrence of amenorrhea of 60 days or longer.
In addition to the exclusion criteria, the screening questionnaire also recorded whether the currently subjectively healthy participants show a history of depression. In this regard, prior depression must either have been previously diagnosed by an expert, or as self-report, in accordance with the criteria for major depression according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).
Retention efforts
Numerous efforts have been undertaken to retain the eligible participants throughout the study. These include personal and consistent contact from the research staff, daily accessibility to the research staff, annual Christmas cards, reminders about the data collection by text message or telephone calls, and regular menopause-specific information on our homepage and social media profiles. Furthermore, participants were given an individual health profile after the evaluation phase and are given various incentives throughout the different time points during the study (e.g. vouchers for massage, wellness and spa treatments, free gym subscription, cosmetics, food vouchers).
Data collection
During the entire screening and study phase, participants are asked to mark bleeding days in their individual paper-and-pencil cycle calendar. This provides an easily accessible method to remember the days of bleeding for the biweekly online mood and cycle diary. Every 2 weeks, participants are reminded to complete the online cycle and mood diary, which assesses specific cycle conditions, menopausal complaints, and mood disturbances over the last 2 weeks. At the beginning of the study phase (t1), further demographic data (e.g. marital status, occupation) and a variety of psychosocial factors were assessed via online questionnaires. Psychosocial measures include psychological traits, physical and mental health outcomes, behavioral aspects, and interpersonal factors. The validated self-report questionnaires assessed in the course of the study can be found in Table 1, which lists the measured constructs, the name of the assessment instrument, the authors, and the number of items of the instrument (Table 1). The questionnaires are completed at the beginning and at the end of the study (t1 and t3). Participants receive the respective links by e-mail. All questionnaires need to be completed consecutively.
Two standardized laboratory visits to the Institute of Psychology, University of Zurich are planned within the study phase, at t1 and t3. Women are instructed a) not to drink caffeinated drinks (e.g. coffee, black or green tea, energy drinks) or alcohol for at least 48 h (2 days) prior to the visit, b) not to engage in intensive physical activity for at least 24 h (1 day) prior to the visit, and c) not to eat, drink or engage in physical effort (e.g., cycling) on the morning of the examination. The laboratory sessions are standardized. Participants arrive at the laboratory at 7:45 am. At the first laboratory visit (t1), a well-instructed psychologist first conducted the German Version of the Structured Clinical Interview for DSM-IV [77] to ensure that all the participants were mentally healthy at the beginning of the study. Otherwise, participants were excluded.
At both laboratory visits (t1 and t3), small capillary blood samples are drawn from the participant’s finger. Blood pressure and pulse (Medisana MTV upper arm blood pressure instrument) are measured and body height and weight are assessed. In addition, the body composition is determined using bioelectrical impedance analysis (BIA, Biocorpus RX4000, Idiag AG, Fehraltdorf, Switzerland). This instrument is a fully digital, phase-sensitive, four-channel impedance-measuring device. Each channel applies a 50 kHz alternating current to measure resistance, reactance and phase angle. To measure the second to fourth digit ratio (2D:4D), the length of the index finger and the ring finger is measured (Digital Caliper Micrometer Vernier Gauge Tool). Accordingly, the grip strength is assessed (Baseline Hydraulic Hand Dynamometer). The laboratory sessions also include a standardized facial photograph. These photos are used for self-rated attractiveness as well as externally rated attractiveness. For the latter, independent and external examiners (N = 10) rate the standardized facial photographs of the participants according to the perceived age, attractiveness and health on a scale from 0 to 100. The assessments are conducted via presentation of the photographs on a computer screen. The examiners have signed a binding declaration of confidentiality.
Within all laboratory sessions, the investigator also asks a short, standardized set of questions about factors and activities potentially biasing the current hormone concentrations. Subjects are instructed to autonomously collect saliva samples in a non-invasive manner with saliva sampling tubes (SaliCaps, IBL International GmbH, Hamburg, Germany). Furthermore, the participants receive written instructions for each step of the saliva sampling. The participants have been provided with 100 SaliCaps (IBL International GmbH, Hamburg, Germany) to collect one daily morning saliva sample for 3 months in total (i.e. 28 daily samples per month during t1, t2 and t3) under standardized conditions. To investigate the cortisol awakening response (CAR) as well as fluctuations throughout the day, four additional saliva samples are collected on the first 2 days of the month of t2 and t3. The additional saliva samples are timed 30 and 60 min after the first saliva sample, at 12:00 pm and before going to bed. The exact sampling times as well as specific information on caffeine or alcohol consumption, sports or current gum bleeding are recorded in a protocol. Participants are reminded to start the saliva sampling at the beginning of t1, t2, and t3.
During the first lab visit, the participants were also introduced to the Ava bracelet (Ava AG, Zürich, Switzerland), which they were asked to wear at night for a total of 6 months. The Ava bracelet assesses raw data of nine different nighttime peripheral physiological parameters: skin temperature, ambient temperature, electrodermal activity, heart rate, heart rate variability, blood circulation of the skin, body acceleration, breathing rate, and sleep phases.
An overview of all biological parameters assessed in the course of the study can be found in Table 2, which lists the sources of the parameters, the label, and the name of the specific parameter (Table 2).
Data handling
A link for the online screening was provided on the study homepage. Women interested in participation were led to the online platform Unipark, where they were asked to provide initial, brief information followed by a declaration of consent. All questionnaires at baseline, t1 and t3 (www.unipark.com), the data analysis of retrospectively assessed relationship experiences (www.qualtrics.com), as well as the mood and cycle diary (www.findmind.ch), are completed online. The participants were explicitly informed about the study design, the aims, the procedure and the expected duration of each part of the assessment. Data security is ensured at all times. All calendars and protocols are paper-and-pencil-based. The online platform Unipark is reliably protected from any external access. The BSI-certified (Bundesamt für Sicherheit in der Informationstechnik [Federal Office for Information Security]) data center is subject to requirements of high data protection and safety in conformity with ISO (International Organization for Standardization) 27,001 based on the IT basic protection. Qualtrics is hosted by trusted data centers that are independently audited using the industry standard Statement on Standards for Attestation Engagements no. 16 (SSAE 16) method. HITECH-updated (Health Information Technology for Economic and Clinical Health Act) HIPAA (Health Insurance Portability and Accountability Act) rules are applied to ensure data protection and security of all customer data. The menstrual cycle and mood diary is recorded via the online platform Findmind (www.findmind.ch). Findmind treats data as confidential information, strives to ensure the privacy of the data and does not share information with third parties. It is operated exclusively on servers in Switzerland and stores all user data. For the dried blood spots (DBS), a sterile disposable lancet (Accu-Chek® Safe-T-Pro Plus) is used to collect up to five drops of blood (about 50 μL per drop), which are spotted onto standardized filter paper (No. 903 Whatman®, DBS Protein Saver Card). The samples are dried and subsequently stored in the laboratory freezer of the Institute of Psychology, University of Zurich at − 20 °C. DBS samples are analyzed in the biochemical laboratory of the Department of Clinical Psychology and Psychotherapy, University of Zurich, Switzerland (genetic and epigenetic analyses) and the Cytolab laboratory in Regensdorf, Switzerland (FSH and LH).
Saliva samples are collected with SaliCap sampling tubes of 2 mL capacity (IBL International GmbH, Hamburg, Germany). Participants are instructed to drool into the tube using a polypropylene straw (passive drool method). After collecting a full month of saliva samples, these can be returned directly to the Institute of Psychology during the study phase or stored at home in the freezer until the second laboratory appointment. The saliva samples are then thawed and centrifuged prior to biochemical analysis, using IBL Saliva Immunoassays (IBL International GmbH, Hamburg, Germany). All saliva samples are analyzed at the Cytolab laboratory in Regensdorf, Switzerland.
The Ava bracelet and its accompanying software is a CE-certified, Class 1 medical device approved by the Food and Drug Administration. It is worn at night on the wrist like a regular watch. The synchronization takes place every morning after waking up, with the corresponding application (Ava fertility tracker). The software runs on Apple and Android phones. The login for the Ava fertility tracker app corresponds to the subjects’ study ID number (personal code). Participants without a smartphone have been provided with an Android phone during the study phase by the University Research Priority Program Dynamics of Healthy Aging (funding source). The data recorded by the Ava bracelet are treated confidentially and in accordance with the privacy policy. Members of the Ava AG have signed a binding declaration of confidentiality. All data handling is subject to the data protection provisions approved by the Swiss Ethics Committee.
Biochemical data analyses
Our biochemical laboratory at the Institute of Psychology, University of Zurich, performs the salivary and DBS analyses. As listed in Table 2, most endocrine parameters are assessed in saliva. Thawed saliva samples are centrifuged and analyzed using enzyme-linked immunosorbent assay (ELISA; IBL International GmbH, Hamburg, Germany). FSH and LH levels are determined in DBS samples (Table 2) using the MILLIPLEX MAP Human Pituitary Magnetic Bead Panel 1 (Merck, Darmstadt, Germany). Intra-assay variation for this kit for FSH and LH is less than 10% and inter-assay variation less than 15%. Sensitivity is 6.40 pg/mL for FSH and 1.34 pg/mL for LH. Sex hormone-binding globulin (SHBG) can be measured in DBS using Human SHBG ELISA Kit (DRG International, Marburg Germany) with intra- and inter-assay coefficients of variability of less than 10% [78]. In terms of immune markers, the Quantikine HS ELISA Human IL-6 (HS600B, R&D Systems, Minneapolis, MN) is recommended for analyzing interleukin 6 (IL- 6; 37) and the sandwich ELISA (BioCheck, Inc., Foster City, CA) for investigating C-reactive protein [79]. Intra- and inter-assay variation for these kits for IL-6 and CRP are less than 15%. Detection rates of these assays are 0.67 pg/ml for IL-6 and 3.00 mg/L for CRP.
The DNA extraction is assessed from DBS samples, as described in Table 2. The Qiagen QIAamp DNA Investigator Kit (Qiagen, Hombrechtikon, Switzerland) is used to extract genomic DNA from three (each 3 mm in diameter) punches of blood-soaked filter paper. These punches are then eluted in 30 μl of RNase-free water, and the DNA concentration is assessed using the Qubit Fluorometer (Thermo Fischer Scientific, Reinach, Switzerland). For epigenetic analyses, we perform a bisulfite conversion of DNA using the EZ 96-DNA Methylation-Gold Kit (Zymo Research, Luzern, Switzerland). The procedure for the next-generation sequencing (NGS) library preparation is described elsewhere by our research group [80].
Statistical data analyses
A variety of statistical analytic strategies are planned for the different measures. Variables will be tested for normal distribution (Kolmogorov-Smirnov test) and homogeneity (Levene’s test). Skewed biological data will be logarithmically transformed [81]. Effect sizes will be estimated using eta-squared [82]. When investigating associations, correlation or partial correlation analyses will be conducted. If assumptions are violated, non-parametric methods will be applied. Regression analyses will be conducted to investigate the strength and form of associations between the various biopsychosocial markers and determinants and resilience- or depression-related factors. Analyses of variance, t-tests or Mann-Whitney U tests, depending on the level of measurement, are planned for the investigation of group differences between participants with and without a history of depression regarding different outcome measures associated with the menopausal transition. Furthermore, factor analyses will be used [81, 83] to examine which of the assessed variables related to resilience can be assigned to a common factor. For the longitudinal data evaluation of specific predictors of perimenopausal depression, multilevel analyses will be conducted [81]. In addition to inference methods, unsupervised methods (e.g. time series clustering methods [84, 85], machine learning algorithms [86]) will be necessary to analyze the massive volume of big data generated by the Ava bracelet.
The level of significance will be two-sided with p < .05, if not stated otherwise. The analysis will be run via the statistical software SPSS 25.0 (IBM, Armonk, NY, USA) and the open source software R (foundation for Statistical Computing, Vienna, Austria).
Results
Of the 1121 women interested in participating in the Swiss Perimenopause Study, a total of 265 (23.64%) were found to be eligible for the study after applying the inclusion and exclusion criteria. Of these, 177 declared their consent to participate. Up until December 2019, 42 women (23.73% of the participants) were reported as dropouts. Excessive effort for study participation was stated as a major cause for dropping out. Hence, the total sample consisted of 135 perimenopausal women.
The sociodemographic data of the participants are shown in Table 4. The participants’ age at the point of study enrollment ranged from 40 to 56 years, with a mean of 48.60 years. Most of the women were Swiss (85.2%) and married (56.3%). With regard to highest educational attainment, 43.0% had completed university education and 37.0% had completed secondary school. In accordance with the STRAW criteria [76], a total of 43.7% were in the early phase of the menopausal transition, while 56.3% were in the late phase at the point of screening. Overall, 60.0% of the women showed no prior depression and 40.0% of the participants reported a history of depression.
Discussion
The Swiss Perimenopause Study was established to investigate specific health-related markers associated with the menopausal transition. The aim of this large longitudinal study is to address the limited scientific understanding of the factors determining whether a woman undergoing the menopausal transition develops clinically relevant symptoms, such as those of a depressive disorder, or whether she manages to successfully adapt to the predominant biopsychosocial changes. Successful adaptation can be defined on a continuum of, for example, higher life satisfaction, lower psychological distress, better general psychological health, and milder menopausal complaints [14]. Thus, the study focuses on the perimenopause as the phase of strong hormonal fluctuations and the prevalence peak of burdensome symptoms.
Strengths of the Swiss Perimenopause Study include the longitudinal design and the in-depth investigation of the perimenopausal stage. Strict inclusion and exclusion criteria are deployed. All participants reported good to excellent subjective health at baseline. The gold-standard STRAW criteria were used to identify perimenopausal women on the basis of bleeding patterns. Individual menstrual cycle data are collected throughout the entire screening and study phase and matched with the assessment of mood changes. An extensive symptom assessment is carried out. Genetic and epigenetic analyses provide the opportunity to investigate inherited physical characteristics as well as gene-environment interactions. Repeated physiological data assessment enables the examination of patterns related to sleep disturbances and vasomotor symptoms. Furthermore, a comprehensive inspection of endocrine processes is planned, to complement the various psychosocial questionnaires applied in our study. Participants were randomly identified from the general population and represent an eligible sample for this longitudinal study on health-related factors of the menopausal transition.
There are a few limitations to consider: The study includes a predominantly well-educated sample, despite efforts to recruit women with different socioeconomic backgrounds. Additionally, the sample mostly consists of Caucasian women, although this corresponds to the distribution of the Swiss population. The initial goal to recruit a balanced sample size of women with and without a history of depression, with a sample size of 80 participants in each group, had to be discarded due to difficulties in enrolling eligible participants with prior depression. A total of 54 (40.0%) women with and 81 (60.0%) women without a history of depression were included in the final sample. Compared to the SWAN or the SMWHS studies, only a relatively small sample size and shorter study duration could be accomplished. However, a sufficiently large number of women has been achieved for all planned analyses, with adequate statistical power. Furthermore, the self-reported nature of all validated psychosocial questionnaires used might lead to a response bias and socially desirable answers. Thus, the evaluation of self-reported data using objective measures, where feasible, will be essential. Moreover, the strict inclusion and exclusion criteria applied in the Swiss Perimenopause Study offers the chance to investigate the actual changes and challenges associated with the perimenopause. However, as this is a very specific sample, the results cannot be applied to other subgroups of the general population such as younger women or postmenopausal women. Additionally, a selection bias has to be considered when interpreting the results. Women agreeing to participate in such a comprehensive examination about the menopausal transition might suffer more from menopausal symptoms, and women with a higher socioeconomic status might show a higher interest in participating in a scientific project.
There has been an increased scientific effort to address questions on the development of menopausal symptoms and depressed mood within the menopausal transition. Longitudinal data on specific factors associated with the menopausal transition are essential to foster our understanding of changes and symptoms experienced by middle-aged women. The Swiss Perimenopause Study, as one of the large studies investigating the menopausal transition, aims to contribute to increased scientific information about the biopsychosocial processes associated with this vulnerable time frame in a woman’s life and thus improve the clinical care of midlife women.
Availability of data and materials
Not available.
Abbreviations
- 2D:4D:
-
Second to Fourth Digit Ratio
- ALSWH:
-
Australian Longitudinal Study on Women’s Health
- App:
-
Application
- BIA:
-
Bioelectrical Impedance Analysis
- BMI:
-
Body Mass Index
- BSI:
-
Federal Office for Information Security (Bundesamt für Sicherheit in der Informationstechnik)
- CAR:
-
Cortisol Awakening Response
- DBS:
-
Dried Blood Spots
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- HIPAA:
-
Health Insurance Portability and Accountability Act
- HITECH:
-
Health Information Technology for Economic and Clinical Health Act
- HUNT:
-
Nord-Trøndelag Health Study
- IBL:
-
Immuno Biological Laboratories
- ISO:
-
International Organization for Standardization
- kHz:
-
Kilohertz
- LH:
-
Luteinizing Hormone (LH)
- MWMHP:
-
Melbourne Women’s Midlife Health Project
- MWHS:
-
Massachusetts Women’s Health Study
- NGS:
-
Next-Generation Sequencing
- POAS:
-
Penn Ovarian Aging Study
- SHBG:
-
Sex hormone-binding globulin
- SMWHS:
-
Seattle Midlife Women’s Health Study
- SSAE:
-
Statement on Standards for Attestation Engagements
- STRAW:
-
Stages of Reproductive Aging Workshop
- SWAN:
-
Study of Women’s Health Across the Nation
References
Buckler H. The menopause transition: endocrine changes and clinical symptoms. Menopause. 2005;11(2):61–5.
Greer G. The change: women, ageing and the menopause. London: Bloomsbury Publishing, London; 2018.
Jaspers L, Daan NMP, Van Dijk GM, Gazibara T, Muka T, Wen KX, et al. Health in middle-aged and elderly women: a conceptual framework for healthy menopause. Maturitas. 2015;81(1):93–8.
Freeman E, Sammel M, Lin H, Gracia C, Pien G, Nelson D, et al. Symptoms Associated With Menopausal Transition and Reproductive Hormones in Midlife Women. Obs Gynecol. 2007;110(2, Part 1):230–40.
Fiacco S, Walther A, Ehlert U. Steroid secretion in healthy aging. Psychoneuroendocrinology. 2018;(June):0–1.
Takahashi TA, Johnson KM. Menopause. Med Clin North Am. 2015;99(3):521–34.
Baker FC, Lampio L, Saaresranta T, Polo-Kantola P. Sleep and sleep disorders in the menopausal transition. Sleep Med Clin. 2018;13(3):443–56.
Greendale GA, Derby CA. Perimenopause and cognition. Obstet Gynecol Clin N Am. 2011;38(3):519–35.
Becker D, Lomranz J, Pines A, Shmotkin D, Nitza E, BennAmitay G, et al. Psychological distress around menopause. Psychosomatics. 2001;42(3):252–7.
O’Neill S, Eden J. The pathophysiology of menopausal symptoms. Obstet Gynaecol Reprod Med. 2017;27(10):303–10. Available from:. https://doi.org/10.1016/j.ogrm.2017.07.002.
Chiang C, Gallicchio L, Zacur H, Miller S, Flaws JA, Smith RL. Hormone variability and hot flash experience: results from the midlife women’s health study. Maturitas. 2019;119(May 2018):1–7.
Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric. 2007;10(3):197–214.
Brown L, Bryant C, Judd FK. Positive well-being during the menopausal transition: a systematic review. Climacteric. 2015;18(4):456–69.
Süss H, Ehlert U. Psychological resilience during the perimenopause. Maturitas [internet]. 2020;131:48–56. Available from. https://doi.org/10.1016/j.maturitas.2019.10.015.
Cutuli JJ, Masten AS. Resilience. In: Lopez S, editor. The encyclopedia of positive psychology. West Sassex: Wiley-Blackwell; 2009. p. 837–43.
Chedraui P, Pérez-López FR, Schwager G, Sánchez H, Aguirre W, Martínez N, et al. Resilience and related factors during female Ecuadorian mid-life. Maturitas. 2012;72(2):152–6.
Maartens LWWF, Knottnerus JAA, Pop VJJ, Maartens LWWF, Leusink LG, Knottnerus JAA, et al. Menopausal transition and increased depressive symptomatology: a community based prospective study. Maturitas. 2002;42(3):195–200.
Maki PM, Kornstein SG, Joffe H, Bromberger JT, Freeman EW, Athappilly G, et al. Guidelines for the evaluation and treatment of perimenopausal depression. Menopause. 2018;25(10):1.
Willi J, Ehlert U. Assessment of perimenopausal depression: A review. J Affective Dis. 2019;249:216–22.
Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci. 2008;33(4):331–43.
Soares CN. Depression and menopause: current knowledge and clinical recommendations for a critical window. Psychiatr Clin North Am. 2017;40(2):239–54.
Woods NF, Mitchell ES. The Seattle midlife Women’s health study: a longitudinal prospective study of women during the menopausal transition and early postmenopause. Women’s Midlife Heal. 2016;2(1):1–16.
McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992 Jan;14(2):103–15.
Woods NF, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle midlife Women’s health study. Res Nurs Health. 1997;20(2):119–29.
Tangen T, Mykletun A. Depression and anxiety through the climacteric period: an epidemiological study (HUNT-II). J Psychosom Obstet Gynecol. 2008;29(2):125–31.
Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med. 2001;52(3):345–56.
Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–90.
Brown WJ, Bryson L, Byles JE, Dobson AJ, Lee C, Mishra G, et al. Women’s health Australia: recruitment for a National Longitudinal Cohort Study. Women Heal. 1999;28(1):23–40.
Dennerstein L, Guthrie J, MSc P, Clark M, BAppSc P, Lehert P, et al. A population-based study of depressed mood in middle-aged, Australian-born women. Menopause. 2004;11(5):563–8.
Kirchengast S, Dottolo E, Praxmarer E, Huber J. Low digit ratio (2D:4D) is associated with early natural menopause. Am J Hum Biol. 2019;32:5–8.
Noser E, Walther A, Ehlert U. Are psychosocial resources associated with perceived facial aging in men? Gerontol Geriatr Med. 2017;3:233372141771487.
Clement U, Löwe B. Validation of the FKB-20 as scale for the detection of body image distortions in psychosomatic patients. Psychother Psychosom Med Psychol. 46(7):254–9.
Schulz P, Schlotz W, Becker P. TICS. Trier Inventar zum chronischen stress [the Trier inventory for the assessment of chronic stress]. Göttingen: Hogrefe; 2004.
Schmitz N, Kruse J, Tress W, Freudenthaler HH, Neubauer AC, Gabler P, et al. Psychometric properties of the general health questionnaire (GHQ-12) in a German primary care sample. Acta Psychiatr Scand [Internet]. 1999;100:462–8. Available from:. https://doi.org/10.1016/j.paid.2008.07.014.
Knoll N, Rieckmann N, Schwarzer R. Coping as a mediator between personality and stress outcomes: a longitudinal study with cataract surgery patients. Eur J Pers. 2005;19(3):229–47.
Hautzinger M, Bailer M. German version of the Center of Epidemiological Studies Depression Scale (CES-D; Allgemeine Depressionsskala; ADS-L). Weinheim: Beltz; 1993.
Freudenthaler HH, Neubauer AC, Gabler P, Scherl WG, Rindermann H. Testing and validating the trait emotional intelligence questionnaire (TEIQue) in a German-speaking sample. Pers Individ Dif [Internet]. 2008;45(7):673–8. Available from:. https://doi.org/10.1016/j.paid.2008.07.014.
Abler B, Kessler H. Emotion regulation questionnaire–Eine deutschsprachige Fassung des ERQ von gross und John [a German version of the ERQ by gross and John]. Diagnostica. 2009;55(3):144–52.
Hilbert A, Tuschen-Caffier B, Karwautz A, Niederhofer H, Munsch S. Eating disorder examination-questionnaire. Diagnostica. 2007;53(3):144–54.
Pöhlmann K, Brunstein JC. GOALS: Ein Fragebogen zur Messung von Lebenszielen [A questionnaire for measuring life goals]. Diagnostica. 1997;43:63–79.
Fiacco S, Palm-Fischbacher S, Campbell J, Ehlert U, Pluess M, Wurmser H, et al. Measuring female intrasexual competition by the scale for intrasexual competition: a validation of the German version. Arch Womens Ment Health. 2019;22(2):259–66.
Pluess M, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke KM, Hellhammer D, et al. Positive life events predict salivary cortisol in pregnant women. Psychoneuroendocrinology. 2012;37(8):1336–40.
Glaesmer H, Grande G, Braehler E, Roth M. The German version of the satisfactionwith life scale (SWLS) psychometric properties, validity, and population-based norms. Eur J Psychol Assess. 2011;27(2):127–32.
Pearlin LI, Menaghan EG, Lieberman MA, Joseph T, Lieberman MA, Mullan JT. The stress process. J Health Soc Behav. 1981;22(4):337–56.
Hauser GA, Schneider HP, Rosemeier PJ, Potthoff P. Menopause rating scale II: the self-assessment scale for climacteric complaints. J für Menopause. 1999;6(4):13–7.
Spitzer C, Hammer S, Löwe B, Grabe HJ, Barnow S, Rose M, et al. Die Kurzform des brief symptom inventory (BSI-18): erste Befunde zu den psychometrischen Kennwerten der deutschen version [the short version of the brief symptom inventory (BSI-18): preliminary psychometric properties of the German translation]. Fortschritte der Neurol Psychiatr. 2011;79(9):517–23.
Glaesmer H, Hoyer J, Klotsche J, Herzberg PY. Die deutsche version des life-orientation-tests (LOT-R) zum dispositionellen Optimismus und Pessimismus [the German version of the life orientation test (LOT-R) on dispositional optimism and pessimism]. Zeitschrift für Gesundheitspsychologie. 2008;16(1):26–31.
Lutz R, Heyn C, Kommer D. Fragebogen zur elterlichen Bindung [FEB; German version of the Parental Bonding Instrument; PBI]. In: Wie Gesund Sind Kranke? Zur Seelischen Gesundheit Kranker. Göttingen: Verlag für angewandte Psychologie; 1995. p. 183–99.
Rammstedt B, John OP. Short version of the big five inventory (BFI-K): development and validation of an economic inventory for assessment of the five factors of personality. Diagnostica. 2005;51(4):195–206.
Klein EM, Brähler E, Dreier M, Reinecke L, Müller KW, Schmutzer G, et al. The German version of the perceived stress scale - psychometric characteristics in a representative German community sample. BMC Psychiatry [Internet]. 2016;16(1):1–10 Available from: https://doi.org/10.1186/s12888-016-0875-9.
Ditzen B, Nussbeck F, Drobnjak S, Spörri C, Wüest D, Ehlert U. Validierung eines deutschsprachigen DSM-IV-TR basierten Fragebogens zum prämenstruellen Syndrom [a German DSM-IV-TR-based questionnaire for the screening of premenstrual symptoms]. Z Klin Psychol Psychother. 2011;40(3):149–59.
Penke L, Asendorpf JB. Beyond global Sociosexual orientations: a more differentiated look at Sociosexuality and its effects on courtship and romantic relationships. J Pers Soc Psychol. 2008;95(5):1113–35.
Ehrenthal JC, Dinger U, Lamla A, Funken B, Schauenburg H. Evaluation of the German version of the attachment questionnaire experiences in close relationships - revised“ (ECR−RD) [evaluation der deutschsprachigen version des Bindungsfragebogens experiences in close relationships - revised” (ECR−RD)]. Psychother Psych Med. 2009;59(6):215–23.
Sander J, Böcker S. The German version of the relationship assessment scale (RAS): a short scale for measuring satisfaction in a dyadic relationship. Diagnostica. 1993;39(1):55–62.
Schumacher J, Leppert K, Gunzelmann T, Strauss B, Brahler E. The resilience scale-a questionnaire to assess resilience as a personality characteristic. Z Klin Psychol Psychiatr Psychother. 2005;53(1):16–39.
Abbott A. Sequence analysis: new methods for old ideas. Annu Rev Sociol. 1995;21:93–113.
Hupfeld J, Ruffieux N. Validierung einer deutschen version der self-compassion scale (SCS-D) [validation of a German version of the self-compassion scale (SCS-D)]. Z Klin Psychol Psychother. 2011;40(2):115–23.
von Collani G, Herzberg PY. Zur internen Struktur des globalen Selbstwertgefühls nach Rosenberg [On the Internal Structure of Global Self-Esteem (Rosenberg)]. Zeitschrift für Differ und Diagnostische Psychol. 2003;24(1):9–22.
Bachem R, Maercker A. Development and psychometric evaluation of a revised sense of coherence scale. Eur J Psychol Assess. 2018;34(3):206–15.
Clayton AH, Goldfischer E, Goldstein I, Derogatis L, Nappi R, Lewis-D’Agostino DJ, et al. Validity of the decreased sexual desire screener for diagnosing hypoactive sexual desire disorder. J Sex Marital Ther. 2013;39(2):132–43.
Berner MM, Kriston L, Zahradnik HP, Härter M, Rohde A. Überprüfung der Gültigkeit und Zuverlässigkeit des Deutschen female sexual function index (FSFI-d) [validity and reliability of the German female sexual function index (FSFI-d)]. Geburtshilfe Frauenheilkd. 2004;64(3):293–303.
Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh sleep quality index in primary insomnia. J Psychosom Res. 2002;53(3):737–40.
Schulz U, Schwarzer R. Soziale unterstützung bei der krankheitsbewältigung: Die Berliner Social Support Skalen (BSSS) [Social Support in Coping with Illness: The Berlin Social Support Scales (BSSS)]. Diagnostica. 2003;49(2):73–82.
Proyer RT, Laub N. The German-language version of the expressions of spirituality inventory-revised: adaptation and initial validation. Curr Psychol. 2017;36(1):1–13.
Klinitzke G, Romppel M, Häuser W, Brähler E, Glaesmer H. Die deutsche version des childhood trauma questionnaire (CTQ) – psychometrische Eigenschaften in einer bevölkerungsrepräsentativen Stichprobe [the German version of the childhood trauma questionnaire (CTQ) – psychometric characteristics in a population representative sample]. Psychother Psychosom Med Psychol. 2012;62(2):47–51.
Bacon ER. Epigenetic regulation of endocrine aging transitions of the Perimenopausal and menopausal brain. Doctoral dissertation: University of Southern California; 2017.
Gardini ES, Chen GG, Fiacco S, Mernone L, Willi J, Turecki G, et al. Differential ESR1 promoter methylation in the peripheral blood—findings from the women 40+ healthy aging study. Int J Mol Sci. 2020;21:1–14.
Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause: Annals of the New York Academy of Sciences. NIH Public Access; 2010. p. 70–85.
Richardson TA, Robinson RD. Menopause and depression: a review of psychologic function and sex steroid neurobiology during the menopause. Prim Care Update Ob Gyns. 2000;7(6):215–23.
Harsh V, Meltzer-Brody S, Rubinow D, Schmidt P. Reproductive aging, sex steroids, and mood disorders. Harv Rev Psychiatry. 2009;17(2):87–102.
Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, et al. Stress-induced changes in human salivary alpha-amylase activity - associations with adrenergic activity. Psychoneuroendocrinology. 2006;31(1):49–58.
Nierop A, Wirtz PH, Bratsikas A, Zimmermann R, Ehlert U. Stress-buffering effects of psychosocial resources on physiological and psychological stress response in pregnant women. Biol Psychol. 2008;78(3):261–8.
Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: an indicator of sympathetic activity? Ann N Y Acad Sci. 2004;1032:258–63.
Gameiro CM, Romão F, Castelo-Branco C. Menopause and aging: changes in the immune system - a review. Maturitas. 2010;67(4):316–20.
Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60.
Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–68.
Wittchen HU, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV - SKID I. [Structured Interview for DSM-IV. SCID I]. Hogrefe, Göttingen: Göttingen; 1997.
Mernone L, Fiacco S, Ehlert U. Psychobiological factors of sexual functioning in aging women – findings from the women 40+ healthy aging study. Front Psychol [Internet]. 2019;10(March):1–13 Available from: https://www.frontiersin.org/article/10.3389/fpsyg.2019.00546/full.
Lacher DA, Berman LE, Chen TC, Porter KS. Comparison of dried blood spot to venous methods for hemoglobin A1c, glucose, total cholesterol, high-density lipoprotein cholesterol, and C-reactive protein. Clin Chim Acta. 2013;422:54–8. Available from:. https://doi.org/10.1016/j.cca.2013.03.032.
Fiacco S, Gardini ES, Mernone L, Schick L, Ehlert U. DNA methylation in healthy older adults with a history of childhood adversity—findings from the women 40+ healthy aging study. Front Psychiatry. 2019;10:1–11.
Field A. Discovering statistics using SPSS : (and sex and drugs and rock “n” roll). 3rd ed. London: Sage;.
Green SB, Salkind NJ, Akey TM. Using SPSS for windows. New Jersey: Upper Saddle River; 2000.
Epskamp S, Fried EI. A tutorial on regularized partial correlation networks. Psychol Methods. 2018;23(4):617–34.
Shirkhorshidi AS, Aghabozorgi S, Wah TY, Herawan T. Big data clustering: A review. Lect Notes Comput Sci. 2014;8583 LNCS(PART 5):707–20.
Aghabozorgi S, Seyed Shirkhorshidi A, Ying WT. Time-series clustering - a decade review. Inf Syst [Internet]. 2015;53:16–38. Available from:. https://doi.org/10.1016/j.is.2015.04.007.
Goodale BM, Shilaih M, Falco L, Dammeier F, Hamvas G, Leeners B. Wearable sensors reveal menses-driven changes in physiology and enable prediction of the fertile window: Observational study. J Med Internet Res. 2019;21:4.
Acknowledgements
We acknowledge the contribution of the participants who have provided data for the Swiss Perimenopause Study. We wish to thank Jessica Grub and our research team for their great support in recruiting the participants for the study. Finally, we warmly thank Sarah Mannion for proof reading the article.
Funding
This research was funded by the University Research Priority Program Dynamics of Healthy Aging of the University of Zurich in Switzerland.
Author information
Authors and Affiliations
Contributions
Conception: UE, JW / HS. Acquisition of data: JW / HS. Drafting the manuscript: JW / HS. Critical revision: UE. Jasmine Willi and Hannah Süss are shared first authors. The author(s) read and approved the final manuscript.
Authors’ information
UE: Project leader of the Swiss Perimenopause Study.
JW and HS: Principal investigators of the Swiss Perimenopause Study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study protocol of the Swiss Perimenopause Study was provided by the Swiss Ethics Committee (KEK-ZH-Nr. 2018–00555). Approved informed consent forms were obtained from each participant of the study before the screening phase (online) and before the study phase (paper and pencil). All participants were informed about the detailed study procedure, the estimated duration of the individual steps, data protection, and the aims of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Willi, J., Süss, H. & Ehlert, U. The Swiss Perimenopause Study – study protocol of a longitudinal prospective study in perimenopausal women. womens midlife health 6, 5 (2020). https://doi.org/10.1186/s40695-020-00052-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40695-020-00052-1