Abstract
Background
Trichoderma atroviride is a competitive soil-borne mycoparasitic fungus with extensive applications as a biocontrol agent in plant protection. Despite its importance and application potential, reference genes for RT-qPCR analysis in T. atroviride have not been evaluated. Light exerts profound effects on physiology, such as growth, conidiation, secondary metabolism, and stress response in T. atroviride, as well as in other fungi. In this study, we aimed to address this gap by identifying stable reference genes for RT-qPCR experiments in T. atroviride under different light conditions, thereby enhancing accurate and reliable gene expression analysis in this model mycoparasite. We measured and compared candidate reference genes using commonly applied statistical algorithms.
Results
Under cyclic light–dark cultivation conditions, tbp and rho were identified as the most stably expressed genes, while act1, fis1, btl, and sar1 were found to be the least stable. Similar stability rankings were obtained for cultures grown under complete darkness, with tef1 and vma1 emerging as the most stable genes and act1, rho, fis1, and btl as the least stable genes. Combining the data from both cultivation conditions, gapdh and vma1 were identified as the most stable reference genes, while sar1 and fis1 were the least stable. The selection of different reference genes had a significant impact on the calculation of relative gene expression, as demonstrated by the expression patterns of target genes pks4 and lox1.
Conclusion
The data emphasize the importance of validating reference genes for different cultivation conditions in fungi to ensure accurate interpretation of gene expression data.
Similar content being viewed by others
Background
Light serves as a crucial environmental cue regulating many cellular processes in filamentous fungi, including growth, development, and metabolism [1]. The ability of organisms to integrate external signals, such as light, contributes to their competitiveness in the environment and constitutes an important driving force of evolution and adaptation [2]. In ascomycetes, responses to light often involve the modulation of characteristic fungal processes, including sporulation, hyphal branching, and the production of secondary metabolites and hydrolytic enzymes [1]. From a transcriptomic point of view, light regulates the expression of numerous genes in various fungi [3,4,5]. Members of the genus Trichoderma are highly competitive soil-borne filamentous fungi and are widely used in agricultural and biotechnological applications, including biocontrol, plant growth promotion, and production of industrial enzymes. Trichoderma atroviride, in particular, is commonly used as a biocontrol agent because of its ability to parasitize and compete against a variety of phytopathogenic fungi [6]. Light profoundly influences the physiology of Trichoderma, affecting growth, conidiation, secondary metabolism, stress response, as well as mycoparasitic activity [7,8,9,10].
Quantitative reverse transcription PCR (RT-qPCR) is a commonly used technique for measuring gene expression levels in a wide range of biological samples. One of the key advantages of RT-qPCR is its high sensitivity and specificity, which allows the detection of even small changes in transcript levels [11]. However, accurate and reliable quantification of gene expression in RT-qPCR experiments requires appropriate normalization of the data to account for differences in RNA quantity, quality, and enzymatic efficiency [12]. Normalization of RT-qPCR data is typically achieved by using reference genes, which are genes that are stably expressed under different experimental conditions and are used to normalize the expression levels of target genes [13]. The use of reference genes is crucial for accurate and reliable gene expression analysis, as it allows the identification of true changes in gene expression levels that are not confounded by experimental variations [14].
Commonly used reference genes for RT-qPCR studies in filamentous fungi are associated with basic cellular structures (e.g. tubulin and actin) and genes involved in basic cellular processes, such as glycolysis (glyceraldehyde-3-phosphate dehydrogenase (gapdh)), synthesis of ribosomal subunits (rRNA), electron transport, and protein degradation (ubiquitin) [15,16,17,18]. These genes have been widely used as reference genes due to their assumed stable expression across different experimental conditions. However, the suitability of these reference genes can be limited under certain conditions and across different fungal species [19,20,21]. The study of gene expression in filamentous fungi is essential for understanding their physiology, development, and interactions with the environment. Yet, identifying stable reference genes for RT-qPCR analysis poses challenges, due to the complex growth morphology and variable gene expression patterns observed in filamentous fungi. In recent years, there has been growing awareness of the importance of appropriate reference gene selection in RT-qPCR experiments, and several statistical algorithms, such as geNorm [22], NormFinder [23], and BestKeeper [24], have been developed to assess reference gene stability across different samples and experimental conditions.
While Brunner et al. [25] have identified and validated reference genes in T. atroviride under conditions such as growth on different carbon sources, development, and confrontation, no investigation has focused specifically on different light conditions in this species. In this context, our study aimed to identify stable reference genes for RT-qPCR based gene expression analysis in T. atroviride grown under two different light conditions, thereby enhancing the accuracy and reliability of transcription analysis in this important model organism. A set of 10 candidate reference genes was evaluated in fungal cultures incubated either in the presence of a white light–dark cycle or complete darkness. Five in silico algorithms including RefFinder [26], GeNorm [22], NormFinder [23], BestKeeper [24] and comparative \(\Delta\)Ct [27] were used to evaluate the expression stability of each candidate gene, and the relative stability level under representative conditions was obtained. In addition, the transcript levels of two genes, a polyketide synthase gene (pks4) and the lipoxygenase-encoding gene lox1, both known to be involved in light-response in Trichoderma [28, 29], were analyzed to demonstrate the effectiveness and reliability of the selected reference genes.
Results
Primer performance analysis
Ten genes were selected as candidate reference genes for T. atroviride P1. The amplification efficiencies, correlation coefficients (\(\text {R}^2\)) and slope values of the candidate reference genes are shown in Table 1.
The specific fragments of all candidate reference genes were successfully amplified from T. atroviride-derived cDNA using the primers given in Table 2. Each PCR reaction resulted in a single product with the expected fragment size confirming the amplification specificity of the primers (data not shown). Moreover, the melting curve of each primer pair showed a single peak, further verifying the specificity of the primer pairs and ruling out the presence of primer dimers (Additional file 1). The amplicon sizes ranged from 112 to 305 bp, and the amplification efficiencies (E) of the primer pairs ranged from 90.26% (rho) to 109.87% (tef1). In addition, all 10 correlation coefficients (\(\text {R}^2\)) were \(\ge \)0.99. These results indicate that the primers designed for all 10 candidate reference genes meet the standards for RT-qPCR and could be used in subsequent experiments.
Expression levels of candidate reference genes
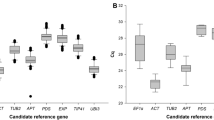
Mean quantification cycle (Cq) values of the ten candidate reference genes ranged from 17.78 to 30.93 in samples derived from cyclic light–dark cultivation conditions and from 15.03 to 27.84 upon cultivation under complete darkness, showing a wide variation of expression levels among the evaluated candidate reference genes (Fig. 1). The average Cq values from all genes indicated that the two genes with the highest (act1) and lowest (tef1) expression levels are basically the same regardless of the evaluated condition.
Expression variability of each of the ten tested reference gene candidates among both tested light conditions, light–dark cycle and complete darkness (dark–dark), presented as box-and-whisker plot. The line across the box indicates the median value. The box indicates the 25th and 75th percentiles, whereas the whisker represents the maximum and minimum values. A Raw expression of each gene in T. atroviride cultivated under light–dark conditions shown as Cq values. B Raw expression of each gene in T. atroviride cultivated under complete darkness shown as Cq values
Expression stability of candidate reference genes
The expression stability of candidate reference genes was estimated using the geNorm [22], NormFinder [23], BestKeeper [24], comparative \(\Delta\)Ct [27], and RefFinder [26] algorithms. With these algorithms, reference genes can be ordered from the most stably expressed, exhibiting the lowest Cq variation, to the least stable. The stability values of the candidate reference genes observed in samples derived from light–dark cultivation (Additional file 2) indicated that tbp and rho were the most stable among the genes tested. Based on the SD values (standard deviation of the Cq value) calculated by BestKeeper, all genes were stably expressed during light–dark cultivation, as indicated by an SD of <1. However, tef1 and gapdh showed the best stability values. According to the ranking by RefFinder, tbp, rho, rpl6e, gapdh, and tef1 emerged as the most stable genes with respect to fungal cultivation under a light–dark cycle. In relation to the less stable genes, all analyzed algorithms classified act1, fis1, btl, and sar1 as the least stable of all candidate genes tested. Similar to the results obtained for growth of T. atroviride under light–dark conditions, all tested genes were stably expressed under conditions of complete darkness, as reflected by SD values ranging from 0.11 to 0.45 (Additional file 3). The RefFinder stability classification of the tested candidate reference genes upon fungal cultivation under complete darkness indicated tef1 and vma1 as the most stable genes. BestKeeper identified tef1 and gapdh, and GeNorm identified tbp and gapdh as the most stable candidates among the tested genes under conditions of complete darkness. Sar1, btl, and fis1 were identified by comparative \(\Delta\)Ct, NormFinder and GeNorm algorithms as the least stable of all candidate genes tested. With the BestKeeper algorithm, act1, rho, and btl emerged as the least stable genes. The general ranking by RefFinder displayed sar1, rho, fis1, and btl as the least stable of all tested candidate genes. In order to accurately identify the most stable genes under both light conditions, all data were combined and jointly analyzed (Fig. 2, Additional file 4). NormFinder and comparative \(\Delta\)Ct identified gapdh as the most stable gene, followed by act1 and tef1. GeNorm identified vma1 and tbp as being most stably expressed with identical stability values (0.371). For BestKeeper, rpl6e displayed the greatest expression stability (SD = 0.91). The overall ranking established by RefFinder revealed that the most stable candidate reference genes were gapdh and vma1. All algorithms identified sar1 and fis1 as the least stable among the tested candidate genes across both cultivation conditions.
Comprehensive gene stability of the tested ten candidate reference genes over both cultivation conditions (light–dark cycle and complete darkness) as calculated by RefFinder. Lower values represent higher stability in the tested samples. See also Additional file 4
Validation of reference gene selection
The impact of selecting different reference genes on the calculation of relative gene expression was evaluated by examining the expression patterns of the target genes pks4 and lox1 in response to light. Two pairs of candidate reference genes, namely gapdh and vma1 (the two most stable reference genes) as well as sar1 and fis1 (the two least stable reference genes), were employed to normalize Cq values. The obtained results revealed that pks4 is expressed only in samples derived from cultivation under light–dark conditions, while no expression was detected upon fungal growth in complete darkness (Fig. 3A). In contrast, lox1 expression levels were higher when T. atroviride was cultivated under complete darkness compared to light–dark conditions (Fig. 3B). Comparative analysis of the expression profiles demonstrated significant variation in gene expression for both target genes when different reference genes were utilized. Notably, when the most stable reference genes were employed, the obtained transcript levels for pks4 and lox1 under light–dark conditions were remarkably higher than when normalized with the least stable reference genes. These findings indicate a direct association between the calculated expression level of a target gene and the reference genes employed for data normalization. Inappropriate selection of reference genes, as demonstrated here for the determination of pks4 and lox1 gene expression, led to an overestimation of their transcript levels, underscoring the importance of validating reference genes prior to their application in gene expression studies.
Relative expression of target genes pks4 and lox1 using validated reference genes including the most (gapdh+vma1) or the least (sar1+fis1) stable reference genes for normalization. A Relative expression of pks4 in T. atroviride cultivated under light–dark conditions (WT LD) or complete darkness (WT DD) normalized to either gapdh+vma1 (blue) or sar1+fis1 (red). B Relative expression of lox1 in T. atroviride cultivated under light–dark conditions (WT LD) or complete darkness (WT DD) normalized to either gapdh+vma1 (blue) or sar1+fis1 (red). Asterisks indicate significant differences (p < 0.05, one-way ANOVA)
Discussion
Our findings demonstrate that gapdh and vma1 were the most stable reference genes across varying light conditions for T. atroviride. These results are in line with previous research indicating that vma1 and gapdh serve as reliable reference genes in various tissues, including other filamentous fungi such as Neurospora crassa [30] and Aspergillus flavus [31], under various cultivation conditions [32, 33]. However, numerous studies have shown that the mentioned housekeeping genes are regulated and exhibit expression variability in response to experimental conditions [34,35,36,37]. Consequently, it remains up to the researcher to judiciously select a reference gene that ensures reliable normalization within a specific experimental context. Paolacci et al. further emphasized the significance of studies aimed at identifying and validating internal control genes suitable for each unique experimental scenario, suggesting that alternative normalization genes may replace traditionally employed ones [38]. This underscores the importance of meticulous selection and validation of reference genes tailored to specific experimental conditions.
The vma1 gene encodes the subunit A of vacuolar ATPase, which plays a critical role in regulating vacuolar pH and ion homeostasis [39]. Likewise, gapdh encodes glyceraldehyde-3-phosphate dehydrogenase, an essential enzyme in glycolysis [40]. Both genes are constitutively expressed and involved in fundamental cellular processes, which may explain their consistent expression patterns even across different light conditions. Consistently, act1, which encodes actin involved in cytoskeletal formation, demonstrated stable expression, as expected for a fundamental cellular component [41]. In T. virens, T. atroviride and T. reesei, the elongation factor encoding gene tef1 maintained constant expression and was used as normalizer evaluating gene expression when these fungi were confronted with the plant pathogen Rhizoctonia solani [42]. Our findings also support tef1 as a good normalizer for T. atroviride strain P1 grown under different light conditions, ranking after gapdh and vma1. In contrast, fis1 and sar1 were identified as the least stable reference genes in our study. The essential role of fis1 in conidiation in other filamentous fungi, such as Magnaporthe oryzae, may explain its instable expression pattern [43]. Interestingly, a study evaluating potential reference genes in T. reesei found sar1 to be the most stable gene for bioreactor cultivation, while act1 did not rank among the most reliable reference genes [16]. In T. atroviride, sar1 turned out to be the most stable reference gene for biocontrol conditions and cultivation on solid media in presence of various carbon sources, alongside act1. Moreover, when comparing cultivation of T. atroviride on solid PDA and liquid PDB, act1 and tef1 were identified as the most stable reference genes [25].
The choice of reference genes for normalization is crucial in gene expression studies, as it directly influences the accuracy and reliability of the results [44]. In our study, the use of sar1 and fis1 as reference genes led to an overestimation of transcript levels of pks4, encoding a polyketide synthase responsible for pigment biosynthesis in T. reesei [28] and T. atroviride, as well as lox1, a lipoxygenase encoding gene known to be involved in the response of T. atroviride to light [29]. This discrepancy demonstrates the inherent variability introduced by the selection of inappropriate reference genes and emphasizes the importance of using stable and reliable normalizers for accurate data interpretation. The overestimation of transcript levels when using unsuitable reference genes can have significant implications on the conclusions drawn and suggested biological role. It can lead to misinterpretation of gene regulation patterns, false identification of differentially expressed genes, and erroneous conclusions about the functional roles of target genes. This has previously been shown in other reports as well, where the choice of unstable genes applied for normalization led to misinterpretation of expression data [45,46,47].
The validation of the tested reference genes in this study also revealed an intriguing expression pattern of the examined target genes pks4 and lox1 in T. atroviride under different light conditions. Specifically, pks4 was found to be transcribed exclusively under light–dark conditions and not under complete darkness, while lox1 exhibited higher expression levels in complete darkness compared to light–dark conditions. The light-induced expression of pks4 suggests its involvement in pigment biosynthesis, which is known to be influenced by the presence of light–dark cycles, consistent with the presence of pigmented spores solely in the presence of light in vivo [48]. On the other hand, the increased expression of lox1 in complete darkness highlights its role in light-response mechanisms that are specifically triggered in the absence of light [29]. To obtain reliable and meaningful results, it is crucial to validate reference genes specific to the experimental conditions and biological system under investigation [49]. Our study emphasizes the importance of using stable reference genes like gapdh and vma1 for normalization in RT-qPCR based gene expression studies in T. atroviride under different light conditions. The use of appropriate reference genes ensures that the expression profiles of target genes are accurately represented, enabling valid interpretations and biological conclusions.
Conclusions
In this study, we assessed the stability of 10 candidate reference genes in T. atroviride upon cultivation under different light conditions using multiple evaluation algorithms. Among the examined genes, the commonly used reference gene sar1 was identified as one of the least stable reference genes across the experimental conditions tested, along with fis1 and btl. Hence, these genes are not recommended for normalization purposes in studies addressing light-dependent gene expression. In contrast, gapdh and vma1 demonstrated consistent and stable transcript levels, making them suitable reference genes for RT-qPCR analysis in T. atroviride under varying light conditions. Therefore, we propose the simultaneous use of gapdh and vma1 as optimal reference genes to enhance the accuracy and reliability of gene expression studies in T. atroviride, particularly when addressing light-mediated processes. We could also show that inappropriate reference gene selection led to overestimation of gene expression levels. Therefore, the validation and careful selection of reference genes are critical to ensure reliable RT-qPCR based gene expression analysis in fungi. This research provides valuable insights into the selection of stable reference genes and underscores the importance of appropriate normalization for accurate gene expression analysis, thus contributing to our understanding of gene regulation and supporting future investigations of the model mycoparasite T. atroviride.
Material and methods
Strains and growth conditions
Trichoderma atroviride strain P1 (ATCC 74058) was used throughout this study. For cultivation, the strain was pre-grown for three days on potato dextrose agar (PDA; Becton, Dickinson and Company, Le Pont De Claix, France) plates at 25 \(^{\circ }\hbox {C}\) under either light–dark (12:12 h cycle; 1040 Lux; Snijders Micro Clima-Series TM Labs Economic Lux Chamber; Snijders Labs, Tiburg, Netherlands) conditions or in complete darkness. In order to ensure consistency of the respective cultivation condition, agar plugs (6 mm diameter) of the actively growing colony margins were propagated twice, after three days each, to the center of fresh PDA plates. For the final cultivation step, the agar plugs from the actively growing colony margins from the pre-cultures were inoculated in quadruplicates centrally on fresh PDA plates covered with a cellophane membrane. Plates were incubated at 25 \(^{\circ }\hbox {C}\) under a light–dark cycle (12:12 h; 1040 Lux) or under complete darkness for 3 days. The biomass was collected, immediately frozen in liquid nitrogen, and kept at − 70 \(^{\circ }\hbox {C}\) for subsequent RNA extraction.
Total RNA extraction and cDNA synthesis
Frozen mycelia were homogenized using the CryCooler (OPS Diagnostics). Total RNA from 1 mg of each sample was extracted using TRIzol Reagent (Invitrogen, Karlsruhe, Germany). Isolated RNA was treated with DNAse I (ThermoFisher Scientific Baltic UAB, Vilnius, Lithuania) and RNA integrity and absence of genomic DNA was further assessed by electrophoresis using 1.5% agarose gels. 1 \(\upmu \hbox {g}\) of treated RNA was reverse transcribed to cDNA using the Revert Aid H- First Strand cDNA Synthesis Kit (ThermoFisher Scientific Baltic UAB, Vilnius, Lithuania) with a 1:1 combination of the provided oligo(dT) and random hexamer primers. The cDNA mixture was diluted 1:10 with ddH2O and stored at -70 \(^{\circ }\hbox {C}\) for subsequent RT-qPCR analysis.
Reference gene selection and primer design
Ten reference genes that were commonly used in eukaryotes were selected to screen for stable gene expression in T. atroviride P1 grown under the described light conditions. These candidate reference genes included sar1 (coding for a SAR/ARF type small GTPase), act1 (actin-encoding), vma1 (vacuolar ATPase subunit 1-encoding), tbp (TATA-binding protein-encoding), gapdh (glyceraldehyde 3-phosphate dehydrogenase-encoding), tef1 (translation elongation factor 1a-encoding), rho (coding for a GTPase activator), btl (beta-tubulin-encoding), rpl6e (ribosomal protein L6e-encoding) and fis1 (coding for a mitochondrial membrane fission protein) selected according to previously published reference gene analysis performed in N. crassa [30], T. reesei [16, 50] and Talaromyces versatilis [51]. Both coding and genomic sequences of each gene were obtained from the JGI T. atroviride P1 database (https://mycocosm.jgi.doe.gov/Triatrov1/Triatrov1.home.html). Primers for act1, btl, fis1, gapdh, rho, rpl6e, tbp, tef1 and vma1 were designed using the NCBI Primer Blast tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/), with an annealing temperature of 60\(^{\circ }\hbox {C}\). Primers for sar1 were obtained from Brunner et al. [25]. Primers were designed to span exon-exon junctions and each primer pair was separated by at least one intron to minimise the amplification of contaminant gDNA. The potential for homo- or hetero dimer formation was examined for each primer pair using the IDT Oligo Analyzer tool (https://www.idtdna.com/pages/tools/oligoanalyzer). Only primers with a \(\Delta\)G value greater than − 7 kcal/mole were used. Primers were obtained from Microsynth AG (Balgach, Switzerland). The specificities of the primers were verified by conventional PCR using cDNA as templates and the amplicons were visualized by electrophoresis on 2.0% agarose gels. The primer sequences are given in Table 2.
RT-qPCR conditions and amplification efficiency
RT-qPCR was performed using the LUNA Universal qPCR Master Mix (New England BioLabs GmbH) in an \(\text {qTOWER}^3\) G cycler (Analytik Jena AG, Jena, Germany). All reactions except the ones for vma1 were performed in a 20 \(\upmu \,\hbox {L}\) mixture containing 10 \(\upmu \,\hbox {L}\) of Master Mix, 4 \(\upmu \,\hbox {L}\) of cDNA template with final amount of 200 ng, 0.8 \(\upmu \,\hbox {L}\) of each primer with final concentration of 400 nM, and 4.4 \(\upmu \,\hbox {L}\) of sterile water. For vma1, 0.4 \(\upmu \,\hbox {L}\) of each primer with a final concentration of 200 nM, and consequently 4.8 \(\upmu \,\hbox {L}\) of sterile water were used per reaction. qPCR analysis included four technical replicates of each sample. Non-template controls (NTC) in which the cDNA was replaced with nuclease free water were also included for each primer pair to exclude possible contaminations. In addition, RT-minus reactions, prepared during cDNA synthesis without adding reverse transcriptase were performed to monitor for possible gDNA amplification. The qPCR cycling conditions were as follows: 95\(^{\circ }\hbox {C}\) for 1 min, followed by 50 cycles of 95\(^{\circ }\hbox {C}\) for 15s and 60\(^{\circ }\hbox {C}\) to 65\(^{\circ }\hbox {C}\) for 30s. Amplicons were verified by subsequent melting curve analysis, performed from 60\(^{\circ }\hbox {C}\) to 95\(^{\circ }\hbox {C}\). Amplification efficiency of each primer pair was evaluated by the standard curve method using serial dilutions of cDNA (1:10, 1:100, 1:1000, and 1:10000) as a template. The efficiencies (E) of corresponding primers were calculated in accordance with the equation \(E = 1+10^{(-1/slope)}*100\).
Stability analysis of candidate reference genes
The expression stabilities of the ten tested candidate reference genes under the two different light conditions were evaluated using RefFinder, which includes the four commonly used in silico algorithms BestKeeper [24], geNorm [22], NormFinder [23], and comparative \(\Delta\)Ct [27]. The candidate reference genes were ranked by each algorithm individually and RefFinder was then applied to calculate a comprehensive value based on the four individual results. The geometric means of the individual ranks were used to determine their expression stability, with the lowest value representing the most stably expressed gene while the highest representing the least stably expressed candidate.
Validation of reference gene stability
To validate the reliability of the selected reference genes, the relative expression levels of the polyketide synthase-encoding gene pks4 and the lipoxygenase-encoding gene lox1 were analyzed in T. atroviride grown in the presence of light or darkness. The nucleotide sequences of pks4 and lox1 were obtained from the JGI T. atroviride P1 database (https://mycocosm.jgi.doe.gov/Triatrov1) and primers (Table 2) were designed using the NCBI Primer Blast tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The RT–qPCR conditions were set up the same as described above. The relative expression of both genes was calculated using qRAT [52] with the two most stable and two least stable candidate reference genes obtained by the comprehensive assessment used as normalizers.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fischer R, Aguirre J, Herrera-Estrella A, Corrochano LM. The complexity of fungal vision. Microbiol Spectr. 2016;4:10–128.
Tisch D, Schmoll M. Light regulation of metabolic pathways in fungi. Appl Microbiol Biotechnol. 2010;85:1259–77.
Chen C-H, Ringelberg CS, Gross RH, Dunlap JC, Loros JJ. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 2009;28:1029–42.
Ruger-Herreros C, et al. Regulation of conidiation by light in Aspergillus nidulans. Genetics. 2011;188:809–22.
Tisch D, Schmoll M. Targets of light signalling in Trichoderma reesei. BMC Genomics. 2013;14:657.
Karlsson M, Atanasova L, Jensen DF, Zeilinger S. Necrotrophic mycoparasites and their genomes. Microbiol Spectr. 2017;5:10–128.
Schmoll M, Esquivel-Naranjo EU, Herrera-Estrella A. Trichoderma in the light of day-physiology and development. Fungal Genet Biol. 2010;47:909–16.
Horwitz BA, Gressel J, Malkin S. Photoperception mutants in Trichoderma: mutants that sporulate in response to stress but not light. Curr Genet. 1985;9:605–13.
Hitzenhammer E, et al. Ypr2 is a regulator of light modulated carbon and secondary metabolism in Trichoderma reesei. BMC Genomics. 2019;20:211.
Rodriguez-Iglesias A, Schmoll M. Protein phosphatases regulate growth, development, cellulases and secondary metabolism in Trichoderma reesei. Sci Rep. 2019;9:10995.
Bustin SA. Quantification of mrna using real-time reverse transcription pcr (rt-pcr): trends and problems. J Mol Endocrinol. 2002;29:23–39.
Derveaux S, Vandesompele J, Hellemans J. How to do successful gene expression analysis using real-time pcr. Methods. 2010;50:227–30.
Radonić A, et al. Guideline to reference gene selection for quantitative real-time pcr. Biochem Biophys Res Commun. 2004;313:856–62.
Guénin S, et al. Normalization of qrt-pcr data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J Exp Bot. 2009;60:487–93.
Bohle K, et al. Selection of reference genes for normalisation of specific gene quantification data of Aspergillus niger. J Biotechnol. 2007;132:353–8.
Steiger MG, Mach RL, Mach-Aigner AR. An accurate normalization strategy for rt-qpcr in Hypocrea jecorina (Trichoderma reesei). J Biotechnol. 2010;145:30–7.
Zhou Y-H, et al. Selection of optimal reference genes for expression analysis in the entomopathogenic fungus Beauveria bassiana during development, under changing nutrient conditions, and after exposure to abiotic stresses. Appl Microbiol Biotechnol. 2012;93:679–85.
Zampieri D, Nora LC, Basso V, Camassola M, Dillon AJP. Validation of reference genes in Penicillium echinulatum to enable gene expression study using real-time quantitative rt-pcr. Curr Genet. 2014;60:231–6.
Dheda K, et al. Validation of housekeeping genes for normalizing rna expression in real-time pcr. BioTechniques. 2004;37:112–4, 116, 118–9.
Lee PD, Sladek R, Greenwood CMT, Hudson TJ. Control genes and variability: absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 2002;12:292–7.
Gutierrez L, et al. The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (rt-pcr) analysis in plants. Plant Biotechnol J. 2008;6:609–18.
Vandesompele J, et al. Accurate normalization of real-time quantitative rt-pcr data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1.
Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-pcr data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–50.
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: bestkeeper-excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–15.
Brunner K, et al. Trichoderma g protein-coupled receptors: functional characterisation of a camp receptor-like protein from Trichoderma atroviride. Curr Genet. 2008;54:283–99.
Xie F, Wang J, Zhang B. Reffinder: a web-based tool for comprehensively analyzing and identifying reference genes. Funct Integr Genomics. 2023;23:125.
Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time pcr. BMC Mol Biol. 2006;7:33.
Atanasova L, Knox BP, Kubicek CP, Druzhinina IS, Baker SE. The polyketide synthase gene pks4 of Trichoderma reesei provides pigmentation and stress resistance. Eukaryotic Cell. 2013;12:1499–508.
Speckbacher V, et al. The lipoxygenase lox1 is involved in light- and injury-response, conidiation, and volatile organic compound biosynthesis in the mycoparasitic fungus Trichoderma atroviride. Front Microbiol. 2020;11:2004.
Cusick KD, et al. Selection and evaluation of reference genes for expression studies with quantitative pcr in the model fungus Neurospora crassa under different environmental conditions in continuous culture. PLoS ONE. 2014;9:e112706.
Caceres I, et al. Deciphering the anti-aflatoxinogenic properties of eugenol using a large-scale q-pcr approach. Toxins. 2016;8:123.
de Jonge HJM, et al. Evidence based selection of housekeeping genes. PLoS ONE. 2007;2:e898.
Løvdal T, Lillo C. Reference gene selection for quantitative real-time pcr normalization in tomato subjected to nitrogen, cold, and light stress. Anal Biochem. 2009;387:238–42.
Raffaello T, Asiegbu FO. Evaluation of potential reference genes for use in gene expression studies in the conifer pathogen (Heterobasidion annosum). Mol Biol Rep. 2013;40:4605–11.
Li Z, et al. Selection of appropriate reference genes for quantitative real-time reverse transcription pcr in Betula platyphylla under salt and osmotic stress conditions. PLoS ONE. 2019;14:e0225926.
Glare EM, Divjak M, Bailey MJ, Walters EH. beta-Actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mrna levels. Thorax. 2002;57:765–70.
Valle-Maldonado MI, et al. Selection of reference genes for quantitative real time rt-pcr during dimorphism in the zygomycete Mucor circinelloides. Mol Biol Rep. 2015;42:705–11.
Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M. Identification and validation of reference genes for quantitative rt-pcr normalization in wheat. BMC Mol Biol. 2009;10:11.
Bowman EJ, Kendle R, Bowman BJ. Disruption of vma-1, the gene encoding the catalytic subunit of the vacuolar h(+)-atpase, causes severe morphological changes in Neurospora crassa. J Biol Chem. 2000;275:167–76.
Sirover MA. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: biochemical mechanisms and regulatory control. Biochimica et Biophysica Acta. 2011;1810:741–51.
Lichius A, Berepiki A, Read ND. Form follows function - the versatile fungal cytoskeleton. Fungal Biol. 2011;115:518–40.
Atanasova L, et al. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genomics. 2013;14:121.
Khan IA, et al. Mitochondrial fission protein mofis1 mediates conidiation and is required for full virulence of the rice blast fungus Magnaporthe oryzae. Microbiol Res. 2015;178:51–8.
Dheda K, et al. The implications of using an inappropriate reference gene for real-time reverse transcription pcr data normalization. Anal Biochem. 2005;344:141–3.
Pinto F, Pacheco CC, Ferreira D, Moradas-Ferreira P, Tamagnini P. Selection of suitable reference genes for rt-qpcr analyses in cyanobacteria. PLoS ONE. 2012;7:e34983.
Li M-Y, et al. Validation and comparison of reference genes for qpcr normalization of celery (Apium graveolens) at different development stages. Front Plant Sci. 2016;7:313.
de Boer ME, et al. Reference genes for qrt-pcr tested under various stress conditions in Folsomia candida and Orchesella cincta (insecta, collembola). BMC Mol Biol. 2009;10:54.
Betina V, Farkas V. Sporulation and light-induced development in Trichoderma. Trichoderma and Gliocladium. 1998;75.
Gutierrez L, Mauriat M, Pelloux J, Bellini C, van Wuytswinkel O. Towards a systematic validation of references in real-time rt-pcr. Plant Cell. 2008;20:1734–5.
Tisch D, Kubicek CP, Schmoll M. New insights into the mechanism of light modulated signaling by heterotrimeric g-proteins: envoy acts on gna1 and gna3 and adjusts camp levels in Trichoderma reesei (Hypocrea jecorina). Fungal Genet Biol. 2011;48:631–40.
Llanos A, François JM, Parrou J-L. Tracking the best reference genes for rt-qpcr data normalization in filamentous fungi. BMC Genomics. 2015;16:71.
Flatschacher D, Speckbacher V, Zeilinger S. qrat: an r-based stand-alone application for relative expression analysis of rt-qpcr data. BMC Bioinform. 2022;23:286.
Funding
Open access funding provided by Austrian Science Fund (FWF). The research was supported by the Austrian Science Fund FWF (Grant No. P32179-B to S.Z.) and the doctoral program BioApp from the University of Innsbruck. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
SZ and DF conceptualized this study. DF and AE conceived this study and AE performed the experiments. DF drafted the manuscript, and AE participated in drafting the manuscript. SZ revised the manuscript and acquired funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Susanne Zeilinger is an Editorial Board Member of Fungal Biology and Biotechnology and was not involved in the peer review of this paper. The authors declare that they have no other competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Melting curves of the ten tested candidate reference genes (sar1, act1, vma1, tbp, gapdh, tef1, rho, btl, rpl6e, fis1) generated by RT-qPCR showing the specificity of the respective primers.
Additional file 2: Table S1.
Expression stability of the tested candidate reference genes in T. atroviride cultivated under light–dark conditions calculated by RefFinder, GeNorm, NormFinder, BestKeeper and comparative \(\Delta\)Ct algorithms. Ranks are according to the value of stability of the respective algorithm.
Additional file 3: Table S2.
Expression stability of the tested candidate reference genes in T. atroviride cultivated under complete darkness calculated by RefFinder, GeNorm, NormFinder, BestKeeper and comparative \(\Delta\)Ct algorithms. Ranks are according to the value of stability of the respective algorithm.
Additional file 4: Table S3.
Overall expression stability of the tested candidate reference genes in T. atroviride cultivated under light–dark and complete darkness calculated by RefFinder, GeNorm, NormFinder, BestKeeper and comparative \(\Delta\)Ct algorithms. Ranks are according to the value of stability of the respective algorithm.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Flatschacher, D., Eschlböck, A. & Zeilinger, S. Identification and evaluation of suitable reference genes for RT-qPCR analyses in Trichoderma atroviride under varying light conditions. Fungal Biol Biotechnol 10, 20 (2023). https://doi.org/10.1186/s40694-023-00167-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40694-023-00167-w