Abstract
Background
During the last decades the frequency and global distribution of toxic cyanobacteria blooms has increased globally, which has been attributed to the eutrophication and climate change. In Chile there have been reports on blooms in aquatic ecosystem in localities with high density population and on the presence of five congeners of microcystins but only two documented toxics blooms with hundreds fish kills. We investigated the presence of toxic cyanobacteria blooms in the Lo Galindo urban lake, Concepción city, and the environmental factors that influence the abundance of cyanobacteria and microcystins concentration. Lo Galindo Lake, is used for various recreational and eventually as a drinking water source.
Results
Toxic blooms of Microcystis aeruginosa are developed in Lo Galindo lake, those that occur throughout the year in a wide range of environmental conditions, forming scums blooms during summer and dispersive blooms in all seasons. There are different microcystin congeners, the most frequent congener was MC-RR (21 %) and the highest concentration corresponded to 115.4 µg L-1 MC-LR.
Conclusions
The dominance and development of the M. aeruginosa blooms in the lake is determined by various environmental factors such as temperature, nutrients, diversity of taxa and wind speed that affect the formation of disperse-type blooms and/or scums; the latter are developed only in summer, coinciding with the highest temperature and concentrations of total microcystins. In the lake the microcystin and different types of congener is highly variable, so special care is recommended to use lake water for consumption and for recreational activities. The emergence and persistence of Microcystis blooms in this body of water are considered a potential health risk for the inhabitants of the area, considering the proximity and the system use by the inhabitants.
Resumen
Antecedentes
Durante las últimas décadas la frecuencia y la distribución global de las floraciones de cianobacterias tóxicas han aumentado a nivel mundial, lo que ha sido atribuido a la eutrofización y al cambio climático global. En Chile existen reportes de floraciones en ecosistemas acuáticos en zonas con alta densidad poblacional y la presencia de cinco congéneres de microcistinas, pero sólo dos floraciones tóxicas que ocasionaron la muerte de cientos de peces. Se investigó la presencia de floraciones de cianobacterias tóxicas en el lago urbano Lo Galindo, ciudad de Concepción, y los factores ambientales que influyen en la abundancia de cianobacterias y concentración de microcistina. Este lago, se utiliza para recreación y como fuente de agua potable alternativa para la ciudad.
Resultados
En el lago se desarrollan floraciones tóxicas de Microcystis aeruginosa, las que se presentan durante todo el año en un amplio rango de condiciones ambientales, formando floraciones tipo acumulativas (scums) durante el verano y dispersivas en todas las épocas del año. Existen diferentes congéneres de microcistina, el más frecuente es MC-RR (21.0 %) y el de mayor concentración MC-LR (115.4 µg L-1).
Conclusión
La dominancia y el desarrollo de las floraciones de M. aeruginosa está determinada por diversos factores ambientales como la temperatura, nutrientes, diversidad de taxa y la velocidad del viento que afectan a la formación de floraciones dispersivas y/o scums, esta última se desarrolla sólo en verano, coincidiendo con las mayores temperaturas y concentraciones de microcistinas totales. La concentración de los congéneres es altamente variable en el lago, por lo que se recomienda especial cuidado en usar el agua para consumo y para actividades recreativas. La aparición y persistencia de las proliferaciones de M. aeruginosa en este cuerpo de agua se consideran un riesgo potencial para la salud de los habitantes de la zona, teniendo en cuenta la proximidad y el uso del sistema por los habitantes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
During the last decades, frequency and global distribution of toxic cyanobacteria blooms have increased worldwide, due to eutrophication and climate change (increased temperature, alteration of rainfall patterns and water bodies residence time) [1]. Cyanobacteria blooms can produce a variety of water quality problems in systems used for human activities, generating loss of biodiversity and recreational spaces, changes in food webs, and produce health effects on humans and animals as a result of toxins production [2].
Blooms of Microcystis spp. (Chlorococcales, Cyanobacteria) are the most common in freshwater bodies around the world and of great concern due to the production of hepatotoxins [3]. This cosmopolitan cyanobacteria form macroscopic colonies composed of thousands of cells, which can regulate their position in the water column by the presence of many intracellular aerotopes [4]. When the water column is stable, colonies float and accumulate in the surface forming a dense layer (as foam) that can cover a fraction or the whole surface of the water body. This dense layer is known as scums and/or hyperscum, whereas when the colonies are dispersed in the water forming green patches they are known as dispersive colonies or simply blooms [5, 6].
The presence of Microcystis blooms in fresh water ecosystems has been explained by a series of factors related to the cells capacity to regulate buoyancy, as well as a consequence of different environmental factors such as: the availability of light, nutrients, temperature and pH [7–11]. Other studies have attributed the blooms occurrence to physical characteristics such as the type of the water body, size, residence time and hydrodynamics (wind, turbulence and stratification) [12–14], as well as the biotic interactions of competition and grazing [15].
Microcystis synthesizes toxic metabolites called microcystins (MCs). These toxins are cyclic peptides that produce human, animal and environmental health problems. More than 90 congeners of this toxin [16] are known and all of them contain the Adda peptide [17, 18]. The microcystins production occurs in the cells that contain functional mcy genes, which are expressed as a response to certain environmental conditions [19]. In the same population both toxic and non-toxic genotypes can be found, but they are phenotypically identical. The factors that control the production of microcystins by producing cells over the non-producing cells in natural systems have been studied by several authors, though no consensus has been found up to date [7, 8, 10, 11, 14].
In South America, the events of toxic cyanobacterial blooms have been under estimated and poorly documented [20]. One of the most common genera reported in blooms is Microcystis, responsible for the intoxication of 131 people and 52 deaths in Caruaru city, Brazil [21], as well as blooms in other areas of Brazil. However, no deaths have been reported other countries of South America, although there are records on cyanobacteria and cyanotoxins presence in Uruguay [5, 12, 22, 23] and Argentina [24–27]. In Chile there have been reports on blooms in aquatic ecosystem in localities with high density population [28–33], and on the presence of five congeners of microcystins (MC-LA, MC-RR, MC-FR, MC-LR and MC-YR) [30, 31], but the only two documented toxics blooms with hundreds fish kills occurred in the Redonda urban lake, Concepción city [32] and in the Laguna Acúleo close to Santiago city [33].
Considering the variety of factors that influence blooms formation and permanence, the identification of the local environmental conditions that allows Microcystis to be dominant and causing blooms is a valuable piece of information to guide management of water bodies. The objective of this study was to investigate the presence of toxic cyanobacteria blooms in the Lo Galindo urban lake, Concepción city, and the environmental factors (biotic and abiotic) that influence the abundance of cyanobacteria and microcystins concentration. This will increase the still scarce knowledge on toxic cyanobacteria blooms in South America, contributing to have a broader view on diversity and concentrations of microcystins in temperate southern hemisphere regions.
Methods
Study area
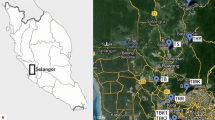
Lo Galindo is a shallow urban lake (maximum depth of 3 m) that corresponds to a non-stratified lake), located in the center of Concepción city, Chile (36°48′01″S and 73°02′31″W). Its area reaches 4.9 Ha and a volume of 62,500 m3. Lake Lo Galindo was monitored during 15 months. Samples were taken every 15 days in one single sampling station, because no significant differences were found in physical and chemical parameters between two or more sampling stations in the lake. Samples were taken by means of a 1 L vertical Niskin bottle at two depths (surface and bottom). These samples were then mixed and an integrated water column sample was obtained.
Abundance of Microcystis aeruginosa and plankton community
The abundance of M. aeruginosa was estimated by the disintegration of the colonies with alkaline hydrolysis (90 °C during 15 min, followed by an intensive mixing) [34]. This method allowed counting the individual cells by standard microscopy with a magnification of 400× using a Neubauer chamber. Results obtained in the counting were expressed as the number of cells per liter (cells L−1). Moreover, the biovolume (mm3 L−1) of M. aeruginosa was calculated using the geometric approximations by Hillebrand et al. [35]. Phytoplankton samples were fixed with 4 ml of 1 % Lugol’s solution and the amount of microalgal cells were quantified by means of the Utermöhl method [36], from sedimentation chambers of known volume in a Zeiss Axiovert 35 inverted microscope. For the chlorophyll a determination, 250 mL of the sample was filtered, the filter was incorporated into a dark plastic jar with 20 ml of 90 % acetone, and the absorbance reading was performed 24 h later for the total extraction of the compound. The determination was carried out through membrane filtration with a 10 AU Turner Designs fluorometer [37]. Zooplankton was collected once a month by filtering a volume of 10 L through a 50 μm plankton net. Concentrated samples were conserved in 95 % ethyl alcohol, subsamples of 5–10 ml of zooplankton were taken and then diluted in water to quantify their abundance in a Sedgwick-Rafter count chamber [38]. These data were registered as the number of zooplankton individuals per liter.
Abiotic factors
The water quality parameters in situ measured were temperature (°C), pH, conductivity (μS cm−1), dissolved oxygen (DO) (mg L−1) with a Multiparameter probe (Hydrolab Quanta model) and transparency (cm) with a Secchi disk. In the case of the chemical parameters, water samples were taken, transferred to the laboratory, stored at 4 °C and analyzed by molecular absorption spectrophotometry and/or ionic chromatography described in Standard Methods 22ª Edition 2012 for total phosphorus (TP) (4500 P), orthophosphate (PO4) (4500 P), total nitrogen (TN) (4500-N C), nitrate (NO3) (4500-NO3), nitrite (NO2) (4500-NO2) and ammonium (NH4) (4500-NH4) [39]. Environmental temperature (°C), wind speed (m s−1) and solar radiation (w m−2) were obtained with a Campbell CR 1000 meteorological station, located at approximately 3 km from the lake.
Microcystins concentration
In order to characterize and quantify the concentration of microcystins, samples were taken in a 1 L amber color glass jar and transferred to the laboratory in cold (4 °C) and dark. These samples were subsequently analyzed by means of high pressure liquid chromatography (HPLC) with a DAD Agilent 1100 detector. Samples were subjected to 3 freeze-thaw cycles in order to lyse the cells and release the toxins. A volume of 600 ml were filtered in a vacuum pump with 0.45 μm pore diameter filters. Solid phase extraction was performed on a C-18 column (54 μm, 6 ml, Oasis) and 1 % methanol (90 %). Separation was carried out using an RP 18 Lichrospher 100 column (5 μm pore, 150 × 4.6 mm) and an eluent system (mobile phase: A water Milli Q and eluent B acetonitrile, both with 0.5 % TFA) during 20 min [40]. The injection volume was 20 μl with a flow of 1 mL min−1. UV detection was carried out at 238 nm and the absorption spectrum of each peak was analyzed in the range of 200–300 nm [40]. The presence of the congeners MC-RR, MC-YR, MC-LR and MC-LA in the samples was confirmed by comparing with the respective analytical standards from Abraxis (USA). Microcystin concentrations were quantified on the basis of the calibration curves. Total microcystin was considered as the sum of the four congeners.
Data analyses
A multiple linear regression model was used to determine the variables that influence on the biovolume of M. aeruginosa in the lake. Independent variables used for this model were adjusted by using the AIC information criterion (Akaike Information Criterion). Subsequently, variables correlated to each other were eliminated. It was verified that the selected variables met normality criteria (by means of the Shapiro Willks test), constant variance (Bresush Pagan test) and independent errors (Durbin Watson test). Richness of taxa (S) (genera and species) and the Shannon-Wiener diversity index (H’log10) were calculated for the microalgae and zooplankton community. The presence of two types of blooms of M. aeruginosa, scums and dispersive during the studied months allowed the comparison of the lake conditions during the events, as well as the identification of the factors that promote these blooms and/or their absence. Therefore, a Pearson correlation was conducted in order to determine significant associations between the abundance of M. aeruginosa, and the concentration of microcystin with environmental parameters by types of blooms and/or its absence. Due to the fact that the cause is not implied by the correlation, a discriminant analysis was used to link biotic and abiotic factors with the dominance of M. aeruginosa during the blooms and/or its absence. A one-way Kruskal Wallis nonparametric variance analysis was used to identify differences among biotic and abiotic parameters by type of bloom and/or its absence. All analyses were performed using R version 2.14, Statistical and Primer 6.0 software.
Results
Microcystis aeruginosa abundance
A total of 30 samples were taken between December 2012 and March 2014 in the Lo Galindo Lake (Fig. 1). A M. aeruginosa scums bloom occurred during the summer of 2013, but it was not evident during the summer of 2014. In this bloom, the number of cells reached a peak of 3.1 × 106 cells L−1, which significantly decreased during the studied period (Kruskal-Wallis test H = 10.5; p < 0.05), without presenting a similar event. Nevertheless, macroscopic colonies of M. aeruginosa dispersed in the water column were observed during all seasons, with an average of 966905 ± 533143 cells L−1 forming blooms of dispersive type (Fig. 2a). During these blooms three peaks of abundance of cells were observed, through without generating a scums bloom. In the months of the early spring the lowest abundance of cells was observed, with an average of 80000 ± 26458cells L−1. These blooms were considered absent, because no colonies were observed with the naked eye.
Microcystins concentration
Microcystins (MC) were detected throughout the year, even in the absence of bloom. A total of 83 % of the analyzed samples were found positive to MC presence. Although no correlation was found between the MC concentration and the number of M. aeruginosa cells (Pearson’s r 2 = 0.07; p > 0.05), significant variations between the concentration occurred during the scums (13.68–84.0 mg L−1), the dispersive blooms (0–115.4 mg L−1) and in blooms absence (0–2.05 mg L−1) (H = 10.5, p < 0.05). The highest concentrations observed in the dispersive blooms occurred during summer (the maximum concentration was reported in February 2014) with significantly different concentrations compared to other seasons of the year (H = 11.5; p < 0.05) (Fig. 2a).
Four different congeners of microcystin were detected: MC-LA, MC-RR, MC-LR and MC-YR. The most common congener in the samples was MC-RR (40 %), its concentration (between 0.5 and 31.1 μg L−1) varied between the types of blooms and its absence (H = 9.1; p < 0.05). The second most common congener was MC-LA (26 %), with concentrations between 0.9 and 44.8 μg L−1, which was significantly higher during the scums (H = 5.1; p < 0.05). Congener MC-YR represented only the 15 % of the samples analyzed as positive and its maximum concentration was 28.3 μg L−1 (Fig. 2b). For the MC-LR and MC-YR congeners presented no significant differences in their concentrations between scums and dispersive blooms and their absence.
The MC cell quota changes in different moments of the year, though these differences were not significant (H = 1.2; p > 0.05). MC cell quota was positively correlated with changes in the concentrations of MC (Pearson’s r 2 = 0.52; p < 0.05) but shows no correlation with Microcystis cells densities (Pearson’s r 2 = 0.06; p > 0.05) (Fig. 2c).
Relation between Microcystis aeruginosa abundance, microcystin concentration and environmental factors
During the sampling period lake in the lake, 71 taxa of algae were identified in the phytoplankton, which belong to six major classes: Chlorophyceae (46 taxa), Bacillariophyceae (16 taxa), Euglenophyceae (3), Cyanobacteria (2), Cryptophyceae (2) and Dinophyceae (2). Chlorophyceae mainly of the genera Monactinus, Pediastrum, Desmodesmus, Monoraphidium and Closterium, Cryptophyceae and Cyanobacteria dominated the lake community (78 %, 10 % and 7 % respectively) (Fig. 3b). Species of the classes Bacillariophyceae, Dinophyceae and Euglenophyceae were present in low abundance (<5 % of total phytoplankton). Chlorophyceae were significantly different during dispersive blooms, especially in spring (H = 4.7; p < 0.05), whereas Cryptophyceae were abundant in winter (H = 1.5; p > 0.05) and Cyanobacteria presented their maximum abundance during the studied summers, mainly in the scums blooms (H = 8.8; p < 0.05). M. aeruginosa was almost the only component of cyanobacteria present throughout the study period. In the scums, the richness of average taxa was 17 ± 8, lower when compared to other months of the year, whereas up to 32 taxa were observed in a dispersive bloom (early summer). However, these differences were not significant. The diversity of taxa (H’log10) was significantly lower during scums (H = 8.4; p < 0.05) (Fig. 3c). In the case of dispersive blooms, colonies of M. aeruginosa were present together with several genera of green algae (Pseudopediastrum boryanum and Desmodesmus communis), diatoms (Aulacoseira granulata and Asterionella formosa), and Cryptomonas ovata.
Biotic factors in Lo Galindo lake. a Biovolume of Microcystis aeruginosa and chlorophyll a. b relative abundance of Phytoplankton (Chloro: Chlorophyceae, Crypto: Cryptophyceae, Dino: Dinophyceae, Bacillar: Bacillariophyceae, Euglen: Euglenophyceae, Cyano: Cyanobacteria). c Biological index S: richness, H’log10: Shannon- Wiener. d Abundance of zooplankton, Crust: crustaceans and Rot: rotifers. S: scums blooms, D: dispersive blooms, A: absence
Zooplankton consisted of crustaceans (genera: Chydorus, Daphnia, Eubosmina and some species of Cyclopidae) and rotifers (Polyarthra, Keratella, Asplanchna, Trichocerca, Brachionus and Filinia). Genera Eubosmina and Polyarthra dominated the community (31 % and 30 %, respectively). During the formation and declining of the scums, individuals of the genus Eubosmina dominated, whereas in the case of dispersive blooms (mainly winter-spring) the greatest abundance consisted of individuals of the genus Polyarthra (53 %) (Fig. 3d). Richness of taxa was greater during summer (S = 10), coinciding with the period of greater abundance of M. aeruginosa and lower during late summer and autumn (S = 5), coinciding with the scums senescence, and high concentrations of total microcystin. However, no significant differences between the seasons were presented (H = 4.0; p > 0.05). Diversity of taxa was higher in late winter (H’log10 = 0.8) and lower in summer (H’log10 = 0.6).
The results of the linear multiple regression model for the M. aeruginosa biovolume showed that parameters such as temperature, TN and diversity of taxa influenced on the abundance in Lake Lo Galindo (Table 1). Chlorophyll a was definitively different during scums, reaching during the study a maximum concentration value of 163.5 μg L−1 (H = 6.0; p < 0.05) (Fig. 3a). The average temperature was 23 ± 2 °C during the bloom, being significantly different compared to the average temperature in other seasons with the dispersive blooms and in absence periods (H = 9.0; p < 0.05). Similarly, the maximum abundance of cells of M. aeruginosa in the scums corresponded to the maximum temperature reported for the lake (25 °C) (Fig. 4a). The pH, together with conductivity and DO presented significant differences respect to the conditions during the dispersive blooms and in their absence (Fig. 4b). The total oxygen concentration was positively correlated with the biovolume of M. aeruginosa (Pearson’s r 2 = 0.94; p < 0.05), and no significant differences were presented respect to its concentration in the dispersive blooms, as well as in their absence (H = 0.3; p < 0.05). The same behavior was observed for the concentrations of NO2, NH4, PO4 and TP (Fig. 4c, d). The scums was presented under low wind conditions (1.4 ± 0.2 m s−1), low precipitations (2.7 ± 2.2 mm) and high solar radiation (338.9 ± 59.0 w m−1), in comparison to dispersive blooms (3.0 ± 1.0 m s−1, 76.0 ± 65.9 mm and 215.9 ± 119.8 w m−1 respectively) (Fig. 4e). Only precipitations (H = 12.9; p < 0.05) and solar radiation (H = 10.8; p < 0.05) presented statistically significant differences during scums blooms, dispersive blooms and their absence. Dispersive blooms of M. aeruginosa were presented in a range of temperatures between 11 °C and 24 °C, with an average of 17 ± 4 °C. Water transparency ranged between 20 and 50 cm and no seasonal variations were presented. These blooms were presented in an average pH of 8.47 ± 0.71, with a concentration of NO3 of 1.5 ± 1.4 mg L−1, significantly different respect to scums (0.2 ± 0.2 mg L−1) and in its absence (4.0 ± 0.4 mg L−1) (H = 12.2; p < 0.05).
Abiotic factors in Lo Galindo lake. a Temperature (Temp.) and water transparency (Trans.). b Conductivity (Cond.), Dissolve oxygen (DO) and pH. c Total nitrogen (TN) and Total phosphorus (TP). d Nitrate (NO3), Nitrite (NO2), Ammonium (NH4), Phosphate (PO4). e Wind speed, rainfall and radiation. S: scums blooms, D: dispersive blooms, A: absence
Results from the discriminant analysis show three groups that differentiate the water conditions when a scums bloom, dispersive bloom or its absence are presented. These were explained by 100 % of variation in the data. The parameters that allowed discriminating between absence and presence of a bloom of M. aeruginosa corresponded to the following factors: temperature, TN, NO3, NH4, PO4, DO, wind speed, and diversity of taxa. Differences between these scums and dispersive blooms would be indicated by parameters such as transparency, TP, NH4, NO3, NO2, pH and diversity of taxa.
The differences in MC concentration were not clearly associated with the water environmental conditions, though the only parameters that are correlated for the scums bloom are temperature (Pearson’s r 2 = 0.7; p < 0.05) and TN (Pearson’s r2 = 0.6; p < 0.05). Finally, in both dispersive bloom and its absence, the MC showed no correlation with any variable measured in this study.
Discussion
Microcystis aeruginosa blooms and environmental factors
M. aeruginosa formed monospecific blooms during all seasons in Lo Galindo Lake. These blooms presented a wide range of environmental conditions and responded positively to the high concentration of nutrients in the lake (especially TN), as well as to seasonal changes of temperature and phytoplankton community. This cyanobacteria was dominant in the summer of 2013, when the temperature reached its annual peak (25 °C) and during this season a bloom of scums type was generated, coinciding with the maximum concentration of PO4 reported in this study, a high concentration of TN, low wind speed and high solar radiation. On the contrary, during the summer of 2014 the number of cells and the visual aspect of the foam (scums bloom) were not reached. This can be explained by this season’s lower temperature (two degrees less) and lower PT and NO2 concentration, 27 % and 90 % less respectively but a highest concentration de NO3 (67 % over the previous year). Apparently these conditions together with an increase in wind speed contributed to the development of macroscopic colonies of M. aeruginosa dispersed throughout the water column and formed blooms of dispersive type.
The highest growth and optimum photosynthetic rates for this species have been reported in temperatures above 25 °C. In water bodies from temperate zones, Microcystis presents different morphological states through the year with the resistance cells recruitment in the sediment during winter (hibernation stage) [4]. When it is below 15 °C the growth is severely limited, though during spring and summer the colonies reinvade the water column giving place to the proliferation in the plankton and the formation of blooms [4]. However, results from the present study indicate the presence of M. aeruginosa and the formation of blooms even at temperatures of 10 °C, consistent with behaviors observed in other urban and hypereutrophic lakes of temperate zones [22, 26, 41]. In these systems the excess of nutrients influence on the colonies of M. aeruginosa persistence, promoting their dominance under unfavorable environmental conditions, such as those in the winter (water mixing driven by the wind and low temperatures) [22], and the typical populations hibernation in the sediment decreases, increasing their permanence in the plankton, which is extended throughout most of the year. Their occurrence in winter and in early spring contributes to maintain the populations in the system and play an important role for the blooms formation in other seasons [6].
Concentration of nitrogen and phosphorus, as well as light has been considered as determining factors for the Microcystis blooms development [7–10]. Cyanobacteria are generally poor competitors for phosphorus in comparison to diatoms and green algae, and in consequence, their greater abundance is favored by an increase in the phosphorus [42, 43]. However, nitrogen is considered more limiting for algae growth by restricting lower levels of competitors and during more time than the phosphorus [43]. In the case of the scums, the concentration of NO3 was lower compared with the concentration in periods of bloom absence, which probably allowed green algae to persist as the dominant class. A high concentration of NO3 favors the eukaryotic plankton growth, whereas at low temperatures along with enough NH4 benefits cyanobacteria such as Microcystis [9]. In dispersive blooms some eukaryotes such as the green algae P. boryanum or D. communis were abundant. These microalgae share similar environmental requirements that allow the coexistence with Microcystis and they are typical companions of shallow eutrophic lakes blooms [44]. Microcystis is dominant in low turbulence, the clustering and formation of surface cells layers is conditioned by the water column stability [22, 26]. This configuration corresponds to a competitive advantage over diatoms and green algae, because it gradually forms a shadow over their competitors and producing light exclusion [42]. Regarding zooplankton, individuals were observed during blooms and, as well as in their absence. In presence of scums, cladocerans were abundant and during dispersive blooms rotifers dominated. These two groups can coexist in blooms of cyanobacteria, conduct also reported in previous studies [45].
Microcystin concentration and environmental factors
Results show high variability in the total MC concentration between seasons and bloom types. The highest total MC concentrations we observed during summer, probably associated with the temperature increase; however, this is not associated with the type of bloom. The cellular microcystin quota shows greater intracellular concentration of toxins in the decline of the scums and in its absence. These MC cellular quotas were significantly correlated with the changes in MC concentrations, but no correlation with M. aeruginosa cells densities was observed. These results are similar to those found in two reservoirs and two lakes near the Loire River itself [14]. Therefore, this study shows that it is not necessary for scums to be present to attain the highest microcystin concentration, this result was in accordance to the no correlation between the MC concentration and the total number of M. aeruginosa cells.
Seasonal variations in microcystins in Lake Lo Galindo show a similar trend, as reported in other eutrophic freshwater systems. This trend has been linked to various factors such as the toxic and non-toxic genotypes proportion, the succession of the species of microalgae and/or the environmental factors dynamics, mainly the TN and/or TP availability [7, 10, 11, 14]. The increment in the temperature (up to a maximum of 25 °C) has been also associated to the concentration of cell toxins in several cyanobacteria genera [7].
The most frequent congeners found in Lo Galindo were MC-RR and MC-LA. Less frequently, MC-LR and MC-YA were also found in lo Galindo. Generally the congeners MC-LR, MC-RR and MC-YR present wide distribution and abundance in the environment, being MC-LR better studied [2, 5, 20, 46–48]. In Central and South America, the following MC congeners were described: MC-LR, MC-RR, MC-FR, MC-YR, MC-AR, MC-LF, MC-hRhR, [Asp3]-MC-LR, [Asp3]-MC-YR and [Leu1]-MC-LR [20]. However, microcystin concentrations are recorded in terms of microcystin LR, which reached the following values: 78.0 MC-LR (mg L−1) in the urban lake Chapultepec [49], 48.6 ± 15 μg L−1 Microcystin-LR in Salto Grande dam, Argentina [50], 0.02 and 8.6 μg L−1 Rio de la Plata estuary [27] and 5.8–2400.0 μg g−1 San Roque Reservoir Uruguay [24]. In Lo Galindo lake, higher concentrations of microcystin LR were found, as well as other congeners with lower concentrations though more frequent as the MC-RR and MC-LA previously reported in the country in the lakes of the Metropolitan Region [30] and in the Biobío Region [31]. In these studies, an effort was made to characterize the toxic peptides using mass spectrometry MALDI-TOF MS, though quantifications of the concentrations of microcystins in the water were not conducted.
Morphological and biochemical aspects in the cell have showed that different chemical structures of the MC congeners lead to different toxicity and toxic effects. On the other hand, Puerto et al. [51] showed through a mice toxicity test, the following sequence: MC-YR > MC-LR > MC-RR. In this sense, MC-RR presents lower toxicity compared to MC-YR. During this study no aquatic organisms died as a result of microcystins presence in Lo Galindo, which could be related to the lower toxic effect of MC-RR, more frequent in the system. MC-LA was also common in Lo Galindo, with higher concentrations compared to MC-RR. In this regard, the MC-LA toxin has been recently reported as dominant in small shallow lakes [52], responsible of sea otters death in California [53], and with a toxicity similar to MC-LR [2, 46, 48].
Most of the water worldwide regulations for drinking water and recreational have been developed mainly on the toxicity and persistence of MC-LR [54], due to the scarce information for other microcystins congeners and the limited availability of this other congeners analytical standards. WHO [54] recommends as precautionary measure for tolerable daily intake (TDI) 1.0 μg L−1 MC-LR and for recreation activities 20 μg L−1 MC-LR. In Lo Galindo, of the nine samples in which MC-LR was detected, eight were equal or higher than 1.0 μg L−1 and only one exceeded 20 μg L−1. Our data show that values exceeding WHO guideline values for drinking and recreational water were exceeded in different periods, so further systematic and expanded data sets are needed. These will be fundamental to help local environmental and human health authorities to avoid possible risks to human health when using the lake either for recreation or as a result of a shortage of water resources in the city, as happened after the earthquake and tsunami on February 2010, when the city dwellers used the lake to meet basic needs. To date, there are no regulations in Chile referred to the water quality in relation to toxins of cyanobacteria [29, 30].
Microcystin concentration, for each congener and total, in natural M. aeruginosa populations during the annual cycle and in different types of blooms is highly variable, both in frequency and concentration, so special care is recommended to use lake water for consumption and for recreational activities. Because of the seasonal variation of environmental conditions and the Microcystis blooms, the potential toxicity of such blooms is difficult to be predicted, making it necessary to continuously monitor the body of water. Therefore, it is necessary to adopt management measures to reduce and prevent the quantity of available nutrients (nitrogen and phosphorous) in the lake. Both presence and frequency of other congeners different from MC-LR should be taken into account when determining regulations and more studies should be conducted related to the congeners of microcystins of greater concentration, mainly with MC-LA and MC-RR. The information generated in this study is considered relevant to generate management measures or actions for the lake restoration, constituting the first effort in Chile to study the problem generated by cyanobacteria blooms as a consequence of the eutrophication of freshwater aquatic systems.
Conclusion
The dominance and development of the M. aeruginosa blooms in the lake Lo Galindo is determined by various environmental factors such as temperature, nutrients, diversity of taxa and wind speed that affect the formation of disperse-type blooms and/or scums; the latter are developed only in summer, coinciding with the highest concentrations of total microcystins.
The combined effect of changes in temperature together with the excess nutrients in the body of water was made clear, providing relevant aspects for understanding the development of cyanobacterial blooms in shallow lakes in temperate areas. The emergence and persistence of Microcystis blooms in this body of water are considered a potential health risk for the inhabitants of the area, considering the proximity and the system use by the inhabitants. This information is considered to be relevant to bring about lake restoration and management actions or measures.
References
Paerl HW, Paul V. Climate change: Links to global expansion of harmful Cyanobacteria. Water Res. 2012;46:1349–63.
Chorus I, Bartram J. Toxic cyanobacteria in water: A guide to their public health consequences monitoring and management. Suiza: Ginebra; 1999.
Komárek J, Komárková J. Review of the European Microcystis-morphospecies (Cyanoprokaryotes) from nature. Czech Phycol Olomouc. 2002;2:1–24.
Reynolds CS, Jaworski GHM, Cmiech HA, Leedale GF. On the annual cycle of the blue-green algae Microcystis aeruginosa Kütz Emend Elenkin Philos. T Roy Soc B. 1981;293:419–77.
UNESCO (2009) Cianobacterias planctónicas del Uruguay Manual para la identificación y medidas de gestión. Montevideo
Zohary T, Robarts RD. Hyperscum and the population dynamics of Microcystis aeruginosa. J Plankton Res. 1990;12:423–32.
Davis TW, Berry DL, Boyer GL, Gobler CJ. The effects of temperature and nutrients on the growth and dynamics of toxic and nontoxic strains of Microcystis during cyanobacteria blooms. Harmful Algae. 2009;8:715–25.
Geoffrey PH, Sarnelle O, White JD, Hamilton SK, Kaul RB, Bressie JD. Nitrogen availability increases the toxin quota of a harmful cyanobacterium Microcystis aeruginosa. Water Res. 2014;54:188–98.
Jacoby JM, Collier DC, Welch EBF, Hardy J, Crayton M. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can J Fish Aquat Sci. 2000;57:231–40.
Lee T, Rollwagen-Bollens G, Bollens SM, Faber-Hammond JJ. Environmental influence on cyanobacteria abundance and microcystin toxin production in a shallow temperate lake. Ecotox Environ Safe. 2015;114:318–25.
Rinta-Kanto JM, Konopko EA, Debruyn JM, Bourbonniere RA, Boyer GL. Lake Erie Microcystis: Relationship between microcystin production dynamics of genotypes and environmental parameters in a large lake. Harmful Algae. 2009;8:665–73.
O’Farrell I, Bordet F, Chaparro G. Bloom forming cyanobacterial complexes co-occurring in a subtropical large reservoir: validation of dominant eco-strategies. Hydrobiologia. 2012;698:175–90.
Romo S, Soria J, Fernandez F, Ouahid Y, Baro-Sola A. Water residence time and the dynamics of toxic cyanobacteria. Freshwater Biol. 2013;58(3):513–22.
Sabart M, Pobel D, Briand E, Combourieu B, Salençon MJ, Humbert JF, et al. Spatiotemporal variations in microcystin concentrations and in the proportions of microcystin-producing cells in several Microcystis aeruginosa populations. Appl Environ Microbiol. 2010;76:4750–9.
Ger KAT, Baxa DV, Lesmeister S, Goldman CR. The effects of dietary Microcystis aeruginosa and microcystin on the copepods of the upper San Francisco Estuary. FreshWater Biol. 2010;55:1548–59.
Puddick J, Prinsep MR, Wood SA, Cary SC, Hamilton DP, Wilkins AL. Isolation and structure determination of two new hydrophobic microcystins from Microcystis sp. (CAWBG11). Phytochem Lett. 2013;6:575–81.
Carmichael WW. Health effect of toxin-producing Cyanobacteria: The CyanoHABs. Hum Ecol Risk Assess. 2001;7:1393–407.
Sivonen K, Jones G (1999) Toxic cyanobacteria in water. In: Chorus I, Bartram J (eds). A guide to their public health consequences monitoring and management. London
Briand E, Bormans M, Quiblier C, Salenc MJ, Humbert JF. Evidence of the cost of the production of Microcystins by Microcystis aeruginosa under differing light and nitrate environmental conditions. Plos ONE. 2012;7:1–10.
Dörr FA, Pinto E, Soares RM, Oliveira F, Azevedo SM. Microcystins in South American aquatic ecosystems: Occurrence toxicity and toxicological assays. Toxicon. 2010;56:1247–56.
Azevedo SMFO, Carmichael WW, Jochimsen EM, Rinehart KL, Lau S, Shaw GR, et al. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology. 2002;181:441–6.
Fabre A, Carballo C, Hernández E, Piriz P, Bergamino L, Mello L, et al. El nitrógeno y la relación zona eufótica/zona de mezcla explican la presencia de cianobacterias en pequeños lagos subtropicales artificiales de Uruguay. Panam JAS. 2010;5:112–25.
Ferrari G, Pérez MC, Dabezies M, Míguez D, Saizar C. Planktic Cyanobacteria in the lower Uruguay River South America. Fottea. 2011;11(1):225–34.
Ame MV, Diaz MD, Wunderlin DA. Occurrence of toxic cyanobacterial blooms in San Roque Reservoir (Cordoba Argentina): a field and chemometric study. Enviro Toxicol. 2003;18(3):192–201.
Ame MV, Wunderlin DA. Effects of iron ammonium and temperature on microcystin content by a natural concentrated Microcystis aeruginosa population. Water Air Soil Pollut. 2005;168:235–48.
Ehrenhaus C, Vigna MS. Changes in the phytoplankton of Lake Planetario after restoration process. Darwiniana. 2006;44:319–28.
Giannuzzi L, Carvajal G, Corradini M, Araujo G, Andrade C, Echenique R, et al. Occurrence of toxic cyanobacterial blooms in Rio de la Plata Estuary Argentina: field study and data analysis. J Toxicol. 2012;2012:373618.
Campos V, Cantarero S, Urrutia H, Heinze R, Wirsing B, Neumann U. Microcystin in cyanobacterial blooms in a Chilean lake. Syst Appl Microbiol. 1999;22:169–73.
Campos V, Lisperguer S, Weckesser J, Vera A, Muñoz D. Cyanobacteria and potential risks of toxicity in continental waters of Chile. Bol Micol. 2005;20:73–81.
Campos V, Muñoz D, Straube M, Lisperguer S, Weckesser J. Péptidos tóxicos y no tóxicos de cianobacterias en cuerpos de agua dulce de la V Región Chile. Bol Micol. 2007;22:95–100.
Neumann U, Campos V, Cantarero S, Urrutia H, Henzie R, Weckesser J, et al. Co-ocurrence of non-toxic (Cyanopeptolin) and toxic (Microcistin) peptides in a bloom of Microcystis sp. from a Chilean Lake. Syst Appl Microbiol. 2000;23:191–7.
Parra O, Avilés D, Becerra J, Dellarossa V, Montoya R. First toxic blue-green algal bloom recorder for Chile: a preliminary report. Gayana Bot. 1986;43:15–7.
Peñaloza R, Rojas M, Vila I, Zambrano F. Toxicity of a soluble peptide from Microcystis sp. to zooplankton and fish. FreshWater Biol. 1990;24:233–40.
Lawton L, Marsalek B, Padisak J, Chorus I (1999) Determination of cyanobacteria in the laboratory. In: Chorus I, Bartram J (eds) Toxic Cyanobacteria in water a guide to their public health consequences monitoring and management, London and New York
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher D, Zohary T. Biovolume calculation for pelagic and benthic microalgae. J Phycol. 1999;35:403–24.
Utermöhl H. Zur vervollkommnung der quantitativen phytoplankton methodik Mitt. Int Ver Theor Angew Limnol. 1958;9:1–38.
Welschmeyer N. Fluorometric Analysis of Chlorophyll a in the Presence of Chlorophyll b and Pheopigments. Limnol Oceanogr. 1994;39(8):1985–192.
Wetzel RG, Likens GE. Limnological Analyses. New York: Springer; 2000.
APHA. Standard methods for examination of water and wastewater. Washington: American public health association; 2012.
ISO 20179 (2005) Water quality Determination of microcystins Method using solid phase extraction (SPE) and high performance liquid chromatography (HPLC) with ultraviolet (UV) detection
Xavier L, Vale M, Vasconcelos VM. Eutrophication, phytoplankton dynamics and nutrient removal in two man-made urban lakes Palácio de Cristal and Serralves, Porto, Portugal. Lake Reserv Manage. 2007;12:209–14.
Okechukwu O, Ugwumba A. Cyanobacteria abundance and its relationship to water quality in the Mid-Cross River floodplain Nigeria. Int J Trop Biol. 2009;57(1–2):33–43.
Stener R. Resource competition during seasonal succession toward dominance by cyanobacteria. Ecology. 1989;70(1):229–45.
Chaudhary BL, Meena L. A environmental hazard - a case study of toxic bloom of Microcystis (Anacystis) spp. in Udaipur lakes Rajasthan (India). J Herb Med Toxicol. 2007;1:55–9.
Pérez-Morales A, Sarma S, Nandini S. Feeding and filtration rates of zooplankton (rotifers and cladocerans) fed toxic cyanobacterium (Microcystis aeruginosa). J Environ Biol. 2014;35:1013–20.
Chorus I, Falconer IR, Salas HJ, Bartram J. Health risks caused by freshwater cyanobacteria in recreational waters. J Toxicol Environ Health. 2000;3:323–47.
Mbukwa EA, Titus AM, Mamba BM. Quantitative Variations of Intracellular Microcystin-LR, −RR and -YR in Samples Collected from Four locations in Hartbeespoort Dam in North West Province (South Africa) During the 2010/2011 Summer Season. Int J Environ Res Public Health. 2012;9:3484–505.
Van Apeldoorn ME, VAN EGMOND HP, GERRIT JA, BAKKER GJI. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007;51:7–60.
Vasconcelos V, Martins A, Vale M, Antunes A, Azevedo J, Welker M, et al. First report on the occurrence of microcystins in planktonic cyanobacteria from Central Mexico. Toxicon. 2010;56:425–31.
Giannuzzi L, Amé MV, Andrinolo D, Bauzá L, Benítez R, Titto E, et al. Cianobacterias como determinantes ambientales de la salud. Argentina: Buenos Aires; 2011.
Puerto M, Pichardo S, Jos A, Cameán AM. Comparison of the toxicity induced by microcystin-RR and microcystin-YR in differentiated and undifferentiated Caco-2 cells. Toxicon. 2009;54:161–9.
Zastepa A, Pick FR, Blais JM. Fate and persistence of particulate and dissolved Microcystin-LA from Microcystis blooms. Hum Ecol Risk Assess. 2014;20:1670–86.
Miller MA, Kudela RM, Mekebri A, Crane D, Oates SC, Timothy M, et al. Evidence for a novel marine harmful algal bloom: cyanotoxin (microcystin) transfer from land to sea otters. Plos ONE. 2010;5:1–11.
WHO (World Health Organization) (2006) Guidelines for Drinking-Water Quality, World Health Organization Press. Geneva
Acknowledgments
These data were generated with the support of the following funding sources: FONIS SA13I20211, VRID No. 212.310.062.1.0, CONICYT doctoral scholarship N°21130171 and CRHIAM/CONICYT/FONDAP/15130015 projects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed to the design of this study, performed the data analysis, and jointly wrote the manuscript. All authors approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Almanza, V., Parra, O., De M. Bicudo, C.E. et al. Occurrence of toxic blooms of Microcystis aeruginosa in a central Chilean (36° Lat. S) urban lake. Rev. Chil. de Hist. Nat. 89, 8 (2016). https://doi.org/10.1186/s40693-016-0057-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40693-016-0057-7