Abstract
Background
Subtle ecological and behavioral mechanisms that enhance reproduction such as nest building by animals may provide useful information of population level processes. Variation in behavioral traits may be observed as phenotypic traits that are shaped by sexual and natural selection. Using ecological sampling of benthic substrata and underwater video recordings, we assessed nest-building behavior through habitat modifications, and size of individuals performing parental care behavior and egg/hatching traits of an abundant temperate reef fish, Chromis crusma.
Results
We identified that only male individuals performed nest building and uniparental care. We noted that nests containing filamentous green or red algae had the highest percent cover of eggs. Using video recordings, we provided evidence of parental care. Male individuals spent nearly 80% of their time inside the nest, aerating the eggs with their fins and mouth, removing unwanted materials, and defending the nest against conspecific, heterospecific, and other predators. The field-collected eggs, characterized by an oil globule and adhesive filaments, hatched after 7 days in the laboratory. The nest-building and parental care behavior of C. crusma lasted for 3 months, and several nests can be constructed throughout the season.
Conclusion
The behavior of building and defending the nests, which is a characteristic of the family members, is a key aspect for the success of the C. crusma; this fish is abundant in kelp ecosystems of the southeastern Pacific.
Similar content being viewed by others
Background
Plants and animals have evolved to ensure the survival of their own future genes, thus affecting the number of offspring for future generations (Gross [2005]). Furthermore, natural selection is expected to favor behaviors that maximize lifetime reproductive success where mixtures of behavioral phenotypes may exist among species and populations (Alcock [2009]). Individuals must therefore perform a series of behaviors to obtain their optimum reproduction over the course of a lifetime (Darwin [1859]). Teleost fish display a wide range of parental care behavior, ranging from no care at all, uniparental care by a female or male, to biparental care (Gross and Sargent [1985]; Blumer [1982]; Webb et al. [1999]). In order to succeed in their offspring's survival, individuals need to evaluate the benefits of conducting care, resulting in variation or termination of parental care behavior within populations (Ochi [1985]; Smith and Wooton [1995]).
The numerous costs associated to finding a mate, competing with conspecifics, courtship, and parental care are often assessed by individuals, allowing behavioral adjustments to minimize these costs (Itzkowitz [1990]; Clotfelter et al. [2006]). Parental care activities occur after fertilization. In fish, a common care activity is fanning, in which the parent moves the pectoral, anal, and/or pelvic fins over the egg clutch, eliminating sediments from the surrounding environment (Goulet [1995]). More sophisticated parenting methods involve the movement of the mouth or branchial cavity, which in turn oxygenates the eggs and removes suspended sediments, or the direct, selective removal of damaged or dead eggs with the mouth (Goulet [1998]; Ochi [1985]). Chasing competitors and predators away from the nest are also important forms of parental care (Amundsen [2003]). Egg characteristics may also be associated with adult behavior. For example, egg size and development time can depend on predominance and duration of parental care (Sargent et al. [1987]; Shine [1978]).
The most noticeable behaviors of several fish species for attracting mates are resource and mate monopolization (Blumer [1982]; DeMartini and Sikkel [2006]; Baylis [1981]). Territorial fishes, for instance, can acquire mates by establishing a temporary breeding territory or nesting site through the formation of structures used to house eggs (Sikkel [1995]; Rushbrook et al. [2008]; Goulet [1998]; Kawase et al. [2013]). Among fishes, nest construction can be divided into three categories: a) excavations - burrows in gravel, sand, or mud (Kawase et al. [2013]); b) simple walls - piling up sand, pebbles and shells, and artificial substrata (Colin [1973]); and c) cement - secreting chemicals from the kidneys to glue together algae, detritus, or sand (Rushbrook et al. [2008]).
In marine benthic substrata, nest builders clean their nesting areas by removing and/or farming preferred substratum species in an area where eggs have been deposited (Unger and Sargent [1988]; Klumpp and Polunin [1989]). Nests can be pivotal for courtship because they may attract the opposite sex and provide shelter for the developing eggs (DeMartini and Sikkel [2006]). Nest quality is expected to differ among individuals who construct them (Clotfelter et al. [2006]). In marine environments, individuals may face several limitations in building nests such as a) bottom topography (e.g., vertical walls versus horizontal surfaces), b) abundance of preferred nest material, c) presence of heterosexual or conspecific breeding pairs (i.e., operational sex ratio), and d) ability to chase predators and competitors away from the nest (Coleman and Wilson [1998]; Taborsky [1994]).
Fishes from the family Pomacentridae are oviparous (DeMartini and Sikkel [2006]), and there are substantial differences in the mating systems within members of this family in both temperate and tropical systems (see Table 1). Nest building along with parental care behavior has been observed in 25 species, 7 of which are temperate (Table 1). The male is responsible for parental care and nest building in 80% of the cases and biparental care in the remaining 20%. Biparental care is present for tropical species only. The majority of the species that build nest do not discriminate the species in the substrata to lay their eggs. However, algal species (usually turf algae) was the preferred site for spawning for six species, three of them are temperate (including Chromis crusma) and two subtropical species. Artificial structures, dead coral, and shells are the preferred sites for six tropical species. Most tropical species deposit their eggs under crevices or rock boulders (Table 1).
Castañeta, C. crusma, is a common and widely distributed damselfish inhabiting the Southeast Pacific Ocean from Santa Rosa Island (5° SL), Ecuador, to Valdivia (39° SL), Chile (Pequeño et al. [2005]). Along the central Chilean coast, individuals are usually found in rock outcrops within depths of between 5 and 35 m and reef areas dominated by large brown macroalgae where they recruit (i.e., Lessonia trabeculata). Adults can reach up to 25 cm in total length (TL) and are often seen in large schools (up to 100 individuals) (Pérez-Matus et al. [2007]). The diet of C. crusma is comprised mainly of planktonic species (Angel and Ojeda [2001]). As a first step to understand variability in parental care and nest-building behavior of an abundant temperate reef fish, we describe the information about the nest and the type of parental care and correlated this behavior with descriptions of the benthic eggs and recently hatched larvae.
Methods
Study site
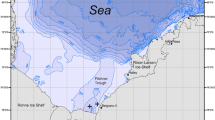
Field observations on nesting behavior were performed during 2011 to 2012 and nest-building behavior was observed during November 2011 through March 2012, which coincides with the spawning season of C. crusma at Zapallar, Valparaíso (32° 33′ SL to 71° 28′ WL) (Figure 1). We searched for and surveyed nests along the depth distribution of C. crusma, within depths of between 5 and 35 m. Large boulders covered with turf and calcareous crustose algae dominated the shallow sections of the study site, where kelp was sparsely distributed from 5 to 15 m. Encrusting sessile fauna such as sponges (Halichondria sp. and Clionopsis platei) and sea anemones (Parazoanthus sp. and Anemonia alicemartinae) as well as filamentous green and red algae dominated the substratum from 15 to 30 m (Figure 1a,b).
Habitat modifications by C. crusma
We searched for demersal nests of C. crusma, defining a nest as an area containing eggs attached to a substratum that was guarded by an individual (Figure 1a,b). At each nest, we described the color of the fish and estimated the size of the nest defender using a 4-cm dive weight positioned 5 cm from the nest. Mean nest size (cm) was calculated by averaging the maximum horizontal and vertical lengths of the nest measured to the nearest 0.5 cm using a measuring tape. We categorized the substratum slope where the nest was located as either a) vertical walls, b) horizontal platforms, or c) intermediate slope platforms. Differences in nest size among the substratum slope were tested using a one-way ANOVA. To meet ANOVA assumptions of normality and homoscedasticity of variance, we used a Cochran c-test and Fligner-Killeen test, respectively (Crawley [2007]). To confirm our prior field observations that nest defenders were males, we randomly collected six adult individuals from six different guarded nests and determined the sex of each individual.
To describe nest habitat and substrate composition around the nest, we selected nests (n = 40) that were guarded by solitary individuals, and three quadrats were each randomly placed inside the nests, at the edge of the nests (along a boundary of the egg clutch), and outside the nests (where eggs are absent). In each quadrat, we recorded the percent cover of sessile benthic species using a 0.01-m2 quadrat with 15 intersection points (random point contact, RPC). We quantified the percent cover of eggs inside the nest. When necessary for confirmation of species identity, eggs and all sessile species were collected by manually removing with a spatula and preserved in 5% buffered formalin. We also used underwater photography (Nikon-D90 with Ikelite Housing and two D125 substrobe; Ikelite Underwater Systems, Indianopolis, IN, USA) on quadrats to further analyze the images on a computer. The same divers performed all sampling by means of self contained underwater breathing apparatus (SCUBA) throughout the study.
To determine differences in the substrata composition inside and outside of nests, as well as among nests, we used multivariate analyses. A Bray-Curtis resemblance matrix was used to carry out the analysis, and a one-factor permutational multivariate analysis of variance (PERMANOVA; Anderson [2001]) was used to test for significant differences in the substratum composition within and among the nests. At each spatial scale, the substratum species compositions were analyzed using similarity percentages (SIMPER), identifying the species that contributed most to the differences between the nest scales (i.e., outside, edge, inside). We estimated the nest quality by evaluating the relationship between species richness (i.e., benthic composition) and the number (i.e., percent cover) of eggs in each nest (Sikkel [1995]).
Parental care: nest-guarding behavior
In order to avoid diver effects on the nest-guarding behavior (individuals tended to leave the nest when divers approached), we set up an underwater video camera (Go-Pro™ Hero 2, GoPro, Inc., San Mateo, CA, USA) anchored with a 2-kg weight. The total dimension of the camera was 8 × 8 cm. The camera was randomly positioned 30 cm from the nests, and the recording time ranged from 25 to 35 min. We placed the camera in eight nests totaling approximately 280 min of recording time. Filming occurred during early egg development stages (2 to 3 days). Images were downloaded to a computer, and we quantified the time that each recorded individual spent performing the following: a) time inside the nest, b) aerating the eggs with the mouth, c) fanning with pectoral or anal fins, d) removing unwanted drift material, and e) defending the nest against intruders.
Embryonic development and larval growth
In order to describe the development time, hatching, and larvae growth of C. crusma, we collected eggs (>50) from more than ten nests at different depths using SCUBA and a scraping knife. The eggs were carefully placed in a 1-L glass container and transported in glass jars with seawater and ice packs to the laboratory (32°56′ SL, 71°33′ WL) (Figure 1c). Once in the laboratory, egg clutches were kept aerated with filtered seawater (0.5 μm) inside 5-L glass jars at 18°C. Embryo development was evaluated on a daily basis until hatching occurred (see below). Recently hatched larvae were transferred into 1-L glass jars at a density of 30 larvae L−1. When the yolk sac was depleted, larvae were fed with Nannochloropsis spp. and rotifers Brachionus plicatilis.
During each monitoring, all eggs and larvae were photographed with a stereomicroscope Olympus SZ-61 equipped with a Motic video camera 2500 (5.0 MP; Olympus Corporation, Tokyo, Japan) connected to a PC with Moticam Image Plus 2.0 software. All measurements (mm) were carried out on fresh eggs (length, maximum width, oil globule diameter) and larvae [body length (from the tip of the snout to the tip of the notochord), pre-anal length, eye diameter, yolk sac length and height, and oil globule diameter]. Egg and yolk sac volume were estimated considering the yolk sac as an ellipsoid (V = 4/3πab2), where a is half of the egg or yolk sac length (mm) and b is half of the height (mm) (Bustos and Silva [2011]). Larval growth was estimated adjusting a least squares linear regression model between body length (BL, mm) and age (days post hatch, dph), BL = α + dphβ, where α and β are the size at hatching and larval growth rate respectively, estimated by the model. All statistical tests were performed using R v 2.14 (R Development Core Team [2012]).
Results
Habitat modifications of C. crusma during the spawning season
According to our observations, reproductive behavior in C. crusma commenced in late October and ended in March (austral spring-summer). During this period, we sampled every 2 weeks, a total of six different sampling days. More than 80 nests were observed, and of these, a total of 39 nests were measured, with a mean diameter of 23.5 cm (±5.2 SD). All adult individuals collected (n = 6) from the nests were males. All had dark grey coloring that differed from their pelagic counterparts and other benthic transients, who were pale grey in color. Our visual estimates revealed that nest defenders ranged from 15.0 to 21.5 cm TL, with a mean of 17.3 cm TL (±2.6 SD). There was no relationship between fish TL and nest diameter (linear regression r2 = 0.08, df = 1, F = 0.09, p value >0.05). Mean nest size measured 21.3 (±2.2 SE), 23.3 (±1.3 SE), and 24.6 (±1.5 SE) cm in vertical walls, intermediate slope, and horizontal platforms, respectively. No significant differences were observed in the size of the nest with respect to the substratum slope (one-way ANOVA, df = 2; F = 0.4, p value >0.05).
In the study area, we recorded a total of 16 benthic species that covered the substratum outside, inside, and at the edge of the nests. Significant differences were found among these categories (one-way ANOVA, df = 2; F = 6.5, p value <0.01). We detected more benthic species at the edge and outside of nests than inside them (Tukey post hoc, p value <0.01, Figure 2). Moreover, outside and edge richness varied significantly among nests, mirroring local changes in the surrounding dominant community structure (i.e., sessile animal or algae-dominated substratum). In contrast, species richness inside of nests was low and similar among nests regardless of the surrounding community. In terms of nest quality, a significant negative relationship was observed between the percent cover of eggs and the number of benthic species (linear regression, r2 = 0.4, df = 1, F = 7.1, p value <0.001). A high number of eggs were present in nests that contained a small number of species (Figure 3).
Defended nest habitats were composed predominantly of filamentous green algae (mostly Cladophoropsis herpestica) or red algae (mostly Pterosiphonia dendroidea or Schottera nicaeensis) and/or crustose algae (Lithothamnion sp.) (Figure 4). The benthic sessile community composition differed inside the nests compared with the species that covered the edge of the nest or outside of them (PERMANOVA, df = 2, pseudo F = 3.8, p value <0.01). Cumulative contributions (SIMPER) of the most influential species indicated that green filamentous algae accounted for 54% and 56% of the differences between inside the nest and outside or edge of the nest, respectively. The percentage cover of these algae was higher inside the nests (Figure 4).
Parental care: nest-guarding behavior
The video recordings of C. crusma (see video, http://vimeo.com/89531693) revealed that males spent between 80% and 95% of their total time inside rather than outside the nests. Fanning was the most frequent activity performed by C. crusma inside the nests, ranging from 78% to 94% of the time. Defending the nest was the second most important activity (7% to 15% of the time). Predators such as the sandperch (Pinguipes chilensis), the triplefin (Helcogrammoides spp), and the rock shrimp (Rhinchocynetes typus) were excluded from the nest by exaggerated swims and accelerated chasing movements. The display of dorsal and pectoral fins was also an aggressive behavior performed by defending males. Only during short (few seconds), aggressive chases to ward-off potential predators and conspecifics would defending males leave the nest. In addition to protective behaviors, males were observed to actively aerate eggs by removing individuals and holding them within an open mouth faced directly in the direction of water flow for several seconds before placing them back in the nest. This was a commonly observed behavior (average of 24.5 events per individual) for the majority (95%) of video/recorded individuals. Finally, the removal of drift material such as dead eggs and other detritus that can affect the normal development of the eggs was also observed.
Embryonic development and larval growth
The demersal eggs of C. crusma were ovoid to elliptical and ranged in size from 0.96 to 1.20 mm (mean: 1.08 mm ± [0.06 SD], n = 50) on the long axis and 0.65 to 0.72 mm (mean: 0.68 ± 0.02 mm SD) in maximum width. Estimated volume ranged from 0.23 to 0.30 mm3 (mean: 0.2 ± 0.02 mm3 SD). Eggs contained one single red to yellow oil globule, 0.18 to 0.31 mm in diameter (0.25 ± 0.02 mm SD). Adhesive filaments attached eggs from their basal poles to the nest (Figure 5). Embryonic development lasted 7 to 8 days, and hatched larvae measured between 2.85 and 3.67 mm in body length (mean 3.30 mm ± 0.18 SD), with a yolk sac of 0.021 ± 0.004 mm3 (range: 0.013 to 0.030 mm3) that was generally depleted after 2 dph. The oil globule was completely absorbed after 7 dph. Both estimated slope (growth rate) and intercept (hatch size) were significantly different from zero (p value β = 0.0014; p value α <0.001).
Photographs of different early developmental stages of castañeta Chromis crusma . Upper left panel, early gastrula; upper right panel, 12-somite stage; lower left panel, pre-hatching stage; lower right panel, recently hatched larvae. Stages according to Kingsford ([1985]).
Discussion
Our results revealed that male individuals of C. crusma set up nesting territories during the austral summer (November through March), which they aggressively defend and actively clean/maintain. Moreover, males frequently undertook numerous ‘incubating’ activities that commonly benefit egg development, including aeration via fin fanning and exposing eggs to direct water flow, as well as egg re-positioning within the nest. The negative correlation between egg abundance and benthic species richness conforming nest substrate suggests that important differences in nest quality occur within a site, which was unrelated with the size (i.e., total length) of the individuals performing the care.
Male individuals responsible for the fate of the fertilized eggs and success during development result in uniparental care (Gross [2005]). Males defend the nest against predators, aerate and fan the eggs, and remove drift materials from the nest during eight consecutive days after which larvae hatch in the laboratory at 18°C. Finally, one oil globule and adhesive filaments characterize C. crusma eggs and many other members of the genera (see Kingsford [1985]), which may explain the upkeep of benthic filamentous algae inside the nest by male individuals (as it allows egg attachment to the surface).
Methods of nest construction by C. crusma coincide with other temperate reef fishes of the family Pomacentridae (see Table 1). For example, the temperate damselfish Hypsypops rubicundus exhibits a similar behavior in the preparation of the nesting site, maintaining a red alga in the middle of the cleaned area, which is approximately 33 to 50 cm in diameter (Sikkel [1995]). In general, temperate reef fish use benthic algae for depositing the eggs while tropical members use dead coral or artificial materials. Tropical members deposit eggs under crevices and boulders presumably to avoid egg predators, which are potentially more abundant in tropical reefs than predators in temperate reefs (Table 1).
Nest construction, in turn, can be crucial to increasing the reproductive success of male individuals. There is striking recent evidence, which suggests that nest and nest-building behavior have evolved under sexual selection (Borgia [1985]; Tomás et al. [2013]; Kawase et al. [2013]). Nevertheless, a problem that has been puzzling behavioral ecologists is the identification of those aspects of the nest that influence the success of the spawning male, and thus determine what affects females in their selection of the spawning site (but see Tomás et al. [2013]). In C. crusma, the identification of nest features that influence female choice can be complicated as is the case with many other fishes which perform parental care; females in many populations preferably spawn in nests that already contain eggs (Hoelzer [1990]). Sex dissimilarities in shape, color, and behavior result from differences in the reproductive achievement of individuals (Clotfelter et al. [2006]). Future research should be directed towards understanding the role of nest features as sexual signals (see Kawase et al. [2013]).
A correlation between benthic sessile species and cover of eggs (as proxy of brood size) was observed in this study. It has been reported that size of the brood is crucial to the survival of offspring (see Ochi [1985]). Sexual selection forces may well fit into this, as male individuals may have acquired information from past investments in nest building by carefully selecting sites where monospecific filamentous algae thrive. Alternatively, males can clean the substratum in such sites maintaining the preferred algae for females to deposit their eggs. The type of benthic substrata (not the bed-rock slope) may also be important for female choice in this species.
Considerable debate exists in the literature surrounding which hypothesis came first, the evolution of egg size (small vs large eggs) or the evolution of parental care. Shine ([1978]) proposed the ‘safe harbor’ hypothesis that predicts the evolution of parental care prior to the evolution of large egg size (selection favors the increase in egg size). Nussbaum and Schultz ([1989]) challenged this by stating that a form of care should be incorporated to reduce the long-term developmental mortality in relation to egg clutch size. Our study is not attempting to resolve this conflict. The high number of egg predators may indicate the high energetic lipid value and volume of C. crusma eggs (an oil globule), which correlates with the amount of energy spent by adults in maintaining predator distance. Differences in egg developmental time exist among tropical and temperate pomacentrids (Table 1). Egg development is similar among temperate reef fish, with time from egg deposit to hatching lasting 6 to 8 days for most temperate species and among tropical pomacentrids with 3 to 5 days for most tropical and subtropical pomacentrids. Some notable exceptions were present in tropical species such as Acanthochromis leucogaster, which exhibited extended egg development time of up to 30 days (Table 1). The longer time for egg development in temperate reef fish may directly affect the cost of parental care of individuals.
The complex life cycle (pelagic larval phase, benthic juvenile and adult) of most marine fishes is characterized by the high mortality of eggs and larvae. A bottleneck of population structure is often observed at the egg and larval stage. Therefore, nest construction and parental care may have important evolutionary consequences that affect local population structures (see Reynolds et al. [2002] for review). Temperate reef fish exhibit a diverse array of reproductive modes, life histories, and parental care systems. With substantial variation among individuals, the reproductive behavior of pomacentrid fishes can be divided into the establishment of a breeding territory, nesting site, construction of nests by building structures used to house eggs, courtship and pair formation, spawning and fertilization, and finally parental care (Limbaugh [1964]; Clarke [1970]; Sikkel [1995]; Turner and Ebert [1962]). To our knowledge, this is the first study which describes parental care carried out by a pomacentrid temperate reef fish in the southeastern Pacific. C. crusma is abundant within the reef system (Pérez-Matus et al. [2007]). Modes of parental care have co-evolved in many taxa, and comparative information about young survivorship from different modes of reproduction is needed. Environmental and demographic parameters influencing parental care patterns are promising lines for future research.
Conclusions
Our results suggest that males Chromis crusma created their nest by removing some species from the substrate, selecting only sessile algae species for egg deposition. The number of sessile species removed may provide insights to the nest quality, which is unrelated to male’s size. As in many other damselfish, egg development is longer in temperate that in tropical species. In laboratory, egg development lasted 7-8 days, which coincides with the time spent by individuals in defending their nest. Males aggressively defended their nest against predators, maintaining eggs by constantly aerating and cleaning eggs by removing drift material and repositioning eggs within the nest. Although this research provides a first step into the understanding of the biology and reproductive behavior of an abundant reef fish of the southeastern Pacific, environmental and demographic (i.e., operational sex ratio) parameters affecting this behavior requires further research.
References
Albrecht H: Behaviour of four species of Atlantic damselfishes from Columbia, South America, ( Abudefduf saxatiles, A. taurus, Chromis multilineata, C. cyanea ; Pisces, Pomacentridae). Z Tierpsychol 1969, 26: 662–676. 10.1111/j.1439-0310.1969.tb01969.x
Alcock J: Animal behavior: an evolutionary approach. Sinauer Associates, Sunderland, USA; 2009.
Amundsen T: Fishes as models in studies of sexual selection and parental care. J Fish Biol 2003, 63 supplement A: 17–52. 10.1111/j.1095-8649.2003.00219.x
Anderson MJ: A new method for non-parametric multivariate analysis of variance. Austral Ecol 2001, 26: 32–46.
Angel A, Ojeda FP: Structure and trophic organization of subtidal fish assemblages on the northern Chilean coast: the effect of habitat complexity. Mar Ecol Prog Ser 2001, 217: 81–91. 10.3354/meps217081
Baylis JR: The evolution of parental care in fishes, with reference to Darwin's rule of male sexual selection. Environ Biol Fishes 1981, 6: 223–251. 10.1007/BF00002788
Blumer LS: A bibliography and categorization of bony fishes exhibiting parental care. Zool J Linn Soc 1982, 75: 1–2. 10.1111/j.1096-3642.1982.tb01939.x
Borgia G: Bower quality, number of decorations and mating success of male satin bowerbirds Ptilonorhynchus violaceus : an experimental analysis. Anim Behav 1985, 33: 266–271. 10.1016/S0003-3472(85)80140-8
Breder CMJ, Rosen DE: Modes of reproduction in fishes. Natural history press, New York; 1966.
Bustos CA, Silva A: Endogenous feeding and morphological changes in hatchery-reared larval palm ruff Seriolella violacea Pisces: Centrolophidae under starvation. Aquacul Res 2011, 42: 892–897. 10.1111/j.1365-2109.2011.02824.x
Clarke TA: Territorial behavior and population dynamics of a pomacentrid fish, the garibaldi, Hypsypops rubicunda . Ecol Monogr 1970, 40: 189–212. 10.2307/1942295
Clotfelter ED, Curren LJ, Murphy CE: Mate choice and spawning success in the fighting fish Betta splendens : the importance of body size, display behavior and nest size. Z Tierpsychol 2006, 112: 1170–1178.
Coleman K, Wilson DS: Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim Behav 1998, 56: 927–936. 10.1006/anbe.1998.0852
Colin PL: Burrowing behavior of the yellowhead jawfish, Opistognathus aurifrons . Copeia 1973, 1: 84–90. 10.2307/1442361
Crawley MJ: The R book. John Wiley & Sons, Ltd, Chichester, West Sussex, England; 2007.
Darwin CR: On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. Murray, London; 1859.
DeMartini EE, Sikkel PC: Reproduction. In The ecology of marine fishes. Edited by: Allen LG, Pondella DJ, Horn MH. University of California Press, Berkele and Los Angeles, California; 2006:483–523.
Fishelson L: Behaviour, socio‐ecology and sexuality in damselfishes Pomacentridae. Ital J Zool 1998, 65 sup1: 387–398. doi:10.1080/11250009809386853 10.1080/11250009809386853
Gittleman JL: The phylogeny of parental care in fishes. Anim Behav 1981, 29: 936–941. 10.1016/S0003-3472(81)80031-0
Goulet D: Temporal patterns of reproduction in the Red Sea damselfish Amblyglyphidodon leucogaster . Bull Mar Sci 1995, 57: 582–595.
Goulet D: Spawning success in the damselfish Amblyglyphidodon leucogaster : the influence of eggs in the nest. Anim Behav 1998, 55: 651–664. 10.1006/anbe.1997.0753
Gross MR: The evolution of parental care. Q Rev Biol 2005, 80: 37–45. 10.1086/431023
Gross MR, Sargent RC: The evolution of male and female parental care in fishes. Am Zool 1985,25(3):807–822.
Hoelzer GA: Male-male competition and female choice in the Cortez damselfish, Stegastes rectifraenum . Anim Behav 1990, 40: 339–349. 10.1016/S0003-3472(05)80929-7
Itzkowitz M: Sex ratio biasing and male reproductive variation in a coral-reef fish. Am Nat 1990, 136: 557–559. 10.1086/285114
Kawase H, Okata Y, Kimiaki I: Role of huge geometric circular structures in the reproduction of a marine pufferfish. Sci Rep 2013, 3: 1–6. 10.1038/srep02106
Kingsford MJ: The demersal eggs and planktonic larvae of Chromis dispilus Teleostei: Pomacentridae in north-eastern New Zealand coastal waters. N Z J Mar Freshwat Res 1985, 19: 429–438. 10.1080/00288330.1985.9516107
Klumpp DW, Polunin NVC: Partitioning among grazers of food resources within damselfish territories on a coral reef. J Exp Mar Biol Ecol 1989, 125: 145–169. 10.1016/0022-0981(89)90040-3
Knapp RA, Warner RR: Male parental care and female choice in the bicolor damselfish, Stegastes partitus : bigger is not always better. Anim Behav 1991, 41: 747–756. 10.1016/S0003-3472(05)80341-0
Limbaugh C: Notes on the life history of two Californian pomacentrids: garibaldis, Hypsypops rubicunda Girard, and blacksmith, Chromis punctipinnis Cooper. Pac Sci 1964, 18: 41–50.
Maddams JC, McCormick MI: Not all offspring are created equal: variation in larval characteristics in a serially spawning damselfish. PLoS One 2012, 7: e48525. 10.1371/journal.pone.0048525
Mapstone GM, Wood EM: The ethology of A budefduf luridus and Chromis chromis Pisces: Pomacentridae from the Azores. J Zool 1975, 175: 179–199. 10.1111/j.1469-7998.1975.tb01395.x
Mizushima N, Nakashima Y, Kuwamura T: Semilunar spawning cycle of the humbug damselfish Dascyllus aruanus . J Ethol 2000, 18: 105–108. 10.1007/s101640070008
Moyer JT: Reproductive behavior of the damselfish Pomacentrus nagasakiensis at Miyake-jima, Japan. Jap J Ichthyol 1975, 22: 151–163.
Murphy BF, Leis JM, Kavanagh KD: Larval development of the Ambon damselfish Pomacentrus amboinensis , with a summary of pomacentrid development. J Fish Biol 2007, 71: 569–584. 10.1111/j.1095-8649.2007.01524.x
Myrberg AA, Bradley JR, Brahy D: R. EA: Reviewed field observations on reproduction of the damselfish, Chromis multilineata Pomacentridae, with additional notes on general behavior. Copeia 1967, 4: 819–827. 10.2307/1441893
Nussbaum R, Schultz D: Coevolution of parental care and egg size. Am Nat 1989, 133: 591–603. 10.1086/284939
Ochi H: Termination of parental care due to small clutch size in the temperate damselfish, Chromis notata . Environ Biol Fishes 1985, 12: 155–160. 10.1007/BF00002768
Pequeño G, Vargas L, Riedermann A: La castañeta Chromis crusma Valenciennes, 1833 en la costa de Valdivia, con comentarios sobre el género Chromis Cuvier, 1814, en aguas chilenas Osteichthyes: Pomacentridae. Invest Mar 2005, 33: 101–107. 10.4067/S0717-71782005000100007
Pérez-Matus A, Ferry-Graham L, Cea A, Vásquez J: Community structure of temperate reef fishes in kelp-dominated subtidal habitats of northern Chile. Mar Fresh Res 2007,58(12):1069–1085. 10.1071/MF06200
R Development Core Team:R: A language and environment for statistical computing. The R Foundation for Statistical Computing, Vienna; 2012.
Reese ES: Ethology and marine zoology. Ann Rev Ocean Mar Biol 1964, 2: 455–488.
Reynolds JD, Goodwin NB, Freckleton RP: Evolutionary transitions in parental care and live bearing in vertebrates. Phil Trans Royal Soc 2002, 357: 269–281. 10.1098/rstb.2001.0930
Ridley M: Paternal care. Anim Behav 1978, 26: 904–932. 10.1016/0003-3472(78)90156-2
Robertson DR: Field observations on the reproductive behaviour of a pomacentrid fish, Acanthochromis polyacanthus . Z Tierpsychol 1973,32(3):319–324. 10.1111/j.1439-0310.1973.tb01108.x
Rushbrook BJ, Dingemanse NJ, Barber I: Repeatability in nest construction by male three-spine sticklebacks. Anim Behav 2008, 75: 547–553. 10.1016/j.anbehav.2007.06.011
Sale PF: The reproductive behaviour of the pomacentrid fish, Chromis caeruleus . Z Tierpsychol 1971, 29: 156–164. 10.1111/j.1439-0310.1971.tb01729.x
Sargent RC, Taylor PD, Gross MR: Parental care and the evolution of egg size in fishes. Am Nat 1987, 129: 32–46. 10.1086/284621
Shine R: Propagule size and parental care: the ‘safe harbor’ hypothesis. J Theor Biol 1978, 75: 417–424. 10.1016/0022-5193(78)90353-3
Sikkel PC: Effects of nest quality on male courtship and female spawning-site choice in an algal-nesting damselfish. Bull Mar Sci 1995, 57: 682–689.
Smith C, Wooton RJ: The cost of parental care in teleost fishes. Rev Fish Biol Fish 1995, 5: 7–22. 10.1007/BF01103363
Taborsky M: Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv Study Behav 1994, 23: 1–100. 10.1016/S0065-3454(08)60351-4
Tomás G, Merino S, Martínez-de la Puente J, Moreno J, Morales J, Rivero-de-Aguilar J: Nest size and aromatic plants in the nest as sexually selected female traits in blue tits. Behav Ecol 2013, 24: 926–934. 10.1093/beheco/art015
Turner CH, Ebert EE: The nesting of Chromis punctipinnis Cooper and a description of their eggs and larvae. Calif Fish Game 1962, 48: 243–248.
Unger LM, Sargent RC: Allopaternal care in the fathead minnow, Pimephales promelas : females prefer males with eggs. Behav Ecol Sociobiol 1988, 23: 27–32. 10.1007/BF00303054
Webb JN, Houston AI, McNamara JM, SzéKely T: Multiple patterns of parental care. Anim Behav 1999, 58: 983–993. 10.1006/anbe.1999.1215
Acknowledgements
We would like to thank Ivo and Checho for providing boat assistance at the study site. This work was supported by Fondecyt under grant number 11110351 to APM. We would also like to thank Pilar Muñoz and Maibe Hermoso for their valuable help with macroalgae identification and laboratory analysis, respectively. This manuscript was improved by the comments and edits of Dr. Evie Wieters and two anonymous reviewers for their contribution in the manuscript. Dr. Sergio Carrasco and Samantha Goyen provided useful comments and edits to earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contribution
TNF and APM conceived the study, participated in the design and coordination of the manuscript, and carried out the field sampling. ML and CB carried out the larval description, development, analysis, and the written section. APM and TNF wrote the paper. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Navarrete-Fernández, T., Landaeta, M.F., Bustos, C.A. et al. Nest building and description of parental care behavior in a temperate reef fish, Chromis crusma (Pisces: Pomacentridae). Rev. Chil. de Hist. Nat. 87, 30 (2014). https://doi.org/10.1186/s40693-014-0030-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40693-014-0030-2