Abstract
Background
Cerebellar mutism syndrome (CMS) or posterior fossa syndrome (PFS) consists of a constellation of neuropsychiatric, neuropsychological and neurogenic speech and language deficits. It is most commonly observed in children after posterior fossa tumor surgery. The most prominent feature of CMS is mutism, which generally starts after a few days after the operation, has a limited duration and is typically followed by motor speech deficits. However, the core speech disorder subserving CMS is still unclear.
Case presentation
This study investigates the speech and language symptoms following posterior fossa medulloblastoma surgery in a 12-year-old right-handed boy. An extensive battery of formal speech (DIAS = Diagnostic Instrument Apraxia of Speech) and language tests were administered during a follow-up of 6 weeks after surgery. Although the neurological and neuropsychological (affective, cognitive) symptoms of this patient are consistent with Schmahmann’s syndrome, the speech and language symptoms were markedly different from what is typically described in the literature. In-depth analyses of speech production revealed features consistent with a diagnosis of apraxia of speech (AoS) while ataxic dysarthria was completely absent. In addition, language assessments showed genuine aphasic deficits as reflected by distorted language production and perception, wordfinding difficulties, grammatical disturbances and verbal fluency deficits.
Conclusion
To the best of our knowledge this case might be the first example that clearly demonstrates that a higher level motor planning disorder (apraxia) may be the origin of disrupted speech in CMS. In addition, identification of non-motor linguistic disturbances during follow-up add to the view that the cerebellum not only plays a crucial role in the planning and execution of speech but also in linguistic processing. Whether the cerebellum has a direct or indirect role in motor speech planning needs to be further investigated.
Similar content being viewed by others
Background
Cerebellar mutism syndrome (CMS) or posterior fossa syndrome (PFS) is characterized by a transient period of cerebellar mutism combined with a range of neurological (motor) disturbances, neuropsychological (cognitive, affective/behavioral) abnormalities and neurogenic speech/language deficits. It is most commonly observed in children and has only been occasionally reported in adults [1–4]. CMS is typically associated with posterior fossa tumor surgery [2], but traumatic brain injury [5], stroke [2, 6] and infections [7, 8] may also cause this syndrome. In a review of 257 children who developed CMS after surgery [9] 62.7% of the cases had a medulloblastoma, 24.9% an astrocytoma, 11.2% an ependymoma, 0.4% a meningioma (ME), 0.4% a germinoma, and 0.4% germ cells. The symptoms of CMS are linked to damage to various parts of the cerebellum and the cerebello-cerebral pathways passing through the brainstem [10]. Immediately after surgery, most patients who later develop CM present ataxia and dysmetria. After a linguistically symptom free interval of some days (generally 1 and a half to 2 days) the patients become mute [9]. Onset of CM is typically accompanied by additional neurological signs and affective/behavioral disorders. Neurological symptoms may consist of oculomotor and oral motor dysfunction, hypotonia, ataxia, paresis, dysmetria, incontinence, tremor and 6th and 7th cranial nerve palsies [11, 12]. Affective/behavioral disorders include irritability and whining (2/3) and apathy (1/2) and tend to resolve before remission of CM [13, 14]. After a period of CM (mean time: 47.6 days/43 days) [9, 15], speech slowly returns and is often characterized by distorted vowels, slowed speech, prolonged phonemes, monopitch/monoloudness, hypernasality, vocal tremor and dysarthria [13, 15–19]. Dysarthria which characterizes motor speech in most of the children after remission of mutism (98.8%) [15, 20] is typically defined as ataxic dysarthria [20]. Only a handful of studies reported that patients affected by CM had non-motor language problems such as a reduced verbal output with short phrases, wordfinding difficulties and disruption of grammar [14, 20, 21]. Neuropsychological studies revealed a range of executive dysfunctions, visuospatial problems and a general intellectual decline during longitudinal follow-up (even 1 year postsurgery) [20, 22]. The residual complex of cognitive, linguistic and affective symptoms in pediatric posterior fossa tumor patients are consistent with the Cerebellar Cognitive Affective Syndrome (CCAS) or Schmahmann’s syndrome [21, 23–25]. Schmahmann’s syndrome consists of a cluster of neuropsychological and affective impairments but do not per se include motor symptoms or CM [26]. However, it might be argued that CM represents the extreme end of adynamic speech on a fluency/dysfluency continuum, which forms part of the linguistic cluster of Schmahmann’s syndrome.
The pathophysiological mechanisms subserving CMS remain to be clarified. The immediate onset of ataxia and dysmetria are likely due to surgical impact on the cerebellum. Secondary processes initiated by the tumor resection seem to play a role in the delayed onset of symptoms [11, 27] and may involve edema of the cerebellum and the cerebellar peduncles, hypoperfusion and subsequent ischemia of the cerebellum, transient dysregulation of neurotransmitter release, crossed cerebello-cerebral diaschisis, and axonal injury. Diaschisis affecting supratentorial brain areas may indeed explain some cognitive symptoms of CMS. Mariën et al. [28] showed that lesions of the right cerebellar hemisphere may result in functional deactivation of supratentorial language areas in the left cerebral hemisphere, and introduced the concept of a ‘lateralized linguistic cerebellum’. Hypoperfusion in frontal regions due to cerebellar lesions may lead to grammatical and executive disorders [29]. SPECT (Single-Photon Emission Computed Tomography) studies have shown hypoperfusion in dominant or bilateral frontal regions during mutism and improvement in blood flow when speech returned [20, 28].
Following remission of CM the cluster of motor speech characteristics are often defined as ataxic dysarthria, since this type of dysarthria is typically found in a context of cerebellar pathology [29]. However, there is no clear-cut typology, which unambiguously classifies the motor speech symptoms as ataxic dysarthria. In an auditory-perceptual analysis of the speech characteristics of 24 children and adolescents, De Smet et al. [16] showed that typical ataxic speech characteristics such as imprecise consonants, excess and equal stress, and irregular articulatory breakdown were only present in a minority of the patients (fewer than 25%). Distorted vowels not associated with irregular articulatory breakdown and/or excess and equal stress were found in seven of the 13 (54%) patients, which confirms that this speech characteristic per se is not distinctive of ataxic dysarthria. As a result, the exact nature of motor speech pathology of CMS remains to be elucidated.
This study investigates the speech and language symptoms following posterior fossa medulloblastoma surgery in a 12-year-old right-handed boy. A detailed description of the motor speech characteristics may help to unravel the puzzling nature and pathophysiology of the CMS.
Case presentation
Case history
Following a 1-year period of frequent episodes of headaches, neck pain and progressive coordination and balancing problems, a 12-year-old right-handed boy (LD) was admitted to hospital. Medical history consisted of repeated falls with a brief loss of consciousness at the age of 2 years. A neurological and cardiological work-up including repeat EEG (electroencephalography) and ECG (electrocardiogram) revealed no abnormalities. Growth and developmental milestones were reported normal. He was born at term after normal gestation and labor and there had been no perinatal or postnatal problems. His scholastic achievements had always been above average levels and there was no familial history of developmental disorder or learning disability. Both his parents and two brothers (14 and 16 years old) were healthy.
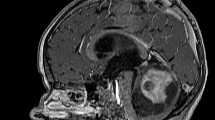
The neurological examination on admission revealed mild hypotonia, dysmetria (finger-to-nose test) and balancing problems (Romberg test). No speech or language problems were observed but in-depth linguistic assessments were not performed. MRI (Magnetic Resonance Imaging) of the brain showed a tumoral mass lesion in the fourth ventricle extending to the foramen magnum, invading the vermis and cerebellar parenchyma bilaterally and exerting mass effect on the tentorium, pons and cerebellum (Fig. 1a-c). The third and lateral ventricles were enlarged due to secondary obstructive hydrocephalus. A ventricular drain was installed and the tumor was removed following a surgical incision from the inion to C7 and of the vermis of 3 centimetres. Subtotal tumor resection was achieved because of tumoral invasion in the brainstem. Anatomopathological examination disclosed a medulloblastoma (grade IV).
(a-i) Preoperative brain MRI (a-c). The white arrow (a; axial Flair sequence) points to the tumor invading the cerebellar parenchyma bilaterally. The lesion appears slightly hyperintense. The 4th ventricle is invaded (white arrowhead), causing a hydrocephalus (dilatation of the lateral ventricles in b). The tumor expands clearly in the vermis as shown on a sagittal image (c). Early postoperative axial FLAIR slice shows postoperative sequelae at the level of dentate nuclei bilaterally (white arrows in d). The hydrocephalus is resolved (e). The 4th ventricle is moderately dilated, including in the rostral direction. The superior medullary velum is visible (white arrow in f). The superior cerebellar peduncles are involved (white arrows in g), with a slight extension towards the corpora quadrigemina at the level of inferior colliculi (white arrows in h). Crus cerebri are spared bilaterally (white arrows in i). R: right side
Postoperative findings: Day one - day four
Early postoperative MRI of the brain revealed subtotal tumor resection with tumoral residue located posteriorly right to the resection cavity, cranial to the vermis. Remission of the hydrocephalus was observed. However, postoperative sequelae included tissue damage at the level of both caudate nuclei, moderate dilation of the 4th ventricle, involvement of the cerebellar peduncles with a slight extension towards the corpora quadrigemina at the level of the inferior colliculi (Fig. 1d-i). Reduction of ventricular enlargement was found as well. In the first 4 days after surgery, the patient adequately produced only a few words (e.g. ‘yes’, ‘mother’) and some short sentences (‘I have to pee’, ‘I know that’). Behavioral initiation was markedly reduced. Drip food was given as oral intake was impossible because of insufficient oral control and sialorrhea.
Clinical neurological examination showed asymmetric peripheral muscle weakness (more severe at the right side than at the left side), but the peripheral reflexes were symmetrically preserved and cranial nerves were unimpaired. Ataxia, hypotonia, dysmetria and akinesia were found. He developed a general state of apathy during the first days after surgery.
Postoperative findings: Day five - week three
Due to leakage of cerebrospinal fluid at the insertion point of the drain and increased hydrocephalus, a new external drain was installed in combination with a ventriculostomy 2 weeks after surgery. The drain was removed one week later. On repeat CT 1 month after surgery (7/10) the ventricular volumes had diminished.
At day 5, he developed akinetic mutism. Auditory comprehension for daily conversation seemed intact since he succeeded to accurately answer yes/no questions in a non-verbal but slightly delayed way using his hands. He had marked difficulties moving his mouth, tongue and eyelids on request (voluntary movements) although automatic movements were carried out flawlessly (e.g. when swallowing or during spontaneous eye-blinking). He could not produce any sounds on command but when coughing or laughing, phonation was clearly audible. Marked presence of automatico-voluntary dissociations clearly indicated buccolabiolingual and eyelid apraxia. A bilateral paresis of the trochlear nerve (right > left) was present and a right paresis of the abducens nerve induced diplopia. After 1 week postsurgery, speech therapy was started to practice orofacial movements and to initiate speech. Under the guidance of the speech therapist peroral food was slowly built up successfully 2 weeks after surgery. Physiotherapy was started which resulted in increased movements of the arms and legs. Ataxia, hypotonia and akinesia were still present at 3 weeks postsurgery.
Aside from apathy, he displayed emotional instability, infantile behavior and he was extremely frustrated and unhappy not being able to speak. A psychiatric evaluation three weeks after surgery confirmed that frustration occurred secondary to mutism.
Postoperative findings: week four - week six
Four weeks after surgery the boy gradually started to speak again. Spontaneous output was severely adynamic (few words/sentences) at week four but became more fluent in the following weeks. The patient spoke with a clear dental articulatory setting: all alveolar sounds (/t/, /d/, /n/ and /l/) were consistently pronounced with the tongue tip against or between the teeth. In addition, the patient had a falsetto voice quality with an average fundamental frequency of 490 Hz. The patient was able to lower his voice on command, but this sounded forced and unnatural. Speech was further characterized by the prolongation of speech sounds (e.g. ‘kammmm’ instead of ‘kam’ (comb)), the devoicing of consonants (e.g. ‘pank’ instead of ‘bank’ (bank), mostly in initial position), hypernasality and self-corrections. In addition, speech tempo was relatively slow (2.5 syllables per second). This is significantly slower than in a reference sample of adult male and female speech in Verhoeven et al. [30] in which articulation rate amounted to 4.23 syllables per second. The speaker’s speech rhythm was quantified by means of the pairwise variability index (PVI) proposed by Ling et al. [31]. This index is based on measurements of vowel duration (vocalic PVI) and the duration of the intervocalic intervals (intervocalic PVI). In this patient, the vocalic PVI amounted to 33.4: this is considerably lower than 65.5, which is the reference value for Dutch suggested in Grabe and Low [32]. However, PVI is between that of French (43.5) and that of Spanish (29.7). This indicates that the patient’s speech rhythm is more syllable-timed than the Dutch stress-timing and this confirms the overall auditory impression of staccato speech.
Aside from staccato speech, none of the typical signs of (ataxic) dysarthria such as distorted vowels, imprecise consonants, excess and equal stress, and irregular articulatory breakdown were observed. By marked contrast, typical characteristics of apraxia of speech (AoS) were found including articulatory groping and segmentation. Formal investigation of motor speech by means of the Diagnostic Instrument Apraxia of Speech (DIAS) [33] was performed at six weeks postsurgery. The DIAS consists of a set of eight tasks that measure the typical characteristics of AoS (Table 1). The tasks include: performance of buccofacial movements (on command, after imitation), repetition of consonants and vowels, repetition of sequential and alternating sequences (diadochokinesis) and repetition of words (non-compounds, compounds, with/without consonant clusters). If three or more characteristics (Ca) are present, AoS is diagnosed. In this patient deviant speech features included a much worser performance for alternating sequences than for sequential sequences (Ca3), articulatory groping (Ca4), initiation problems (Ca5), syllable segmentations (Ca6), cluster segmentations (Ca7), and an articulation complexity effect (Ca8). As six out of the eight typical features were met, AoS was diagnosed (Table 1). Although buccolabiolingual praxis had improved, apraxia was still formally diagnosed by means of the DIAS [33]; articulatory groping was observed and the performance of articulatory movements improved when imitating (see Table 1).
Additional linguistic and cognitive assessments were carried out at 6 weeks postsurgery on the basis of the ScreeLing [34], the Clinical Evaluation of Language Fundamentals (CELF) [35], the Boston Naming Test-NL (BNT) [36, 37] and verbal fluency tasks (phonological, semantic fluency) (Table 1). The ScreeLing revealed normal phonological (24/24, cut-off 19) and semantic functions (21/24 cut-off 19) while syntax (18/24, cut-off 19) was impaired. The CELF yielded defective results on all five indexes (receptive language index: percentile 8.1, expressive language index: percentile 6.3, language content index: percentile 1.6, language form index: percentile 5.5, language memory index: percentile: 0.4). Visual confrontation naming (BNT: 38/60, z = −2.22) and verbal fluency (phonological: z = −2.90, semantic: z = −3.10) were also impaired (see Table 1 for detailed results). In summary, linguistic test results indicated a general language production/perception impairment (CELF, indexes 1–4) with prominent wordfinding difficulties (BNT) and syntactic impairments (ScreeLing). In addition, he presented verbal working memory deficits (CELF, index 5) and verbal executive dysfunctions (verbal fluency).
Although the right hemiparesis improved, the boy was bounded to a wheelchair. Hypotonia, ataxia and diplopia persisted. Affective/behavioral symptoms diminished but overall frontal behavioral disinhibition with infantile features (continuously laughing, (falsetto voice), interdental articulatory setting) were still present 6 weeks after surgery. The infantile behavior decreased when the family was not present in the room.
Postoperative findings: week seven - month six
A grade IV medulloblastoma was treated by means of a program of radiotherapy (boost of 30.6Gy in 17 extra fractions of 1.8 Gy) and a preventive program of craniospinal radiotherapy (23.4Gy in 13 fractions of 1.8Gy). During the radiotherapy concomitant chemotherapy was given (Vincristine 1.5 mg/mt 1×/week * six cycli). During the entire period of radio and chemotherapy patient suffered from frequent headaches, nausea and infections which made extensive cognitive assessment impossible.
He was admitted to a rehabilitation center in between the four cycli of chemotherapy where he entered a multidisciplinary therapy program (speech therapy, physiotherapy, ergotherapy). Clinical neurological investigations at 6 months postsurgery showed almost symmetrical strength with remission of the right hemiparesis. The patient started walking again but ataxia remained. Speech and language improved but slowed speech, articulatory groping, segmentation and wordfinding difficulties were still observed. No formal language tests were performed. He spoke with a normal tonal voice but the falsetto voice reappeared occasionally. After 8 months planned neurocognitive examinations were cancelled because of regrowth of the tumor.
Discussion
This patient with CMS not only shares a number of overt similarities but also some differences with the complex of symptoms that constitute CMS as generally described in the literature. As in most cases with CMS, this patient developed cerebellar mutism (CM) combined with neurological symptoms, neuropsychological disturbances and speech deficits following posterior fossa tumor surgery [1–4]. Postoperatively, a spectrum of neurological symptoms were found that consisted of: ataxia, dysmetria, hypotonia, akinesia, diplopia (trochlear and abducens paresis) and a bilateral paresis which affected the right body-side more prominently. The boy became mute 4 days after partial resection of a medulloblastoma in the posterior fossa. During these first 4 days after surgery, reduced verbal output and a general state of apathy were found. The onset of CM was accompanied by apraxic deficits (oral and eyelid apraxia) as well as affective and behavioral disturbances (emotional instability, infantile behavior, irritability). After 4 weeks of CM, speech slowly returned. Although behavioral and affective symptoms diminished, infantile behavior persisted. At 6 weeks postsurgery, formal assessment of speech (DIAS) was consistent with a diagnosis of AoS. Ataxic dysarthria was formally excluded since no vowel distortions, imprecise consonant production, excess and equal stress and irregular articulatory breakdown were found. The neurolinguistic work-up further showed distorted language production and perception, wordfinding difficulties, grammatical disturbances and verbal fluency deficits. After 6 months, speech and language as well as motor skills improved but did not return to preoperative level.
Although the neurological and neuropsychological (affective, cognitive) symptoms of this patient are typical for CMS [13–20, 22], speech and language symptoms were markedly different from what is typically described in the literature [20]. Non-motor language deficits are only rarely reported in the CMS literature. Indeed, after a careful review of 167 cases published between 1972 and 2006, De Smet et al. [15] identified 165 (98.8%) patients with postoperative motor speech disorders and only a handful of cases with non-motor language deficits [38]. The language deficits described in the study of Riva and Georgi [38] consisted of agrammatic, hypospontaneous language. However, language symptoms in the CMS population have not been systematically investigated by means of formal instruments and might therefore have been overlooked [28]. In this patient syntactic, wordfinding and verbal fluency deficits were identified as the most prominent language deficits. Only two studies similarly described language deficits following posterior fossa tumor surgery. In the study of De Smet et al. [20] 4 patients affected by CM suffered from adynamic speech production, impaired verbal fluency, word-finding difficulties and grammatical disturbances at 1.5–6 months postoperatively. Levisohn et al. [21] showed that 11 out of 19 posterior fossa tumor patients (regardless of the presence/absence of CM) had word-finding difficulties/severe language problems within 2 years from surgery. The linguistic symptoms seem to form part of the Cerebellar Cognitive Affective Syndrome (CCAS) or Schmahmann’s syndrome [21, 23–25] that refers to a constellation of neuropsychological (visuo-spatial, executive), affective and linguistic impairments that are caused by cerebellar lesions. PFS, CM, CCAS are related conditions that have been used interchangeable in studies, which makes it hard to compare the results of different studies.
In the posterior fossa literature motor speech symptoms following CM are typically defined as ataxic dysarthria in posterior fossa literature [13, 15–19]. Reviewing the data on 283 childhood cases to chart the mode of recovery of motor speech production after CMS, De Smet et al. [20] showed that 98.8% of the reviewed cases displayed motor speech impairments. However, perceptual speech analysis in 24 children and adolescents (of whom 12 developed CMS) disclosed that the most prominent speech deficits consisted of distorted vowels, slow rate, voice tremor, and monopitch and that typical ataxic speech characteristics such as imprecise consonants, excess and equal stress, and irregular articulatory breakdown were only present in half of the patients (54%) [16]. De Smet et al. [16] concluded that motor speech disturbances following cerebellar tumor surgery do not necessarily resemble ataxic dysarthria. Acoustic/phonetic speech analyses and DIAS assessment [33] in this patient revealed the following characteristics: high pitch (falsetto voice), dental articulatory setting, hypernasality, prolongation of phonemes, slowed/staccato speech, devoicing of consonants, frequent self-corrections, seeking behavior/articulatory groping, initiation problems, syllable segmentations, cluster segmentations, worse performance for alternating sequences than for sequential sequences and an articulation complexity effect. Although hypernasality, prolongation of phonemes and slowed staccato speech are symptoms that overlap between ataxic dysarthria and AoS, there is ample evidence to conclude that this patient’s deviant speech production is consistent with AoS in the absence of ataxic dysarthria [29, 39]. First, DIAS results clearly matched a typological diagnosis of AoS as six out of the eight typical AoS features were met (seeking behavior/articulatory groping, initiation problems, syllable segmentations, cluster segmentations, worse performance for alternating sequences than for sequential sequences and an articulation complexity effect). Second, patients with AoS typically have problems with irregular voicing or devoicing [40] and frequently correct themselves [41], which was also the case in this patient (devoicing of consonants, self-corrections). Third, the three most typical symptoms of ataxic speech (i.e. imprecise consonants, excess and equal stress and irregular articulatory breakdown) were not observed in this patient. The origin of the high pitch falsetto voice and dental articulatory setting are unclear. These symptoms are neither typical of AoS nor of ataxic dysarthria. Tumor surgery may cause vagal neuropathy and as such vocal fold paralysis [42] inducing a compensatory falsetto or paralytic falsetto [43, 44]. However, ENT (ear, nose, throat) investigations did not reveal vagal neuropathy or vocal fold paralysis. The falsetto voice and the dental articulatory setting might therefore reflect phenomena linked to the childish/infantile behavior of this patient.
As a result it is hypothesized that the CM in this patient was more likely induced by an underlying speech planning and organization deficit (apraxia) and not by a pure motor disorder (dysarthria). AoS and ataxic dysarthria share some semiological similarities (e.g. irregularity, slowness), but these may reflect more universal aspects (e.g. motor slowness) [29]. The role of the cerebellum in ataxic dysarthria is generally acknowledged [45], whereas a possible role of the cerebellum in AoS remains to be elucidated. According to Ziegler [29] cerebellar lesions are not implicated in the origin of AoS. The primary lesion sites that have been assigned to AoS are Brodmann area 44 of Broca’s area, the left inferior premotor and motor cortex, and the left anterior insular cortex [46–48]. However, the aforementioned clinical symptoms of this case’s speech clearly match with the concepts of AoS put forward by Ziegler [29]: inconsistent speech movement aberrations resulting in inconsistent sound distortions and phonemic errors, slowed speech, breakdown of speech rhythm due to groping false starts, inter-/intrasyllable pauses, scanned rhythm, frequent self-correction. Consequently, in this case AoS was formally diagnosed and related to the cerebellar lesion. In addition, functional neuroimaging and clinical studies have recently shown that the cerebellum is also involved in speech motor control, which is required for the planning and execution of speech [49–52]. Nevertheless the exact role of the cerebellum in motor planning versus motor execution of speech is still unclear [29, 53]. Whether the cerebellum has a direct or indirect role in the motor speech planning network might be further investigated using SPECT reflecting distant functional effects in anatomically connected cerebral regions primarily involved in motor speech planning such as Broca's area, the language dominant inferior premotor and motor cortex and the anterior insula [46–48].
This case might be the first example that clearly demonstrates AoS following posterior fossa tumor surgery. Therefore, the findings add to the view that the cerebellum plays a crucial role in the planning and execution of speech. Whether the cerebellum has a direct or indirect role in motor speech planning needs to be further investigated. Although residual speech symptoms following CMS are frequently defined as ataxic dysarthria, there is no consensus on the core speech characteristics that follow CM [9, 12, 16, 54, 55]. Therefore, this case presenting apraxic rather than dysarthric speech deficits provides novel evidence that might add to current insights in the core features of motor speech and language disturbances in CMS.
Limitations and future directions
No in-depth speech and language assessments were performed in the preoperative phase and after the 6 week follow-up period. No preoperative assessments were carried out because the presence of obstructive hydrocephalus required immediate surgical intervention. Longer-term postoperative assessments were missing due to referral to another hospital, time-consuming treatment plans and regrowth of the tumor. No motor scales were formally conducted but a close clinical neurological follow-up was performed. The use of functional neuroimaging might enable to further unravel the direct or indirect role of the cerebellum in motor speech planning.
Conclusion
CMS consists of a constellation of neuropsychiatric, neuropsychological and neurogenic speech and language deficits. It is most commonly observed in children after posterior fossa tumor surgery. The most prominent feature of CMS is mutism, which generally starts after a few days after the operation, has a limited duration and is typically followed by motor speech deficits. On the pathophysiolgical level the complex range of symptoms unambiguously reflects crucial involvement of the cerebellum and the cerebro-cerebellar pathways in disrupted cognitive and affective processes. However, the core speech characteristics of CMS and the exact role of the cerebellum in motor speech planning versus execution are still unclear. In this article, a detailed description is provided of the motor speech and language deficits following posterior fossa tumor surgery in a 12-year-old right-handed boy. In-depth analyses of motor speech production revealed features consistent with a diagnosis of AoS while ataxic dysarthria was absent. Future research is needed to confirm these findings and to explore the precise role of the cerebellum in motor speech planning processes.
Abbreviations
- AoA:
-
Apraxia of speech
- BNT:
-
Boston naming test
- C:
-
Cervicular
- Ca:
-
Characteristics
- CCAS:
-
Cerebellar cognitive affective syndrome
- CELF:
-
Clinical evaluation of language fundamentals
- CM:
-
Cerebellar mutism
- CMS:
-
Cerebellar mutism syndrome
- CT:
-
Computed tomography
- DIAS:
-
Diagnostic
- ECG:
-
Electrocardiogram
- EEG:
-
Electroencephalography
- ENT:
-
Ear nose and throat
- Gy:
-
Gray
- MRI:
-
Magnetic resonance imaging
- NL:
-
Nederlandse versie (Dutch version)
- PFS:
-
Posterior fossa syndrome
- PVI:
-
Pairwise variability index
- SPECT:
-
Single-photon emission computed tomography
References
Baillieux H, De Smet HJ, Lesage G, Paquier P, De Deyn PP, Mariën P. Neurobehavioral alterations in an adolescent following posterior fossa tumor resection. Cerebellum. 2006;5:289–95.
Baillieux H, Weyns F, Paquier P, De Deyn PP, Mariën P. Posterior fossa syndrome after a vermian stroke: a new case and review of the literature. Pediatr Neurosurg. 2007;43:386–95.
De Smet HJ, Mariën P. Posterior fossa syndrome in an adult patient following surgical evacuation of an intracerebellar haematoma. Cerebellum. 2012;11:587–92.
Mariën P, De Smet HJ, Wijgerde E, Verhoeven J, Crols R, De Deyn PP. Posterior fossa syndrome in adults: a new case and comprehensive survey of the literature. Cortex. 2013;49:284–300.
Fujisawa H, Yonaha H, Okumoto K, Uehara H, Ie T, Nagata Y, et al. Mutism after evacuation of acute subdural hematoma of the posterior fossa. Childs Nerv Syst. 2005;21:234–6.
Mariën P, Verslegers L, Moens M, Dua G, Herregods P, Verhoeven J. Posterior fossa syndrome after cerebellar stroke. Cerebellum. 2013;12:686–91.
Dimova PS, Bojinova VS, Milanov IG. Transient mutism and pathologic laughter in the course of cerebellitis. Pediatr Neurol. 2009;41:49–52.
Parrish JB, Weinstock-Guttman B, Yeh EA. Cerebellar mutism in pediatric acute disseminated encephalomyelitis. Pediatr Neurol. 2010;42:259–66.
Gudrunardottir T, De Smet HJ, Bartha-Doering L, van Dun K, Verhoeven J, Paquier P, et al. Chapter 11: Posterior Fossa Syndrome (PFS) and Cerebellar Mutism. In: Mariën P, Manto M, editors. The Linguistic Cerebellum. Academic Press; 2016. p. 257–314.
Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: review of the literature. Childs Nerv Syst. 2011;27:355–63.
Pollack IF. Neurobehavioral abnormalities after posterior fossa surgery in children. International Review of Psychiatry. 2001;13(4):302–12.
Steinbok P, Cochrane DD, Perrin R, Price A. Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg. 2003;39:179–83.
Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the Posterior Fossa Syndrome in children after cerebellar tumour surgery. Cortex. 2010;46:933–46.
Di Rocco C, Chieffo D, Frassanito P, Caldarelli M, Massimi L, Tamburrini G. Heralding cerebellar mutism: evidence for pre-surgical language impairment as primary risk factor in posterior fossa surgery. Cerebellum. 2011;10:551–62.
De Smet HJ, Baillieux H, Catsman-Berrevoets C, De Deyn PP, Mariën P, Paquier PF. Postoperative motor speech production in children with the syndrome of “cerebellar” mutism and subsequent dysarthria: a critical review of the literature. Eur J Paediatr Neurol. 2007;11:193–207.
De Smet HJ, Catsman-Berrevoets C, Aarsen F, Verhoeven J, Mariën P, Paquier PF. Auditory-perceptual speech analysis in children with cerebellar tumours: a long-term follow-up study. Eur J Paediatr Neurol. 2012;16:434–42.
Mei C, Morgan AT. Incidence of mutism, dysarthria and dysphagia associated with childhood posterior fossa tumour. Childs Nerv Syst. 2011;27:1129–36.
Morgan AT, Liégeois F, Liederkerke C, Vogel AP, Hayward R, Harkness W, et al. Role of cerebellum in fine speech control in childhood: persistent dysarthria after surgical treatment for posterior fossa tumour. Brain Lang. 2011;117:69–76.
Ozimek A, Richter S, Hein-Kropp C, Schoch B, Gorissen B, Kaiser O, et al. Cerebellar mutism--report of four cases. J Neurol. 2004;251:963–72.
De Smet HJ, Baillieux H, Wackenier P, De Praeter M, Engelborghs S, Paquier PF, et al. Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology. 2009;23:694–704.
Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–50.
Palmer BW, Boone KB, Lesser IM, Wohl MA. Base Rates of “Impaired” Neuropsychological Test Performance Among Healthy Older Adults. Arch Clin Neuropsychol. 1998;13:503–11.
Kossorotoff M, Gonin-Flambois C, Gitiaux C, Quijano S, Boddaert N, Bahi-Buisson N, et al. A cognitive and affective pattern in posterior fossa strokes in children: a case series. Dev Med Child Neurol. 2010;52:626–31.
Manto M, Mariën P. Schmahmann’s syndrome - identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias. 2015;2:2.
Sadeh M, Cohen I. Transient loss of speech after removal of posterior fossa tumors--one aspect of a larger neuropsychological entity: the cerebellar cognitive affective syndrome. Pediatr Hematol Oncol. 2001;18:423–6.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79.
Wang MC, Winston KR, Breeze RE. Cerebellar mutism associated with a midbrain cavernous malformation. Case report and review of the literature. J Neurosurg. 2002;96:607–10.
Mariën P, Engelborghs S, Fabbro F, De Deyn P. The lateralized linguistic cerebellum: A review and a new hypothesis. Brain Lang. 2001;79:580–600.
Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, et al. Consensus paper: Language and the cerebellum: an ongoing enigma. Cerebellum. 2014;13:386–410.
Verhoeven J, De Pauw G, Kloots H. Speech rate in a pluricentric language: a comparison between Dutch in Belgium and the Netherlands. Lang Speech. 2004;47:297–308.
Ling LE, Grabe E, Nolan F. Quantitative characterizations of speech rhythm: syllable-timing in Singapore English. Lang Speech. 2000;43:377–401.
Grabe E, Low E. Durational variability in speech and the Rhythm Class Hypothesis. In C. Gussenhoven and N. Warner (eds.) Papers in Laboratory Phonology 7. Berlin: Mouton de Gruyter; 2002. p. 173–200.
Feiken J, Jonkers R. Diagnostisch Instrument voor Apraxie van de Spraak (DIAS) (Diagnostic Instrument for Apraxia of Speech). Houten: Bohn, Stafleu en Van Logum; 2012.
Visch-Brink EG, van de Sandt-Koenderman MWME, El Hachioui H. ScreeLing. Houten: Bohn, Stafleu en Van Logum; 2010.
Wiig EH, Semel E, Secord WA. Clinical Evaluation of Language Fundamentals (CELF) - fifth edition. Pearson: Amsterdam, The Nederlands; 2013.
Mampaey E, Storms G, Mariën P. Boston Naming Test (BNT). In: Bouma A, Mulder J, Lindeboom J, Schmand B, editors. Handboek neuropsychologische diagnostiek. Amsterdam, The Netherlands: Pearson Assessment; 2012.
Mariën P, Mampaey E, Vervaet A, Saerens J, De Deyn PP. Normative data for the Boston Naming Test in native Dutch-speaking Belgian elderly. Brain Lang. 1998;65:447–67.
Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–61.
Mariën P, Manto M. The Linguistic Cerebellum. London: Academic Press, Elsevier; 2016.
Archambault D, Bergeron M. Is devoicing a phonetic or phonemic problem? A case study of a patient with Apraxia of speech. J Neurolinguistics. 1990;5:265–84.
Ogar J, Slama H, Dronkers N, Amici S, Gorno-Tempini ML. Apraxia of speech: an overview. Neurocase. 2005;11:427–32.
Vachha B, Cunnane MB, Mallur P, Moonis G. Losing Your Voice: Etiologies and Imaging Features of Vocal Fold Paralysis. J Clin Imaging Sci [Internet]. 2013 [cited 2015 Dec 8];3. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3690671/.
Iengo M, Villari P, Cavaliere M, De Clemente M, Merolla F. Anatomo-functional study of 37 patients with monolateral chord paralysis. Acta Otorhinolaryngol Ital. 2000;20:23–33.
Lundy DS, Casiano RR. “Compensatory falsetto”: effects on vocal quality. J Voice. 1995;9:439–42.
Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31:265–72.
Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–61.
Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–87.
Ottomeyer C, Reuter B, Jäger T, Rossmanith C, Hennerici MG, Szabo K. Aphemia: an isolated disorder of speech associated with an ischemic lesion of the left precentral gyrus. J Neurol. 2009;256:1166–8.
Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–41.
Mariën P, Verhoeven J, Engelborghs S, Rooker S, Pickut BA, De Deyn PP. A role for the cerebellum in motor speech planning: evidence from foreign accent syndrome. Clin Neurol Neurosurg. 2006;108:518–22.
Mariën P, Verhoeven J. Cerebellar involvement in motor speech planning: some further evidence from foreign accent syndrome. Folia Phoniatr Logop. 2007;59:210–7.
Shuster LI, Lemieux SK. An fMRI investigation of covertly and overtly produced mono- and multisyllabic words. Brain Lang. 2005;93:20–31.
Mariën P, van Dun K, Verhoeven J. Cerebellum and apraxia. Cerebellum. 2015;14:39–42.
Korah MP, Esiashvili N, Mazewski CM, Hudgins RJ, Tighiouart M, Janss AJ, et al. Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;77:106–12.
Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev. 2008;14:221–8.
Acknowledgments
Elke De Witte is a postdoctoral fellow of the Research Foundation - Flanders (FWO).
Funding
This study was funded by the Research Foundation - Flanders (FWO).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Author’s contribution
EDW: study design, clinical data collection, data analysis, data interpretation, literature search, writing, IW: clinical data collection, clinical follow-up of patients, review of clinical data, writing, DDS: description of MRI, figures, writing, GD: review of neurosurgical data, figures, writing, MM: review of neurosurgical data, writing, JV: data analysis, data interpretation, writing, MM: neuroimaging data interpretation, figures, writing, PM: study design, clinical data collection, data analysis, data interpretation, literature search, writing. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Ethics approval and consent to participate
This study was approved by the ethical committee of the Vrije Universiteit Brussel. Written informed consent was obtained from the patient for participation in the study. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
De Witte, E., Wilssens, I., De Surgeloose, D. et al. Apraxia of speech and cerebellar mutism syndrome: a case report. cerebellum ataxias 4, 2 (2017). https://doi.org/10.1186/s40673-016-0059-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40673-016-0059-x