Abstract
Background
Stem CO2 efflux (ES) plays a critical role in the carbon budget of forest ecosystems. Thinning is a core practice for sustainable management of plantations. It is therefore necessary and urgent to study the effect and mechanism of thinning intensity (TI) on ES.
Methods
In this study, five TIs were applied in Larix principis-rupprechtii Mayr 21-, 25-, and 41-year-old stands in North China in 2010. Portable infrared gas analyzer (Li-8100 A) was used to measure ES and its association with environmental factors at monthly intervals from May to October in 2013 to 2015. In addition, nutrients, wood structure and nonstructural carbon (NSC) data were measured in August 2016.
Results
The results show that ES increased with increasing TI. The maximum ES values occurred at a TI of 35 % (3.29, 4.57 and 2.98 µmol∙m-2∙s-1) and were 1.54-, 1.94- and 2.89-fold greater than the minimum ES value in the CK stands (2.14, 2.35 and 1.03 µmol∙m-2∙s-1) in July for the 21-, 25- and 41-year-old forests, respectively. The ES of the trees in low-density stands was more sensitive to temperature than that of the trees in high-density stands. Soluble sugars (SS) and temperature are the main factors affecting ES. When the stand density is low enough as 41-year-old L. principis-rupprechtii forests with TI 35 %, bark thickness (BT) and humidity should be considered in addition to air temperature (Ta), wood temperature (Tw), sapwood width (SW), nitrogen concentration (N) and SS in the evaluation of ES. If a change in stand density is ignored, the CO2 released from individual 21-, 25- and 41-year-old trees could be underestimated by 168.89 %, 101.94 % and 200.49 %, respectively. CO2 release was estimated based on the stem equation in combination with the factors influencing ES for reference.
Conclusions
We suggest that it is not sufficient to conventional models which quantify ES only by temperature and that incorporating the associated drivers (e.g. density, SS, SW and N) based on stand density into conventional models can improve the accuracy of ES estimates.
Similar content being viewed by others
Introduction
Forest ecosystems play a pivotal role in the global carbon cycle, and the balance between carbon assimilation by forests and its release through respiratory processes is important in regulating the rate of CO2 accumulation in the atmosphere (Dixon et al. 1994). Among the largest C flux pathways in forests is emission of CO2 from stem surfaces (ES; Ryan et al. 2009); for example, Yang et al. (2016) estimated that annual global ES of forests was 6.7 +/‒ 1.1 Pg per year, comparable in magnitude to burning of fossil fuels. Despite considerable recent research, the mechanisms and controls of ES at the individual tree and forest stand level are not fully understood, constraining our ability to predict responses of this flux to changes in climate, forest composition and structure, and other potential drivers (Trumbore et al. 2013; Tu et al. 2017).
Although respiration by living cells in the stem (RS) is the dominant process contributing to ES in trees, interpretation of ES measurements is complicated by CO2 transport processes either or both to and away from the chamber location, primarily in xylem sap (Ft; Saveyn et al. 2008; Salomón et al. 2016b). The principal environmental factor regulating cellular respiration is temperature because the rate of most enzyme-catalyzed biochemical reactions increases exponentially with temperature. This effect is often represented using the Q10 convention which specifies the proportional increase in reaction rate (or flux) for every 10ºC increase in temperature, i.e. the temperature sensitivity. Both cell maintenance and growth respiration can contribute to the total RS, and maintenance respiration (Rm) is much more temperature sensitive than growth respiration (Rg; Adu-Bredu et al. 1997). Soil nutrient availability also might influence RS because cellular respiration often depends upon tissue nutrient (especially N) concentration (Stockfors and Linder 1998; Vose and Ryan 2002).
Regarding internal biotic factors influencing ES, the mass of living tissues directly determines RS (Gruber et al. 2009; Fan et al. 2017). Three principal tissues are inner bark, cambium and parenchyma in the sapwood. Typically inner bark is thought to comprise the largest proportion of RS (Gruber et al. 2009). Bark thickness can also influence ES through its resistance to CO2 diffusion from stem to atmosphere (Steppe et al. 2007). On a whole-tree basis both ES and RS increased with gross production and stem growth rate (Yang et al. 2016), and phloem girdling studies have also demonstrated that locally higher stem growth above the girdle results in higher ES and RS (Maier et al. 2010; Yang et al. 2019). However, this relationship is complicated by the local increase in substrate availability above the girdle which may stimulate RS independently of growth; Maier et al. (2010) suggested that increased RS with higher substrate supply could reflect up-regulation as a means to avoid end-product suppression of photosynthesis. In sum, the environmental and biotic factors affecting ES are complex, resulting in challenges to predicting the response of this important C flux pathway to global change and forest management (Trumbore et al. 2013).
Pre-commercial forest thinning is a standard and widely adopted silvicultural practice to enhance the growth in value of a forest stand by directing the wood production into the most valuable trees while maintaining favorable stem form (Kerr and Haufe 2011). Routine thinning is conducted in young, even-aged forest stands of both natural and plantation origin. After thinning the growth of the remaining trees increases and the crowns of individual trees gradually expand increasing gross photosynthesis (Binkley et al. 2004). Opening the canopy increases the penetration of solar radiation and alters the microclimate in the forest (Aussenac 2000). These changes in forest environment, structure, growth and physiological activity would be expected to cause profound responses in ES, but to our knowledge few detailed studies have been reported (Lavigne 1987). Because a large proportion of the world’s forests is managed by thinning, it is important to understand both the response of ES to thinning and the underlying mechanisms.
The objective of this study was to quantify the effect of different levels of pre-commer cial thinning on individual tree and whole-stand ES of Larix principis-rupprechtii, a widely-distributed tree species in both natural and plantation forests of northern China (Yao et al. 2013). Three plantations were thinned at four different intensities (15 %‒35 % removal) in 2010, and ES was measured monthly during the warm season in 2013‒2015 using a Li-8100 A CO2 flux system. Due to the loose bark of Larix principis- rupprechtii, DBH growth rate can not accurately reflect the stem growth rate. Therefore, we use the sapwood growth rate to represent the stem growth rate. There were five primary aims of this study: (1) to clarify the variation in ES with thinning intensity (TI); (2) to explore the mechanism by which TI influences ES; (3) to evaluate the effect of various influencing factors on ES; (4) to determine the TI-related response and mechanism of temperature sensitivity in ES; and (5) to evaluate the degree of error in the estimations of annual tree-scale and stand-scale CO2 efflux if the effect of stand density on ES in the three forest ages is not considered and establish an estimation equation for ES that incorporates the factors influencing ES.
Materials and methods
Site description
The study was conducted at the Saihanba National Forest Park (SNFP, 42°02′‒42°36′ N; 116°51′‒117°39′ E, altitude 1600‒1800 m) of Weichang Manchu and Mongolian Autonomous county in Hebei Province, northern China. The climate is semiarid monsoon climate. The mean annual temperature was ‒1.5 °C; and average annual rainfall was 433 mm. The precipitation from June to August accounts for 55 % of the total annual precipitation (Fig. 1). The soil is classified as gray forest soil (Xi 1994). The soil parent materials are eluvium, alluvium, and saprolite. The thickness of the organic surface horizon is approximately 3‒8 cm in all stands (Zhao et al. 2018b). The stand is dominated by L. principis-rupprechtii (Yao et al. 2013).
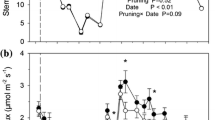
Monthly mean precipitation, air temperature (Ta) and soil temperature (Ts) in the Saihanba National Forest Park (SNFP) (1996−2013). Error bars indicate the standard error, n = 9 (Zhao et al. 2018b)
Experimental design
In our study, to be more convincing, the experimental site included 21- (young larch), 25- (immature larch), and 41-year-old (mature larch) artificial forests of L. principis-rupprechtii. Fifteen permanent plots of 400 m2 (20 m × 20 m) with essentially the same site and stand conditions were randomly established in larch plantations for each forest age, a total of forty-five plots. According to stand volume calculation, the five TIs were expressed as the percentage of felled trees to the total volume of trees in each plot: 0 % (unmanaged, control), 15 % (weak thinning, TI 15 %), 20 % (moderate thinning, TI 20 %), 30 % (strong thinning, TI 30 %) and 35 % (very strong thinning, TI 35 %; Table 1; Shen 2001). In 2010, we randomly implemented these five TIs in fifteen plots for each forest age, with three replicates for each intensity. To minimize edge effects, a 6-m-wide buffer area was established around the inner edge of each plot. The rate of ES was monitored in three model L. principis-rupprechtii trees per standard plot.
Stem CO2 efflux measurements
We used a portable infrared gas analyzer-Li-8100 A (LI-COR, Lincoln, Nebraska, USA) with 8100 − 103 chamber to monitor ES at breast height (approximately 1.3 m) of 45 sample trees. The 8100 − 103 chamber was sealed over the collar with gasket, and the chamber volume seals with its own gasket when it closes. Use gaskets to minimize leaks and wind effects. The installation steps were as follows: polyvinyl chloride (PVC) collars (with diameters of 7.5 cm) were fixed with environmentally friendly neutral silicone adhesive onto the tested parts of the stems. After drying, the residual silicone adhesive was removed, and the areas between the stem and the PVC collars were sealed. During the measurement, the Li-8100 A chamber was fixed onto the PVC collar with a string. Before the measurement, the seal was checked by exhaling on and around the chamber and the collar to ensure no rapid change in CO2 concentration in real time (in a properly sealed chamber, the CO2 concentration should increase by no more than 2 ppm) and by the leak test function of the Li8100A. After the measurement, the change in the CO2 concentration and the coefficient of variation (CV) value recorded in the Li-8100 A were analysed to ensure the sealing of the Li-8100 A and collar and the accuracy of the data. More details of instrument tightness testing methods are described in Reference (Zhao et al. 2018b). Our previous study found that the daily mean ES could be determined from measurements taken from 9:00 to 11:00 during the daily dynamic monitoring of ES over a one-year period (Zhao et al. 2018a). During the study period, nine days with roughly homogeneous weather conditions were selected from every month, and the ES at each measurement point was measured from 9:00 to 11:00. The air temperature (Ta), wood temperature (Tw, 1 cm below bark) and relative humidity (RH, %) were simultaneously measured with a Li-8100 A instantaneous digital thermometer. The air moisture content (Ma, mmol∙mol‒1) was measured using the Li-8100 A system. The temperature and moisture data were acquired simultaneously with the ES measurements. The measured area of the stem was calculated by the ellipse area formula: fix a piece of white paper on the surface of the stem to be measured and press it with the treated PVC pipe. The indentation area on the white paper is the measured area of the stem.
Analysis of wood structure, nutrient content and nonstructural carbon
In August 2016, before the leaves turned yellow, ES values were measured, and nine increment cores were taken at a height of 1.3 m from the stems of forty-five trees in each stand age using a wood core sampler. Three cores measured the wood structure by LINTAB 5 tree-ring measurement station (RINNTECH, Heidelberg, Germany) after polished smooth. The growth rate of sapwood is equal to the width of sapwood after thinning divided by the number of annual rings. The other three cores were dried at 70 °C for 48 h, milled and analysed of the nutrient content. The N and P were analyzed by means of an AA3 HR AutoAnalyzer (Seal Analytical Ltd., Southampton, UK); and K were analyzed by Lumina3300 (Aurora Biomed Inc., Vancouver, Canada). The remaining three cores were immediately placed in a cooler and transported to our laboratory. SS and starch were analyzed through an enzymatic assay (McCleary et al. 1997; Yang et al. 2019).
Data analysis
During dormancy, ES is dominated by maintenance respiration, and growth respiration is negligible (Adu-Bredu et al. 1997). In this study, during the cold season (November to April), the respiration rates were too low and the variation too high to meet the standard criterion, which states that the CV of the measurements should be below 2, so we were unable to obtain reliable measurements of ES. Thus, the ES values in October were used to represent the ES values during dormancy. The period from May to October 2013‒2015 was selected for monitoring ES, and the ES values during the period from November to April were estimated using the mature tissue method with Ta and the change in ES resulting from a 10 °C increase in temperature (Q10) (Amthor 1989).
The assumptions of normality and homogeneity of the variance in the data for each variable were verified using Shapiro-Wilk test and the Levene test. The variables that did not conform to these assumptions were mathematically transformed using logarithms or reciprocal functions. We performed statistical analysis separately for each stand age to statistically analyze the effects of TI and each factor (environmental factors, nutrient content, wood structure and NSC) on ES.
Pearson’s rank correlations and ordinary least square regression (OLS) were used to determine the relationships between the ES and each factor (including the stand density caused by the five TIs). The relative weight method was used to compare the weights of each prediction variable and closely approximated the average increase in R-square obtained by adding a predictor variable across all possible submodels (Johnson 2004; Johnson and Lebreton 2004; Lebreton and Tonidandel 2008). The quantitative relationships between ES and the factors were determined and modeled by all-subsets regression analysis, during which the variance inflation factor (VIF) was used to test the multicollinearity of predicted variables. Based on a comparison of the weights of predictive variables with multicollinearity problems, the predictive variables with smaller weights were excluded, and a new equation was established. Then, the goodness of fit between the old and new models was compared by analysis of variance (ANOVA) and the Akaike information criterion (AIC). The temperature coefficient (Q10) was computed by ES and temperature; the surface area was estimated by the circular truncated cone surface area equation. Estimation of yearly stand-scale and tree-scale Es and calculation of Q10 were done according to Zhao et al. (2018b).
Statistical analysis was performed with the R v.3.5.0 statistical software (R Foundation for Statistical Computing, Vienna, Austria) and at a P < 0.05 significance level. All figures were created using Origin 8 (OriginLab, Northampton, MA, USA) and the R v.3.5.0 statistical software.
Results
Thinning intensity variation in E S and influencing factors
As shown in Fig. 2, the ES increased with increasing TI for the 21-, 25- and 41-year-old forests. The ES of each stand was the highest in July of each year; the maximum ES values occurred at TI 35 % (3.29, 4.57 and 2.98 µmol∙m‒2∙s‒1) and were 1.54-, 1.94- and 2.89-fold greater than the minimum ES values at control (2.14, 2.35 and 1.03 µmol∙m‒2∙s‒1) in July for the 21-, 25- and 41-year-old stands, respectively. The seasonal ES variation range in the 21-, 25- and 41-year-old forests increased with increasing TI. The amplitudes of variation at TI 35 % were the highest (2.26, 3.57, and 2.55 µmol∙m‒2∙s‒1), and the amplitudes of variation at control were the lowest (1.74, 1.69, and 1.12 µmol∙m‒2∙s‒1). In the 21- and 25-year-old stands, there were significant differences in ES under different TIs (P < 0.05) in July, August and September. In the 41-year-old stands, except for in May and October, there were significant differences in ES under different TIs (P < 0.05).
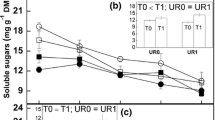
In the 21-year-old forest stands, the variables with significant differences among the five TIs were SR (P = 0.012) and ST (P = 0.002); the maximum SR values occurred at TI 35 % (13.73 cm2∙yr‒1), which was significantly higher than other plots (Fig. 3); the maximum ST values occurred at TI 15 % (19.25 mg∙g‒1) (Fig. 4). In the 25-year-old forest stands, the variables with significant differences under different TIs were Ta (P = 0.020), HS (P = 0.015) and SR (P = 0.060); the maximum Ta values occurred at TI 35 % (14.44ºC) (Fig. 5); HS showed a single peak curve with thinning intensity, and the maximum value appeared at TI 20 % (0.64) (Fig. 5). In the 41-year-old forest stands, the variables with significant differences were Ta (P = 0.029), BT (P = 0.001), SW (P = 0.023), HS (P = 0.000) and SR (P = 0.054); BT showed “V” shape with the increase of thinning intensity, the minimum value is 0.40 cm, which appears in TI 20 % (Fig. 3).
Thinning intensity (TI) variations in the stem nutrient concentrations (nitrogen concentration (N), phosphorus concentration (P) and potassium concentration (K)) and nonstructural carbon (soluble sugar content (SS) and starch content (ST)) of 21- (a), 25- (b) and 41-year-old (c) Larix principis-rupprechtii forests. Error bars indicate the standard error, n = 9.
Effects of environmental factors, nutrient content, wood structure and nonstructural carbon on E S in forests of different ages
In the 21-year-old forest, ES was significantly correlated with Ta (r = 0.57, P < 0.01), SW (r = 0.39, P = 0.03), N (r = 0.51, P < 0.01) and starch (r = 0.37, P = 0.04) when the data from all TIs over the 21-year-old forests were included, as shown in Fig. 6a. The results of the model selections based on the all-subsets regression analysis and VIF testing (VIF of SW = 12.82, VIF of SR = 7.28, SW and SR exhibit multicollinearity) demonstrated that the best model used Ta, density, BT, SW, HS, N and starch to explain the TI variation in ES in the 21-year-old forest stands (R2 = 0.905) (Table 2).
Correlogram of the correlations among stem CO2 efflux (ES) and the variables in the environmental factors (air temperature (Ta), air moisture content (Ma), relative humidity (RH) and wood temperature (Tw)), nutrient content (nitrogen concentration (N), phosphorus concentration (P) and potassium concentration (K)), wood structure (bark thickness (BT), sapwood width (SW), width ratio of heartwood to sapwood (HS) and sapwood growth rate (SR)) and nonstructural carbon (soluble sugar content (SS) and starch content (ST)) for 21- (a), 25- (b) and 41-year-old (c) Larix principis-rupprechtii. Rows and columns have been reordered using the angular order of eigenvectors. The blue color and hashing that goes from lower left to upper right represents a positive correlation between the two variables that meet at that cell. Conversely, a red color and hashing that goes from the upper left to the lower right represents a negative correlation. The darker and more saturated the color, the greater the magnitude of the correlation. The cross sign indicates that the P value of the correlation coefficient of two variables was less than 0.05
The Tw (r = 0.66, P < 0.01), Ma (r = 0.48, P = 0.01) and SS (r = 0.49, P = 0.01) were significantly correlated with ES in 25-year-old forests (Fig. 6b). Tw was significantly correlated with Ta (r = 0.43, P < 0.01), Ma (r = 0.86, P = 0.02) and RH (r = 0.76, P < 0.01). The results of the model selections based on the all-subsets regression analysis and VIF testing demonstrated that the best model used Tw, density, BT, HS, SR, N, K and SS to explain the TI variation in ES in the 25-year-old forest stands (R2 = 0.863) (Table 2).
In the 41-year-old forest, the Ma (r = 0.53, P < 0.01), Ta (r = 0.37, P = 0.04), density (r = ‒0.65, P = 0.01), N (r = 0.46, P = 0.01) and SS (r = 0.54, P < 0.01) were significantly correlated with ES (Fig. 6c). RH was significantly correlated with Ma (r = 0.87, P < 0.01). The results of the model selections based on the all-subsets regression analysis and VIF testing demonstrated that the best model used Ta, Ma, density, BT, HS, N and SS to explain the TI variation in ES for the 41-year-old forests (R2 = 0.907) (Table 2).
The above models were compared with the equation fitting ES with all 14 predictive variables by ANOVA and AIC for 21- (AIC: 28.03 VS 39.60, P = 0.60), 25- (AIC: ‒6.94 VS ‒2.13, P = 0.78) and 41-year-old (AIC: ‒70.24 VS ‒54.70, P = 0.75) forests.
Q10 change in thinning intensity
Exponential fitting of the annual ES and Ta at different TIs was conducted to evaluate the mechanism by which temperature affects ES (Table 3). For the three stand ages, the ES of the trees with lower density was more sensitive to temperature than that of the trees with higher density. In the 25- and 41-year-old forest stands, the ES of TI 35 % was the most sensitive to temperature (Q10 = 3.33 and 3.86). In the 21-year-old forest stands, the ES of the TI 30 % stand was the most sensitive to temperature (Q10 = 3.26), and the Q10 of the TI 35 % stand was 3.22, which was only 1.23 % lower than that of the TI 30 % stand. The Q10 values of TI 35 % in the three forest ages were 1.25, 1.23 and 1.45 times higher than those in control, respectively.
Relative weight of impact factor to E S
As shown in Fig. 7, fourteen factors affecting ES, including environmental factors, nutrient content, wood structure and NSC, could explain 90.9 %, 87.5 % and 89.3 % of the ES in the three forest ages, respectively. The five variables with the highest weight for ES in the 21-year-old stand were SS (14.51), starch (14.46), Ta (10.72), SW (9.78) and N (9.37); thus, the main environmental, nutrient content, wood structure and NSC factors that influenced the TI-related variations in ES were Ta, SW, N and SS, respectively. In the 25-year-old forest, the five highest weighted factors for ES were Tw (28.37), SS (15.21), Ma (13.81), RH (9.73) and N (8.06); thus, the main environmental, nutrient content, wood structure and NSC factors that influenced the TI-related variations in ES were Tw, SW (5.03), N and SS, respectively. In the 41-year-old forest, the five highest weighted factors for ES were SS (17.16), BT (16.48), Ma (16.07), RH (10.53) and Ta (10.37); thus, the main environmental, nutrient content, wood structure and NSC factors that influenced the TI-related variations in ES were Ma, BT, N (7.57) and SS, respectively.
The amount of CO2 released from different density stands
As shown in Table 4, the maximum stand-scale CO2 efflux value occurred at TI 35 % (10.86 ± 0.37 t CO2∙ha‒1∙yr‒1) and was 1.45-fold greater than the minimum ES value at control (7.47 ± 0.23 t CO2∙ha‒1∙yr‒1) in the 21-year-old forest. In the 25-year-old forest, the maximum stand-scale CO2 efflux occurred at control, with a value of 15.89 ± 0.54 t CO2∙ha‒1∙yr‒1, and the minimum occurred at TI 30 %, with a value of 10.08 ± 0.42 t CO2∙ha‒1∙yr‒1. In the 41-year-old forest, the maximum stand-scale CO2 efflux occurred at TI 35 % (5.87 ± 0.31 t CO2∙ha‒1∙yr‒1), similar to the 21-year-old forest, and the minimum occurred at control, with a value of 3.80 ± 0.10 t CO2∙ha‒1∙yr‒1. Under five TIs, the stand-scale CO2 release of the 25-year-old stand was the highest among the three forest ages, and the CO2 release of the 41-year-old stand was the lowest. There were significant differences in the stand-scale CO2 release among the five TIs for all three forest ages (P < 0 01). The tree-scale CO2 efflux increased with increasing TI for all three forest ages. The tree-scale CO2 release of TI 35 % (4.84 ± 0.22, 10.40 ± 0.49 and 12.35 ± 0.39 kg CO2∙plant‒1∙yr‒1) increased by 168.89 %, 101.94 % and 200.49 % compared with that of control (1.80 ± 0.21, 5.15 ± 0.27 and 4.11 ± 0.13 kg CO2∙plant‒1∙yr‒1) for the 21-, 25- and 41-year-old forests, respectively. Additionally, the tree-scale CO2 emissions were significantly different among the five TIs for three forest ages (P < 0.01).
Discussion
Seasonality and TI variation in E S
The current study found that ES increased with TI. Previous studies have presented that thinning could significantly promote the growth of tree DBH (Zhao et al. 2016) and that DBH growth could promote ES increase (Robertson et al. 2010; Yang et al. 2012; Araki et al. 2015; Tu et al. 2017), supporting our research results. There are several factors accounting for these complex relationships between ES and TI. Thinning reduces tree density and opens the canopy, thus changing the microclimatic conditions by allowing greater penetration of solar radiation and wind into forest stands (Brèda et al. 1995; Aussenac 2000). In conifer forests, low-density stands with more open canopies are usually sunnier, hotter and drier than high-density stands (Meyer et al. 2001; Rambo and North 2009; Ma et al. 2010; Martín-Benito et al. 2010; Moreno-Gutierrez et al. 2011). Consequently, there are significant differences in temperature under different TIs, thereby supporting the above point of view. ES and temperature are strongly positively correlated (Bown and Watt 2016; Darenova et al. 2018; Salomón et al. 2019). Therefore, temperature is one of the mechanisms by which thinning affects ES. Additionally, thinning may increase photosynthetic production because of the lower competition for light, water and mineral nutrients (López-Serrano et al. 2005; Han et al. 2006; Barbour 2007; Forrester et al. 2012; Forrester and Baker 2012). These changes could affect the carbohydrate levels of the remaining trees, and the accumulation of SS could increase the stem respiration rate (Yang et al. 2019). As shown in Fig. 6, thinning increased the SS and promoted the carbon emission rate of stem. Moreover, a decrease in stand density could lead to an enhancement of transpiration (Forrester 2015) and an acceleration of the transport of CO2 in the catheter. The ES at a given location includes the CO2 generated by trunk and root respiration and transported in the sap flow (Teskey et al. 2008; Bloemen et al. 2014) and the CO2 exhaled by trunk cells at the measured location (Araki et al. 2015). Thus, an increase in CO2 transport accelerates the outward discharge of CO2 in trunk sap flow (Saveyn et al. 2008; Bužková et al. 2015; Salomón et al. 2016b, 2019), eventually leading to an increase in ES. Therefore, we speculate that changes in the microenvironment (temperature and humidity), transpiration tension and NSC content are key to the increase in ES caused by thinning. Future research exploring the relationship between growth respiration and maintenance respiration under different TIs and the above influencing factors could be of great significance for explaining the relationship between thinning and ES.
The present research shows that Ta, Tw, N and SW are the predominant environmental, nutrient content and wood structure factors that influenced on the TI variations in ES in the 21- and 25-year-old forests. These findings also agree with our earlier observations, which were calculated by a different method (Zhao et al. 2018b). Contrary to expectations, the study of ES under different TIs in the 41-year-old forest showed that the main influencing environmental factor was Ma, while the main influencing wood structural factor was BT. These inconsistencies might be explained as follows: the density of the 41-year-old forest stands was only approximately 25 %‒33 % that of the 21-year-old and 25-year-old stands (Table 1). This lower stand density can result in drought conditions in the forest environment (Moreno-Gutierrez et al. 2011), especially in the thinned 41-year-old forest, which is more water deficient than other stands. When the forest environment is arid, the xylem CO2 concentration becomes more sensitive to changes in air humidity, as has been reported for Eucalyptus tereticornis (Salomón et al. 2019), Quercus pyrenaica (Salomón et al. 2016a), and Populus deltoides (Saveyn et al. 2008). These studies demonstrate the dependency of the xylem CO2 concentration on water availability; the change in environmental humidity in the forest can greatly increase the content of CO2 in the xylem and promote the metabolism of the trunk. Furthermore, thinning can promote an increase in tree diameter (Zhao et al. 2016). Trunk BT increased by 1‒2 mm per 10 cm increase in trunk diameter (Paine et al. 2010). The thickening of the bark helps improve trunk water retention (Teskey et al. 2008). However, the change in BT also hinders the diffusion of xylem CO2 to the atmosphere (Aubrey and Teskey 2009; Tu et al. 2017), which makes the BT a limiting factor for ES and makes ES more sensitive to changes in BT. Therefore, we can conclude that the main environmental factors affecting forest ES are Ta and Tw and that the main tree structure factor is SW. However, when the environmental humidity in a forest decreases to a certain threshold, the xylem CO2 concentration of trees sharply decreases (Salomón et al. 2019), resulting in a decrease in forest ES. At this time, the main environmental factor affecting ES changes from temperature to Ma, and the tree structure factor changes from SW to BT. Therefore, the effects of humidity and BT should be considered when a low-density forest is used to study the changes of ES.
According to the weight ranking of the influencing factors of ES, SS is not only the main influencing factor in NSC but also plays an important role in all the influencing factors of ES in our study. The study of loblolly pine by Maier et al. (2010) showed that SS is an important factor controlling the rates of ES and explains much of the temporal and spatial variation in ES in forest ecosystems, which is consistent with our results. Thinning enhances photosynthesis, and photosynthates are retained in the form of NSC (primarily SS and starch) (Hoch and Körner 2003). Although the photosynthesis and NSC concentration of the needles increased after thinning (Li et al. 2018), the responses of the NSC concentrations of the inner bark and xylem to thinning were not statistically significant. According to theory, the plant NSC pool increases when the assimilated C exceeds the demand for C but decreases when the assimilated C fails to meet demand (Sala et al. 2012). Hence, when the tree growth is slow and the SS is in surplus, the NSC could be stored in the form of starch, whereas when the growth is rapid or conditions are dry, part of the starch could be converted to SS to promote respiration (Rodríguez-Calcerrada et al. 2014; Sevanto et al. 2014; Cui et al. 2017), and the allocation of a plant’s internal carbohydrates could present a sink-driven pattern (Lambers et al. 2008). Therefore, we speculate that in a stand with a high TI, trees give priority to assigning more NSC to growth and therefore increase the availability of trunk NSC to occupy more growth space. Although the trunks of trees in stands with a high TI exhibit more NSC, the respiratory consumption of NSC also increases, resulting in lower proportions but constant concentrations of NSC in the trunk. An increase in TI can promote an increase in the DBH of trees, which also confirms this explanation. Therefore, although thinning does not cause a difference in SS, SS, like temperature, is an important factor affecting ES.
Response of Q10 to TI
Q10 for the three forest ages ranged from 2.58 to 3.86, comparable with that observed in other species (Yang et al. 2012, 2019; Rodríguez-Calcerrada et al. 2014; Tarvainen et al. 2014; Darenova et al. 2018). Close inspection of Table 3 shows that Q10 basically increases with increasing TI. In contrast to our study, a previous study suggested that thinning reduces Q10 (Lavigne 1987). The differences in the trends probably result from the limited measuring periods (July and September) and TI levels in Lavigne (1987) and from differences among tree species. Thinning can improve the light conditions of a stand, and enhancement of photosynthesis can increase the temperature sensitivity of ES (De Roo et al. 2019). Thinning can increase the transpiration of trees by reducing stand density and then accelerate the flow velocity of trunk sap flow; faster sap flow velocity can increase Q10 (Maier et al. 2010; Zhu et al. 2012; Yang et al. 2019). In addition, thinning can enhance the metabolic activity of trunk cells and carbohydrate availability (Lambers et al. 2008; Sala et al. 2012) and increase the SS content in the trunk (Fig. 3), thereby promote the rapid growth of tree diameter, so the improvements in cell metabolic activity (Saveyn et al. 2007), carbohydrate availability (Yang et al. 2019) and soluble sugar content in stem partly explain the increase in Q10. Significantly, thinning not only improved the growth respiration of trees, but also greatly improved the maintenance respiration, and the temperature sensitivity of maintenance respiration was far greater than that of growth respiration (Adu-Bredu et al. 1997). We speculate that the increase of maintenance respiration after thinning led to the increase of temperature sensitivity of ES. Therefore, the thinning-induced increase in Q10 might be partly due to the potential effects of photosynthesis, sap flow, soluble sugar content, metabolic activity of living cells and carbohydrate availability and the increase of Rm.
CO2 effluxes for each TI
The range of our annual tree-scale estimates of ES for 21- to 41-year-old L. principis-rupprechtii trees (1.80 to 12.35 kg CO2∙plant‒1∙yr‒1; Table 4) are similar to those for yellow poplar (Fan et al. 2017), red oak and red maple (Reinmann et al. 2016). Our study shows that if the change in stand density is ignored, the CO2 releases of individual trees in the three forest age groups could be underestimated by 168.89 %, 101.94 % and 200.49 %, respectively. Thinning not only promotes the release of CO2 via stem respiration but also increases the diameter of the trees and therefore the area of the trunk. Thus, the release of CO2 from the trunk increases considerably. On the stand scale, the promoting effect of TI on the CO2 release from the 21- and 41-year-old stands was weakened, and the CO2 release at the stand scale in 25-year-old stands decreased with the increase in TI. This trend is mainly because although thinning promotes ES and increases the trunk area of individual trees, it reduces the number of trees per unit area. Nevertheless, we know from the results of this study that, regardless of tree scale or stand scale, the release of CO2 under different TIs varies significantly. Therefore, in the estimation of stem CO2 release, stand density is a factor that cannot be ignored, especially in the calculation of individual tree trunk respiration carbon release. Temperature has a greater effect on ES than other factors, but Ta and Tw can only explain 11.60 %‒35.56 % of R2. Therefore, temperature is not sufficient to quantify ES accurately, and the effect of other factors, such as stand density, on ES must be integrated into models simulating ES in forest ecosystems. To estimate the carbon release from the trunk more accurately, we used the ES estimation equation combined with stand density and other factors for reference in Table 2.
Conclusions
In this study, to clarify the effects of TI on ES and Q10 of Larix principis-rupprechtii and the related mechanisms and to evaluate the error in CO2 emission estimates at the annual tree scale and stand scale without considering the effects of stand density on ES, five TIs were applied to Larix principis-rupprechtii stands with three forest ages of 21, 25 and 41 years, and the ES, environmental, nutritional, wood structure and NSC factors were monitored. The main findings are as follows: (1) Thinning can increase ES by improving the microenvironment (temperature and humidity), transpiration tension and NSC content. (2) SS, like temperature, is a main factor affecting ES. When the stand density is low enough, BT and humidity should be considered in addition to Ta, Tw, SW, N and SS in the evaluation of ES. (3) The temperature sensitivity of ES increases with increasing TI, and photosynthesis, sap flow, metabolic activity of living cells and carbohydrate availability may be potential influencing factors. (4) If the change in stand density is ignored, the CO2 release of individual 21-, 25- and 41-year-old trees could be underestimated by 168.89 %, 101.94 % and 200.49 %, respectively. (5) The CO2 estimation based on the stem equation was simulated for reference.
Abbreviations
- TI:
-
Thinning intensity
- ES :
-
Stem CO2 efflux
- Ta :
-
Air temperature
- Tw :
-
Wood temperature
- Ma :
-
Air moisture content
- RH:
-
Relative humidity
- BT:
-
Bark thickness
- SW:
-
Sapwood width
- HS:
-
Width ratio of heartwood to sapwood
- SR:
-
Sapwood growth rate
- N:
-
Nitrogen concentration
- P:
-
Phosphorus concentration
- K:
-
Potassium concentration
- SS:
-
Soluble sugar content
- ST:
-
Starch content
References
Adu-Bredu S, Yokota T, Hagihara A (1997) Temperature effect on maintenance and growth respiration coefficients of young, field-grown hinoki cypress (Chamaecyparis obtusa). Ecol Res 12:357–362
Amthor JS (1989) Respiration and crop productivity. Springer-Verlag, New York
Araki MG, Kajimoto T, Han Q, Kawasaki T, Utsugi H, Gyokusen K, Chiba Y (2015) Effect of stem radial growth on seasonal and spatial variations in stem CO2 efflux of Chamaecyparis obtusa. Trees-Struct Funct 29:499–514
Aubrey DP, Teskey RO (2009) Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol 184:35–40
Aussenac G (2000) Interactions between forest stands and microclimate: ecophysiological aspects and consequences for silvicultura. Ann For Sci 57:287–301
Barbour MM (2007) Stable oxygen isotope composition of plant tissue: a review. Funct Plant Biol 34:83–94
Binkley D, Stape JL, Ryan MG (2004) Thinking about efficiency of resource use in forests. For Ecol Manage 193:5–16
Bloemen J, Agneessens L, Van Meulebroek L, Aubrey DP, McGuire MA, Teskey RO, Steppe K (2014) Stem girdling affects the quantity of CO2 transported in xylem as well as CO2 efflux from soil. New Phytol 201:897–907
Bown HE, Watt MS (2016) Stem and soil CO2 efflux responses of Pinus radiata plantations to temperature, season, age, time (day/night) and fertilization. Cienc Investig Agrar 43:95–109
Brèda N, Granier A, Aussenac G (1995) Effect of thinning on soil and tree water relations, transpiration and growth in an oak forest (Quercus petraea (Matt.) Liebl.). Tree Physiol 15:295–306
Bužková R, Acosta M, Dařenová E, Pokorný R, Pavelka M (2015) Environmental factors influencing the relationship between stem CO2 efflux and sap flow. Trees-Struct Funct 29:333–343
Cui ZY, Yang ZJ, Xu DP, Xi RC, Zhang NN, Liu XJ, Hong Z (2017) Stem respiration and chemical composition in Dalbergia odorifera plantations differing in soil moisture content. Aust J For Sci 134:347–365
Darenova E, Acosta M, Pokorny R, Pavelka M (2018) Variability in temperature dependence of stem CO2 efflux from Norway spruce trees. Tree Physiol 38:1333–1344
De Roo L, Bloernen J, Dupon Y, Salomon RL, Steppe K (2019) Axial diffusion of respired CO2 confounds stem respiration estimates during the dormant season. Ann For Sci 76:52
Dixon RK, Solomon AM, Brown S, Houghton RA, Trexier MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Fan HL, McGuire MA, Teskey RO (2017) Effects of stem size on stem respiration and its flux components in yellow-poplar (Liriodendron tulipifera L.) trees. Tree Physiol 37:1536–1545
Forrester DI (2015) Transpiration and water-use efficiency in mixed-species forests versus monocultures: effects of tree size, stand density and season. Tree Physiol 35:289–304
Forrester DI, Baker TG (2012) Growth responses to thinning and pruning in Eucalyptus globulus, Eucalyptus nitens, and Eucalyptus grandis plantations in south eastern Australia. Can J For Res 42:75–87
Forrester DI, Collopy JJ, Beadle CL, Warren CR, Baker TG (2012) Effect of thinning, pruning and nitrogen fertiliser application on transpiration, photosynthesis and water-use efficiency in a young Eucalyptus nitens plantation. For Ecol Manage 266:286–300
Gruber A, Wieser G, Oberhuber W (2009) Intra-annual dynamics of stem CO2 efflux in relation to cambial activity and xylem development in Pinus cembra. Tree Physiol 29:641–649
Han QM, Araki M, Chiba Y (2006) Acclimation to irradiance of leaf photosynthesis and associated nitrogen reallocation in photosynthetic apparatus in the year following thinning of a young stand of Chamaecyparis obtusa. Photosynthetica 44:523–529
Hoch G, Körner C (2003) The carbon charging of pines at the climatic treeline: a global comparison. Oecologia 135:10–21
Johnson JW (2004) Factors affecting relative weights: The influence of sampling and measurement error. Organ Res Method 7:283–299
Johnson JW, LeBreton JM (2004) History and use of relative importance indices in organizational research. Organ Res Method 7:238–257
Kerr G, Haufe J (2011) Thinning practice: a silvicultural guide. Forestry Commission 54
Lambers H, Chapin FS, Pons TL (2008) Plant physiological ecology. Springer, New York
Lavigne MB (1987) Differences in stem respiration responses to temperature between balsam fir trees in thinned and unthinned stands. Tree Physiol 3:225–233
LeBreton JM, Tonidandel S (2008) Multivariate relative importance: Extending relative weight analysis to multivariate criterion spaces. J Appl Psychol 93:329
Li R, Yang Q, Zhang W, Zheng W, Wang S (2018) Response of nonstructural carbohydrates to thinning and understory removal in a Chinese fir [Cunninghamia lanceolata (Lamb.) Hook] plantation. Trees-Struct Funct 32:801–808
López-Serrano FR, De Las Heras J, González-Ochoa AI, GarcíaMorote FA (2005) Effects of silvicultural treatments and seasonal patterns on foliar nutrients in young post-fire Pinus halepensis forest stands. For Ecol Manage 210:321–336
Ma S, Concilio A, Oakley B, North M, Chen J (2010) Spatial variability in microclimate in a mixed-conifer forest before and after thinning and burning treatments. For Ecol Manage 259:904–915
Maier CA, Johnsen KH, Clinton BD, Ludovici KH (2010) Relationships between stem CO2 efflux, substrate supply, and growth in young loblolly pine trees. New Phytol 185:502–513
Martín-Benito D, Del Río M, Heinrich I, Helle G, Cañellas I (2010) Response of climate-growth relationships and water use efficiency to thinning in a Pinus nigra afforestation. For Ecol Manage 259:967–975
McCleary BV, Gibson TS, Mugford DC (1997) Measurement of total starch in cereal products by amyloglucosidase-alpha-amylase method: collaborative study. J AOAC Int (USA)
Meyer CL, Sisk TD, Covington WW (2001) Microclimatic changes induced by ecological restoration of Ponderosa pine forests in Northern Arizona. Restor Ecol 9:443–452
Moreno-Gutierrez C, Barbera GG, Nicolas E, De Luis M, Castillo VM, Martinez-Fernandez F, Querejeta JI (2011) Leaf delta 18O of remaining trees is affected by thinning intensity in a semiarid pine forest. Plant Cell Environ 34:1009–1019
Paine CET, Stahl C, Courtois EA, Patiño S, Sarmiento C, Baraloto C (2010) Functional explanations for variation in bark thickness in tropical rain forest trees. Funct Ecol 24:1202–1210
Rambo TR, North MP (2009) Canopy microclimate response to pattern and density of thinning in a Sierra Nevada forest. For Ecol Manage 257:435–442
Reinmann AB, Templer PH (2016) Reduced winter snowpack and greater soil frost reduce live root biomass and stimulate radial growth and stem respiration of red maple (Acer rubrum) trees in a mixed-hardwood forest. Ecosystems 19:129–141
Robertson AL, Malhi Y, Farfan-Amezquita FILIO, Aragao LEO, Silva Espejo JE, Robertson MA (2010) Stem respiration in tropical forests along an elevation gradient in the Amazon and Andes. Global Change Biol 16:3193–3204
Rodríguez-Calcerrada J, Martin-StPaul NK, Lempereur M, Ourcival JM, del Rey MC, Joffre R, Rambal S (2014) Stem CO2 efflux and its contribution to ecosystem CO2 efflux decrease with drought in a Mediterranean forest stand. Agric For Meteorol 195:61–72
Ryan MG, Cavaleri MA, Almeida AC, Penchel R, Senock RS, Luiz Stape J (2009) Wood CO2 efflux and foliar respiration for Eucalyptus in Hawaii and Brazil. Tree Physiol 29:1213–1222
Sala A, Woodruff DR, Meinzer FC (2012) Carbon dynamics in trees: feast or famine? Tree Physiol 32:764–775
Salomón RL, Valbuena-Carabaña M, Gil L, McGuire MA, Teskey RO, Aubrey DP, González-Doncel I, Rodríguez-Calcerrada J (2016a) Temporal and spatial patterns of internal and external stem CO2 fluxes in a sub-Mediterranean oak. Tree Physiol 36:1409–1421
Salomón RL, Valbuena-Carabaña M, Teskey R, McGuire MA, Aubrey D, González-Doncel I, Rodríguez-Calcerrada J (2016b) Seasonal and diel variation in xylem CO2 concentration and sap pH in sub-Mediterranean oak stems. J Exp Bot 67:2817–2827
Salomón RL, Steppe K, Crous KY, Noh NJ, Ellsworth DS (2019) Elevated CO2 does not affect stem CO2 efflux nor stem respiration in a dry Eucalyptus woodland, but it shifts the vertical gradient in xylem [CO2]. Plant Cell Environ 42:2151–2164
Saveyn AN, Steppe K, Lemeur R (2007) Drought and the diurnal patterns of stem CO2 efflux and xylem CO2 concentration in young oak (Quercus robur). Tree Physiol 27:365–374
Saveyn A, Steppe K, McGuire MA, Lemeur R, Teskey RO (2008) Stem respiration and carbon dioxide efflux of young Populus deltoides trees in relation to temperature and xylem carbon dioxide concentration. Oecologia 154:637–649
Sevanto S, Mcdowell NG, Dickman LT, Pangle R, Pockman WT (2014) How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ 37:153–161
Shen GF (2001) Silviculture. China Forestry Press, Beijing
Steppe K, Saveyn A, McGuire MA, Lemeur R, Teskey RO (2007) Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct Plant Biol 34:785–792
Stockfors JAN, Linder S (1998) Effect of nitrogen on the seasonal course of growth and maintenance respiration in stems of Norway spruce trees. Tree Physiol 18:155–166
Tarvainen L, Räntfors M, Wallin G (2014) Vertical gradients and seasonal variation in stem CO2 efflux within a Norway spruce stand. Tree Physiol 34:488–502
Teskey RO, Saveyn A, Steppe K, Mcguire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32
Trumbore SE, Angert A, Kunert N, Muhr J, Chambers JQ (2013) What’s the flux? Unraveling how CO2 fluxes from trees reflect underlying physiological processes. New Phytol 197:353–355
Tu J, Wei X, Fan H, Wu J, Hao L, Pei Q (2017) Disentangling critical drivers of stem CO2 efflux from Pinus elliottii trees in Subtropical China. Agric For Meteorol 237:296–302
Vose JM, Ryan MG (2002) Seasonal respiration of foliage, fine roots, and woody tissues in relation to growth, tissue N, and photosynthesis. Global Change Biol 8:182–193
Xi CP (1994) Soil classification. China Agricultural Press, Beijng
Yang JY, Teskey RO, Wang CK (2012) Stem CO2 efflux of ten species in temperate forests in Northeastern China. Trees-Struct Funct 26:1225–1235
Yang JY, He YJ, Aubrey BP, Zhuang QL, Teskey RO (2016) Global patterns and predictors of stem CO2 efflux in forest ecosystems. Global Change Biol 22:1433–1444
Yang Q, Zhang W, Li R, Zheng W, Yang J, Xu M, Wang S (2019) Effects of girdling on stem CO2 efflux and its temperature sensitivity in Chinese fir and sweetgum trees. Agric For Meteorol 268:116–123
Yao YT, Zhang JZ, Hu JF (2013) Larix principis-rupprechtii. Agricultural science and technology press of China, Beijing
Zhao KJ, Ji FL, Liu YW, Liu XL, Jia ZK, Ma LY (2016) Growth of Larix principis-rupprechtii with thinning and pruning. J Zhejiang Agric For Univ 33:582–589
Zhao KJ, Dong BQ, Jia ZK, Ma LY (2018a) Effect of climatic factors on the temporal variation of stem respiration in Larix principis-rupprechtii Mayr. Agric For Meteorol 248:441–448
Zhao KJ, Zheng MX, Fahey TJ, Jia ZK, Ma LY (2018b) Vertical gradients and seasonal variations in the stem CO2 efflux of Larix principis-rupprechtii Mayr. Agric For Meteorol 262:71–80
Zhu LW, Zhao P, Cai XA, Zeng XP, Ni GY, Zhang JY, Yu MH (2012) Effects of sap velocity on the daytime increase of stem CO2 efflux from stems of Schima superba trees. Trees-Struct Funct 26:535–542
Funding
This project received funding from National Natural Science Foundation of China (No. 31870387), China Scholarship Council.
Author information
Authors and Affiliations
Contributions
Kuangji Zhao: Design of the study, data collection, data analysis, and manuscript writing. Timothy J. Fahey: Guide the revision of manuscript. Xiangzhen Wang and Jie Wang: Data collection; Fang He and Chuan Fan: Data analysis and polish the manuscript. Zhongkui Jia and Xianwei Li: Supervision of the scientific experiments. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, K., Fahey, T.J., Wang, X. et al. Effect of thinning intensity on the stem CO2 efflux of Larix principis-rupprechtii Mayr. For. Ecosyst. 8, 63 (2021). https://doi.org/10.1186/s40663-021-00346-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40663-021-00346-4