Abstract
Background
The ‘Khasi hill sal’ forest ecosystem in Meghalaya, India represents the easternmost limit of sal distribution. We tested if tree diversity and compositional heterogeneity of this ecosystem was higher than other sal-dominated forests due to moister environment.
Methods
Vegetation was sampled in 11 transects of 10 m width and up to 500 m length covering 5.2 ha area. All stems ≥ 10 cm girth at breast height were enumerated.
Results
We found a pattern of mixed dominance of Shorea robusta (sal) and Schima wallichii and co-dominance of Pinus kesiya and Careya arborea. The Shannon’s diversity index (H′) was 3.395 nats. This value is remarkably high and competitive to that of moist sal forests of eastern Himalayan foothills and sal-dominated forests of Tripura. A high value of H′ was manifested by: a) high species richness (S = 123), b) good equitability (70.6%), c) ‘fair’ resource apportionment, and d) abundance of rare species (84% species with less than one per cent of total individuals, 67% species with two or less individuals ha−1 and 59% species with one or less individuals ha−1). The compositional heterogeneity was ‘fair’ (Whittaker’s βw = 3.15). The presence of Fagaceae with six species commanding 4.3% of importance value (IVI) and of a pine (P. kesiya) in sal forest was remarkable. As many as 58 species showed ‘low density (≤ 10 individuals ha−1), uniform dispersion’, five species achieved ‘higher density (> 10 individuals ha−1), uniform dispersion’ and six of the top 10 species were ‘clumped’. The forest showed an exponential demographic curve illustrating ‘good’ regeneration of an expanding community. Vertical stratification was simple with a poor canopy and fair subcanopy, which together with low basal area (15.65 m2 · ha−1 for individuals ≥ 10 cm gbh) indicated logging of mature sal trees in the past.
Conclusions
The ‘Khasi hill sal’ forest ecosystem is richer in alpha and beta diversity than most sal-dominated forests, but past logging has reduced basal area. Selective removal of small timber and firewood, slash-and-burn agriculture and recurrent burning of forest floor are the principal anthropogenic factors controlling forest structure and regeneration of species.
Similar content being viewed by others
Background
Sal (Shorea robusta)-dominated forest ecosystems occur mainly in India, Nepal, Bangladesh and Bhutan (Troup [1921]), and as a single forest formation, occupy maximum geographical area of nearly 12 million ha in South Asia (Tiwari [1995]; Gautam and Devoe [2006]). The exceptional presence of trees of sal is reported from Myanmar and southwestern China, but sal-dominated forests are unknown. Sal forests occur in areas receiving 100 cm or more annual rainfall on alluvial and lateritic soils. In central India, sal predominates in Vindhya and Satpura ranges. The eastern limit of natural range of sal forests is in the State of Assam in northeast India. Nearly 2000 km long arc of sal distribution at the foothills (terai) of Himalaya extends from Shivalik hills in Himachal Pradesh to Sonitpur district in northern reaches of Brahmaputra valley. In the south of Brahmaputra river, sal forests extend eastward up to Nagaon district (Dutta and Devi [2013a], [b]). In Meghalaya, sal forests occur on foothills of the plateau and are predominant on northern slopes. Our study sites in Ri-Bhoi district represent a community of sal, which is a continuum of the eastern limit of sal forests in adjacent Nagaon district. On the south of Meghalaya, sal forests occur mainly in central part of Bangladesh (Rahman [2011]) and in the State of Tripura of India (Majumdar et al. [2012], [2014]). The sal forests in Garo-Khasi-Jaintia hill range of Meghalaya form a part of the Patkai complex of mountains. In these hills, sal occurs up to about 900 m, which is probably the highest altitude known for occurrence of sal. These sites also represent an ecotone between two ecoregions, viz., Brahmaputra valley semievergreen forests and Meghalaya subtropical forests (Olson and Dinerstein [1998]). This zone is characterized by gradual disappearance of sal and occurrence of pine (Pinus kesiya).
After the dawn of forestry management in Bengal in 1865 by the colonial government (Shankar et al. [1998a]), sal forests were the principal source of timber for railway sleepers, house building and furniture. Areas blanked by extraction of sal trees were planted with teak (Tectona grandis), especially in eastern and northeastern India (Tewari et al. [2014]). In most sal forests, S. robusta is generally the most dominant species commanding up to three-fourth of density and importance value and even greater proportion of basal area. Historically, sal forests were regarded ‘species poor’ as not many other species and their individuals were suitable for timber extraction. A study from foothills of Darjeeling Himalaya showed that the diversity of tree species is much higher in sal forests than previously hypothesized (Shankar [2001]). Subsequent studies from the same region confirmed the trend (Kushwaha and Nandy [2012]).
The large expanse of sal forests in South Asia encompasses a variety of climate: dry habitats in west to moist habitats in east, plain to foothills, high to moderate temperatures and low to high latitudes in the northern tropical region of Indian subcontinent. These variations have caused differences in floristic composition and structure of sal forests. Although an appreciable number of studies on sal forests are available, many sal-dominated forests still need inventory. In this study, we analyze the patterns of dominance, diversity and dispersion of tree species in a previously undocumented ‘Khasi hill sal’ forest in Meghalaya, which not only represents the easternmost limit of sal forests, but also occupies the highest altitudes among sal-dominated forests. We presumed that tree diversity and compositional heterogeneity (beta diversity) would be greater in sal forests of Meghalaya due to moister environment as compared to sal forests of central and northern India. We collated phytosociological attributes of sal forests of similar environment in eastern and northeastern India to compare and discuss our results. The study produced a pattern of mixed dominance of species, uniform dispersion of most rare species and a high value of Shannon’s diversity index (H′) which is close to the highest value known from sal forests.
Methods
Study area
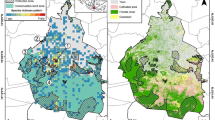
The hilly State of Meghalaya lies between 24°02′ and 26°07′ N latitude and 89°48′ and 92°51′ E longitude and covers a geographical area of 22429 km2 (Figure 1). The most area of Meghalaya is included in ‘Indo-Burma’ global hotspot of biodiversity (Myers [2003]). The low elevations all along the periphery of Meghalaya plateau experience fairly high temperature, whereas the higher elevations in the centre of the plateau have the benefit of moderate temperature (Figure 1). The average annual temperature is 21.8°C and rainfall is 3200 mm at Nongpoh (572 m) close to the study sites (Anonymous [2014]). The warmest month of the year is August with an average temperature of 25.9°C and the coolest month is January with an average temperature of 15.1°C. The southwest monsoon moving from the Bay of Bengal causes heavy rainfall on the southern aspect of the plateau and then it diminishes northward, creating a rainfall gradient (Shankar et al. [1993]). About 80% of the rain falls during rainy season and the most of the remainder falls in spring, rendering the winter cool and dry (Shankar et al. [1991]). The climate is controlled by Asia-Pacific monsoon with following distinct seasons: spring (March–April), rainy (May–September), autumn (October–mid-November) and winter (mid-November–February).
Geographical location of 11 sampled transects in Ri-Bhoi district of Meghalaya. The locations of transects and their names are shown in the map. The ombrothermic diagram depicts the climate of the study sites based on long-term data for Nongpoh which is the administrative headquarters of the Ri-Bhoi district.
The incredible variation in geology, topography and climate has resulted in formation of red sandy loam, clay loam and alluvial soils. The red loam soils occur in the central part of the Garo Hills and on the uplands of central and eastern Meghalaya. Alluvial soils occur at foothills and along river courses along the northern, western and southern border of the State. The most soils are lateritic (oxisol) type derived from Archaen gneisses, schists and granites (Gnasser [1964]). The soils of Meghalaya are acidic with pH varying from 3.8 to 6.9 (Shankar et al. [1991]).
Meghalaya has six principal forest types: i) tropical evergreen forest, ii) tropical moist mixed deciduous forest, iii) ‘Khasi hill sal’ forest, iv) Khasi-Jaintia subtropical pine forest, v) Khasi subtropical mixed broadleaved forest, and vi) Khasi subtropical oak-dominated forest. The sal forest studied here corresponds to ‘Khasi hill sal’ forests [category 3C/C1 Ia(ii)] of the seminal work of Champion and Seth ([1968]) on forest types of India. Champion and Seth ([1968]) considered ‘Khasi hill sal’ forest as ‘true climax’ and remarked that continued burning for jhum (slash-and-burn agriculture) is extending its territory on higher altitudes where Schima wallichii is a major component. Champion and Seth ([1968]) did not record presence of pine (P. kesiya) in this forest type probably because they observed this forest below 650 m altitude. Our study has plots in the upper limits of ‘Khasi hill sal’ forest, between 500 and 900 m, where it forms an ecotone with subtropical pine forest which is a predominant vegetation type ranging from 1000 to 1900 m altitude. Champion and Seth ([1968]) also remarked that ‘Khasi hill sal’ is closely allied to Himalayan sal forests.
Methodology
Field sampling
The vegetation was sampled in 5.2 ha area of 11 transects in Ri-Bhoi District in Meghalaya. Each transect had a width of 10 m and a length up to 500 m depending on the accessibility of the sampling site (Table 1). A transect encompasses contiguous subplots of 500 m2 (i.e., 10 m × 50 m). All individuals (stems) ≥ 10 cm girth at breast height (1.37 m above the ground level) were included in enumeration. Each stem was measured for girth (cm), height (m) and damage (top broken, lopping, disease etc.) following Murali et al. ([1996]) and Shankar et al. ([1998b]). The voucher specimens of species were collected, packed in polythene bags, dried in a herbarium press and processed to put up on the herbarium sheets following Jain and Rao ([1976]).
The plant species were identified and their habits verified from the regional floras (Hooker [1872]–1897; Kanjilal et al. [1934]–1940; Balakrishnan [1981] & 1983; Haridasan and Rao [1985] & 1987). The herbarium at the Botanical Survey of India, Eastern Circle, Shillong was consulted for identification. The accepted names of species as well as family were adopted from The Plant List ([2013]).
The species were classified into large tree, medium tree, small tree, shrub, woody climber and scandent shrub following Shankar ([2001]). The species in emergent canopy, subcanopy and understory were considered as large tree, medium tree and small tree, respectively. The multi-stemmed species with < 30 cm girth were considered as shrub and the climbers ≥ 10 cm girth were labelled as woody climber. The perennial species which exhibited climbing nature at the top were scandent shrubs.
Data analysis
The occurrences of a species in contiguous subplots of 500 m2 were taken into account for calculation of frequency (Mueller-Dombois and Ellenberg [1974]). The density of a species in a hectare was determined by dividing the count of individuals in all transects by the total area sampled. The stand density was the sum of the densities of all species in the community. The basal area of each individual was calculated from its respective girth and in multi-stemmed individuals, basal area of each stem was calculated separately (Shankar [2001]). The basal areas of all individuals of a species were summed to arrive at the total basal area of the respective species. The stand basal area was the sum of the basal areas of all species in the community. For each species, the values of frequency, density and basal area were converted into relative values by dividing respectively by sums of frequencies, densities and basal areas of all species. The importance value index (IVI) of a species was computed by summing up relative density, relative frequency and relative basal area (Curtis and McIntosh [1950]).
The synthetic characters of the community were computed following standard methods. The dispersion of the species was studied by variance-to-mean ratio (Greig-Smith [1983]). A ratio of 1.0 indicates a random dispersion, < 1 a uniform dispersion and > 1 an increasingly clumped dispersion. The diversity indices were calculated using IVI values of individual species in the community. The Shannon’s diversity index was calculated following Shannon ([1948]) as:
The Simpson’s index of dominance was calculated following Simpson ([1949]) as:
The Pielou’s index of evenness was calculated following Pielou ([1975]) as:
where n i = IVI of ith species, N = sum of IVI of all species, S = number of species in the community, H′ max is logeS.
The beta diversity (β) which refers to compositional heterogeneity among sampling units (i.e., places in a region, or transects in a vegetation type) was calculated from species presence-absence data of sampled transects following Whittaker ([1972]) as follows:
where γ is total species diversity at regional or landscape level and the mean species diversity at the local or within-habitat scale. The beta diversity is zero if all species in all samples are same and is maximum if there is no overlap of species among all samples.
The population structure of the forest (pool of all species) as well as of individual species was studied in nine 30 cm wide girth classes, viz., <30, 30 – < 60, 60 – < 90, 90 – < 120, 120 – < 150, 150 – < 180, 180 – < 210, 210 – < 240 and ≥ 240 cm. Similarly, the height structure of the forest (pool of all species) as well as of individual species was studied in six 5 m wide height classes, viz., <5, 5 – < 10, 10 – < 15, 15 – < 20, 20 – < 25 and ≥ 25 m.
The data were statistically treated and graphically plotted in MS-Excel. The rank-abundance plot used natural log-transformed values of IVI. A linear fit was applied to rank-abundance plot to delineate resource sharing pattern by the species in the community (Magurran [1988]). An exponential curve was fitted to depict stand’s population structure (girth and height class distributions) and a chi-square test of goodness of fit evaluated the curve fit. The transects were clustered using paired-group algorithm and Bray-Curtis similarity measure in PAST software Version 2.17c (Hammer et al. [2001]).
Results
Of 4800 stems sampled in 5.2 ha area of 11 transects, 16 stems of Musa paradisiaca, 42 clumps of bamboos, and 153 cut stems were excluded and the remaining 4589 individuals were included in further analysis (Table 1). In all, 367 individuals were multi-stemmed (forked) with 597 forks which were accounted for calculation of basal area only (Table 1). Hence, 3992 live individuals were of 10 cm or more girth and of these, 2331 individuals were 30 cm or more girth.
Floristic composition
The woody layer of ‘Khasi hill sal’ forest exhibited a total of 3992 individuals of ≥ 10 cm girth in 5.2 ha sampled area. These individuals represented 123 species in 89 genera and 47 families (Table 2). A total of 119 species could be identified with certainty up to species level, two up to genus level, and two up to family level (Table 2). As much as 27 families had one species, nine families two species, one family four species, three families five species and seven families more than five species. Leguminosae had 11 species followed by Moraceae (10), Lauraceae (9), Phyllanthaceae (9), Rubiaceae (8), Fagaceae (6) and Malvaceae (6). At generic level, Leguminosae topped with eight genera followed by Malvaceae with six genera (Table 2). Majority of species (46) had small tress, 36 had medium trees, and 29 species had large trees. There were 5 shrubs, 4 scandent shrubs and 3 woody climbers.
Three families accounted for almost 50.0% of total individuals (Figure 2a): Dipterocarpaceae (25.7%), Theaceae (17.4%) and Pinaceae (6.6%). The next 17 families, each with one per cent or more but less than six per cent individuals, contributed 45.0% individuals. The remaining 27 families, each with less than one per cent individuals, contributed only 5.4% individuals. Three families (Clusiaceae, Magnoliaceae and Rosaceae) had only one individual each and another five families (Araliaceae, Boraginaceae, Pandanceae, Proteaceae and Styracaceae) had only two individuals each (Table 2).
Pattern of dominance of plant families, in decreasing order, in ‘Khasi hill sal’ forests of Meghalaya in terms of: a) concentration of individuals, b) accumulation of basal area, and c) importance value index. Only top 25 families are shown and the remaining families are truncated from the graphs. Nearly all truncated families had less than 1 per cent contribution in all cases.
The top three families accounted 61.7% basal area (Figure 2b): Dipterocarpaceae (25.8%), Theaceae (22.2%) and Pinaceae (13.7%). The next 10 families, each with one per cent or more but less than six per cent basal area, contributed 30.8% basal area. The remaining 34 families, each with less than one per cent basal area, contributed only 7.5% basal area.
The top three families accounted 43.2% of total importance value (Figure 2c): Dipterocarpaceae (19.4%), Theaceae (16.1%) and Pinaceae (7.8%). The next 17 families, each with one per cent or more but less than six per cent importance value, contributed 50.1% importance value. The remaining 27 families, each with less than one per cent importance value, shared only 6.6% importance value.
The ‘Khasi hill sal’ forests harbour many rare and some threatened species. We could not determine with certainty if any of 123 species were endemic to the area of study or to the State of Meghalaya. However, some species appeared in IUCN Redlist (IUCN [2014]): Lower Risk/Least Concern ver. 2.3 (Alstonia scholaris, Engelhardtia spicata, S. robusta and Toona ciliata), Least Concern ver. 3.1 (Bauhinia purpurea, Holarrhena pubescens, P. kesiya and Spatholobus parviflorus) and Data Deficient ver. 2.3 (Mangifera indica).
Species richness and diversity
Species varied from 23 to 39 per transect with a mean of 29.6 and coefficient of variation 17.9% (Table 1). The most species common to any two transects were 20 between T3 and T8 and the least were six between T1 and T11. Nearly 51% species occurred in a single transect, 18% in two, 9% in three, 6% in four and 16% in six or more transects (Figure 3). S. robusta and S. wallichii occurred in all transects. The cluster analysis yielded a Cophenetic correlation value of 0.8638 (Figure 4). T7 was the most distinct transect in terms of species composition. The remaining transects broadly clustered into two groups: T1, T2, T6, T9, T10 and T11 in one and T3, T4, T5 and T8 in another. The similarity within transects of both groups was of nearly same magnitude (Figure 4).
Whittaker’s species richness index was 33.9, Shannon’s diversity index (H′) was 3.395 nats (or 4.898 bits), maximum diversity (H′max) was 4.81, Pielou’s evenness or homogeneity index (E) was 0.706 and Simpson’s dominance index (D) was 0.076. The compositional heterogeneity at landscape level as measured by Whittaker’s beta diversity (βw) was 3.15.
Density, basal area and importance value index
The stand density and basal area of individuals ≥ 10 cm girth were 767.7 ha−1 and 15.65 m2 · ha−1, respectively (Table 3). These values were 448.3 ha−1 and 13.79 m2 · ha−1 for individuals ≥30 cm girth. The basal area of an average individual was 203.9 cm2 (which is equal to 50.6 cm girth) for individuals ≥ 10 cm girth and 307.6 cm2 (which is equal to 62.2 cm girth) for individuals ≥ 30 cm girth.
Six species with 100 or more individuals each accounted for 61.2% of total individuals: S. robusta (1026 individuals), S. wallichii (693), P. kesiya (264), Careya arborea (211), Semecarpus anacardium (135) and Callicarpa arborea (114). The next 37 species, each with ten or more but less than hundred individuals, contributed 33.3% individuals. The remaining 80 species, each with less than ten individuals, shared only 5.5% individuals. Most species were rare: 103 species with less than one per cent of total individuals, 82 species with two or less individuals per hectare and 72 species with one or less individual per hectare (Table 3).
Twelve species, with at least 1 m2 basal area, accounted 80.2% of total basal area: S. robusta (20.97 m2), S. wallichii (18.07 m2), P. kesiya (11.16 m2), C. arborea (3.38 m2), Erythrina stricta (2.12 m2), Syzygium cumini (1.75 m2), Castanopsis lanceifolia (1.71 m2), S. anacardium (1.35 m2), Castanopsis purpurella (1.29 m2), Aporosa octandra (1.19 m2), Callicarpa arborea (1.19 m2) and Vitex peduncularis (1.10 m2). The next 43 species, each with ≥ 0.1 but < 1 m2, contributed 17.3% basal area. The remaining 68 species, each with < 0.1 m2, shared only 2.5% basal area (Table 3).
Thirteen species, with at least five or more IVI value, accounted 68.7% of total IVI: S. robusta (58.2), S. wallichii (48.2), P. kesiya (23.3), C. arborea (14.5), Callicarpa arborea (9.5), S. anacardium (8.7), S. cumini (8.5), A. octandra (7.2), Phyllanthus emblica (6.5), Lagerstroemia parviflora (5.8), C. lanceifolia (5.2), V. peduncularis (5.2) and E. spicata (5.1). The next 27 species, each with more than one but less than five IVI value, contributed 21.5% IVI. The remaining 83 species, each with less than one IVI value, shared only 9.8% IVI (Table 3).
The abundances of species (importance value index) followed a fair lognormal pattern of resource sharing with a few common species with high abundance (S. robusta, S. wallichii, P. kesiya and C. arborea), several intermediate species with moderate abundance and some very rare species with very low abundance (Figure 5). The rank-abundance plot was significantly (p < 0.01) explained by a linear fit with R2 = 0.912 which was marginally higher (R2 = 0.954) if top four species were excluded (Figure 5).
Pattern of distribution of species abundances in ‘Khasi hill sal’ forest of Meghalaya in a rank-abundance plot based on ln-transformed importance value index (IVI) of species. A fit of unweighted simple least square was significant (solid line, y = –0.037x + 1.924, n = 123, R2 = 0.912). On exclusion of top four species, the fit was marginally better (dashed line, y = –0.034x + 1.688, n = 119, R2 = 0.954). The top five species in descending order of IVI are: S. robusta, S. wallichii, P. kesiya, C. arborea and Callicarpa arborea.
Spatial dispersion of species
The variance-to-mean (V/M) ratio revealed that 63 species showed ‘uniform’ dispersion, none had ‘random’ dispersion and 23 had ‘clumped’ dispersion (Tables 3 and 4). The dispersion pattern of 37 species was ‘obscure’ due to zero variance as these species appeared in a single sampling unit (Table 4). The top four dominant species in terms of IVI, S. robusta, S. wallichii, P. keisya and C. arborea were clumped (Table 3). However, among 14 species with a density of ten or more individuals per hectare, six were uniform and the rest clumped (Table 3). Most species with less than ten individuals per hectare were uniform, but few were clumped.
Stand structure and regeneration
The population structure exhibited an exponential decline in concentration of individuals (R2 = 0.939, p < 0.001, Durbin-Watson statistic = 1.939), at initially faster rate and then at increasingly slower rate, from a lower to the next higher girth class (Figure 6a), indicating ‘good’ regeneration at community level. The girth class 10 to < 30 cm comprised 41.6% individuals comprising mostly saplings of trees (94.5%) and few shrubs (1.4%) and climbers (4.1%).
Stand structure of trees ≥ 10 cm girth in ‘Khasi hill sal’ forest in Meghalaya: a) structure in horizontal space, in nine girth classes, follows an exponential decline with increasingly slower rate as the size of girth increases, and b) vertical stratification in six height classes follows a unimodal exponential decline at relatively steady rate. The girth classes (cm) are: 1, ≥ 10 to < 30; 2, ≥ 30 to < 60; 3, ≥ 60 to < 90; 4, ≥ 90 to < 120; 5, ≥ 120 to < 150; 6, ≥ 150 to < 180; 7, ≥ 180 to < 210; 8, ≥ 210 to < 240; 9, ≥ 240. The height classes (m) are: 1, ≥ 0 to < 5; 2, ≥ 5 to < 10; 3, ≥ 10 to < 15; 4, ≥ 15 to < 20; 5, ≥ 20 to < 25; 6, ≥ 25.
The vertical stratification showed an average height of 8.18 ± 4.43 m of individuals having ≥ 10 cm girth and 10.98 ± 3.58 m of individuals with ≥ 30 cm girth. The distribution of individuals in height classes showed a unimodal exponential pattern with mode in 5 to 10 m height class (R2 = 0.990, p < 0.001, Durbin-Watson statistic = 2.687), and then a decline to the next higher class (Figure 6b). About 69.4% individuals were less than ten metre in height, 30.2% individuals between ten and twenty metre height and only 0.4% individuals with more than twenty metre height shaping a ‘cuspidate pyramidal’ structure. The maximum height recorded was 30 m. Only 28 individuals of six species attained a height of 20 m or more: Caryota obtusa (1 individual), C. purpurella (1), Entada rheedii (2), P. kesiya (1), S. robusta (12) and S. wallichii (11).
The dominant species in the community showed a broadly similar pattern of population structure and regeneration (Figure 7). S. robusta, predominant in canopy, showed a better regeneration than other species as it had only marginally lower number of individuals in 10 to 30 cm than in 30–60 cm girth class (Figure 7a), and similarly in < 5 m than in 5 to 10 m height class (Figure 7b). S. wallichii (Figures 7c, 7d), and C. arborea (Figures 7e, 7f) showed greater paucity of younger individuals in 10 to 30 cm girth class. P. kesiya showed a lack of regeneration with total absence of younger individuals in 10 to 30 cm girth class (Figure 7g, h).
Girth and height structure of four dominant species in ‘Khasi hill sal’ forest in Meghalaya. The girth and height classes are as defined in Figure 6.
Discussion
Floristics and species richness
The tree of sal is native to South Asia (Troup [1921]). Generally, sal is the dominant tree in the forests where it occurs. The canopies of sal-dominated forests appear similar across regions, but vary in floristic composition, patterns of species diversity, coverage of basal area, canopy height and regeneration of species. Historically, foresters viewed sal forests ‘species poor systems’ and utilized for timber extractions. However, a case of high tree diversity in a sal forest was reported from Mahananda wildlife sanctuary in foothills of eastern Himalaya in the beginning of this century (Shankar [2001]). Further investigation spanning larger geographical area of eastern Himalayan terai (including Darjeeling, Jalpaiguri and Coochbehar) endorsed that sal forests of this region harbour high levels of woody species diversity (Kushwaha and Nandy [2012]). Floristic inventories in sal forests vary dramatically in plot size, sampled area, and measurement threshold of minimum stem diameter, imposing a restriction in comparison of species richness (Table 5). Nonetheless, studies on sal-dominated forests offer a range from 17 species in 0.5 ha (Dutta and Devi [2013a]) to 152 species in 4 ha (Majumdar et al. [2014]) in eastern and northeastern India (Table 5) and from three species in 0.3 ha (Singh et al. [1995]) to 177 species in 24 ha in India (Pandey and Shukla [2003]). In this study, ‘Khasi hill sal’ forests revealed 123 species in 5.2 ha sampled area. The species richness in ‘Khasi hill sal’ forests appears close to 134 species in 3.2 ha sample area in moist sal forests of eastern Himalayan terai (Kushwaha and Nandy [2012]).
In ‘Khasi hill sal’ forests, species richness of individuals ≥ 10 cm girth is principally due to trees (90.2%). The shrubs (4.1%) and climbers (5.7%) contribute little to species richness. The dominance of trees over other growth forms (shrubs, scandent shrubs and climbers) is common in lowland forests in India, primarily due to recurrent fire and grazing. In sal-dominated lowland forests composed of 87 species in Darjeeling terai, trees were 87.4%, shrubs 5.7% and climbers 6.9% (Shankar [2001]). However, in taungya-raised, mature sal plantations in Gorakhpur, Pandey and Shukla ([2003]) observed greater proportion of shrubs and lianas.
The ‘Khasi hill sal’ forests display an evergreen physiognomy as the three most dominant species are evergreen. Although the foliage becomes prominently thin during winter, most trees are never rendered naked. At most, the deciduous phase lasts one to two weeks for individual trees of S. robusta. Corroborating other studies (Shankar et al. [1998a]; Shankar [2001]; Pandey and Shukla [2003]; Kushwaha and Nandy [2012]), Euphorbiaceae (including Phyllanthaceae) and Leguminosae were the most speciose families with 14 and 11 species, respectively. These were followed by Moraceae (10 species), Lauraceae (9), Rubiaceae (8), Fagaceae (6) and Malvaceae (6). The families of predominantly tropical distribution (megatherms) were commanding dominance although some families of predominantly montane environment (microtherms) such as Fagaceae, Pentaphylacaceae, Primulaceae, Symplocaceae and Theaceae were also present. Among these, Theaceae with one species (S. wallichii) commanded nearly 16.1% IVI value. In eastern and northeastern India, S. wallichii occurs throughout lowland and lower montane forests with varying dominance (Shankar et al. [1998a]; Majumdar et al. [2014]). An obvious difference between ‘Khasi hill sal’ forests and most other sal forests listed in Table 5 is the representation of Fagaceae with six species commanding 4.3% of IVI. Fagaceae is also represented, by two species (Castanopsis tribuloides and Quercus spicata) in sal forests of Darjeeling terai (Shankar [2001]) and by two species (Castanopsis armata and Lithocarpus spicata) in Tripura (Majumdar et al. [2012]), but with only one per cent or less contribution to IVI. Yet another striking difference is the occurrence of pine.
Patterns of species diversity and evenness
The value of Shannon diversity index (H′ = 3.395 nats with 123 species) of ‘Khasi hill sal’ forests was close to so far known highest value of H′ = 3.59 nats among natural sal forests of India from Mahananda wildlife sanctuary in Darjeeling with 87 species in 2 ha (Shankar [2001]). However, the present value was greater than H′ = 3.10 with 134 tree species from moist sal forests of eastern Himalayan terai (Kushwaha and Nandy [2012]). A recent study from ‘moist plain sal forests’ occurring below 100 m altitude in South district of Tripura (Majumdar et al. [2014]) has revealed values of H′ greater than those from Darjeeling in three of five sal associations studied: S. robusta–Diperocarpus turbinatus (H′ = 3.93), S. robusta–C. arborea (H′ = 3.73) and S. robusta–S. wallichii (H′ = 3.82). Another association S. robusta–Artocarpus chama is rather close (H′ = 3.42). Pandey and Shukla ([2003]) recorded a value of H′ = 3.96 nats from taungya-raised sal plantations of Gorakhpur with 208 species including herbs. A value of H′, as high as recorded in the present study, is a manifestation of high species richness (S = 123) coupled with fair equitability in the community (Pielou’s E = 0.706). As compared to the maximum diversity (H′max = 4.81), the realized H′ is 70.6% in this study. A reasonably high equitability of resource apportionment among species is evident as the resource distribution in the community follows a lognormal pattern.
The compositional heterogeneity of ‘Khasi hill sal’ forests is ‘fair’ as beta diversity βw is 3.15 of a maximum possible value of ten in this case. The heterogeneity of a landscape is a function of the differentiation in species composition across samples (transects) and of the distances among the samples which spread spatially from 25°43′ N to 25°57′ N latitude and 91°46′ E to 92°00′ E longitude (Figure 1). By Simpson’s measure of D, the probability that two trees drawn at random from the sample population are from the same species is as high as 7.6 per cent.
Patterns of species dispersion in community
The present study supported ‘low density, uniform dispersion’ of trees as 58 species with a density of ≤ 10 individuals ha−1 out of 86 species (excluding 37 species with obscure dispersion) were uniformly dispersed. The uniform dispersion of abundant species is uncommon in natural communities, but five species, viz., M. esculenta, C. tribuloides, C. armata, Q. lineata and S. tetragonum could achieve ‘higher density (> 10 individuals ha−1), uniform dispersion’ in this study. Nonetheless, 6 of the 10 most important species were ‘clumped’. The clumping was more pronounced in trees than in shrubs and climbers. Uniform dispersion of a large number of species meant smaller differences in climate and habitat diversity across sampled transects. The conclusion from a tropical dry forest, ‘rare species more clumped than common species’ (Hubbell [1979]), was not strongly supported in our study. The pattern of dispersion of species in this forest was in contrast with that noticed in sal forest of Darjeeling wherein only 2 of 87 species were uniform, nearly one-half clumped and the remaining random or near-random, presumably due to greater habitat diversity (Shankar [2001]).
Patterns of abundances (density, basal area and importance value)
A stand density of 767.7 ha−1 of individuals having ≥ 10 cm girth and that of 448.3 ha−1 of individuals with ≥ 30 cm girth was well within the range revealed by the sal forests (Table 5). The density values of individuals of ≥ 30 cm girth were close to the values from eastern Himalayan terai (Shankar [2001]; Kushwaha and Nandy [2012]), Gorakhpur (Pandey and Shukla [2003]), Tripura (Majumdar et al. [2012], [2014]) and Doboka Reserve Forest (Dutta and Devi [2013b]). Apparently, there appears a great consistency in stocking pattern of stems of 30 cm or more girth in sal forests of eastern region of India.
The individuals below 30 cm girth comprise not only saplings of tree species present in higher girth class but also saplings of immigrant or reappearing species. In addition, shrubs, scandent shrubs and climbers that will probably never exceed a girth of 30 cm also occur (Shankar [2001]). Hence, < 30 cm girth class is not only an indicator of regeneration of tree species, but also an accumulator of smaller life forms.
The basal area of 15.6 m2 · ha−1 for individuals with ≥10 cm girth and of 13.8 m2 · ha−1 for individuals with ≥30 cm girth was substantially lower than the range depicted by the sal forests of the region (Table 5). The studies from Darjeeling (Shankar [2001]), Kamrup (Deka et al. [2012]), Tripura (Majumdar et al. [2012]) and Gorakhpur (Pandey and Shukla [2003]) suggest a value of basal area around 26 m2 per hectare. The values exceptionally higher than this occur in managed plantations such as in Garo Hills (Kumar et al. [2006]), in well stocked sal forests with large girth trees as in moist sal forests of eastern Himalayan terai (Kushwaha and Nandy [2012]) or a result of overestimation due to inadequate and biased sampling (Dutta and Devi [2013a], [b]). The maximum girth of S. robusta was below 150 cm which is far lower than the potential maximum girth this tree could attain in pristine environment. Apparently, anthropogenic interference in form of past logging of trees by the foresters for supply of sal logs, and small timber extraction for households (especially poles) have eliminated large girth trees from the forest.
The ‘Khasi hill sal’ forest showed a mixed dominance of S. robusta and S. wallichii and co-dominance of P. kesiya and C. arborea. These species respectively had a density of 197.3, 133.3, 50.8 and 40.6 individuals ha−1, a basal area of 4.0, 3.5, 2.1 and 0.7 m2 · ha−1, and an IVI of 58.2, 48.2, 23.3 and 14.5 (Table 3). In sal forests, mixed dominance of two or more species is not common as S. robusta alone weaves the framework of the forest. S. robusta commanded three-fourth of IVI in Kamrup (Deka et al. [2012]), three-fourth of density in Gorakhpur (Pandey and Shukla [2003]), two-third of IVI in Hojai (Dutta and Devi [2013a]), one-half of IVI in eastern Himalayan terai (Kushwaha and Nandy [2012]), and one-third of density in Tripura (Majumdar et al. [2014]) and Doboka (Dutta and Devi [2013b]). Mixed dominance of species in a sal-dominated forest occurs in Darjeeling (Shankar [2001]). S. wallichi as second or third dominant species occurs in eastern Himalayan terai (Shankar [2001]; Kushwaha and Nandy [2012]), Garo Hills (Kumar et al. [2006]), Kamrup (Deka et al. [2012]) and Tripura (Majumdar et al. [2014]).
Stand structure and regeneration
The ‘Khasi hill sal’ forest showed a healthy demographic curve with an exponential fit, illustrating ‘fair’ regeneration of an expanding community. Clearly, the forest community is able to maintain continual supply of juveniles (stems in 10 to < 30 cm girth size) to higher girth classes in a pool of species despite anthropogenic stresses in form of wood extraction, fire and grazing. However, the individuals in < 30 cm girth class were less than two-fold of those in 30 to < 60 cm girth class, indicating stressed regeneration. Evidently, 24% (29) species were lacking regeneration as they were present in ≥ 30 cm girth class but not in < 30 cm girth class. All these species were rare: 27 species with one or less stems per hectare and two large trees (C. purpurella and Lithocarpus elegans) with > 1 but < 5 stems per hectare. The remaining 76% (94) species were present in both < 30 and ≥ 30 cm girth classes and apparently regenerating well. Of these, 69 species were rare (with one or less stems per hectare) and 25 species with more than one stem per hectare were not so rare. In a pool of species, plenty of juveniles of some species could be compensating for the paucity of juveniles of other species and the species might be replacing each other temporarily in cyclic succession. These observations are in line with the classic ‘Mosaic Theory of Regeneration’ (Aubréville [1938]) developed from patterns in mixed tropical forests on the Ivory Coast (see Richards [1952]).
The selective removal of poles and collections of firewood, forage and non-timber forest products (NTFPs) was visible during field enumeration and an impact of these activities was evident. A noticeable low number of individuals of S. wallichii and P. kesiya in 10 to < 30 cm girth class is attributable to preferential removal of pole size individuals for house building. Firewood is mostly collected from S. wallichii and P. kesiya although other tree species were also present.
The ‘Khasi hill sal’ forests are of low height. Almost 69% of individuals are below 10 m and only 0.4% individuals attain 20 m or more height. The vertical stratification is ‘cuspidate pyramidal’ with: i) emergent canopy (negligible with few stems ≥ 20 m, 6 species), ii) subcanopy (well developed with tree dominance in 10 to < 20 m space, 76 species), and iii) understory (robust and predominated by small trees, shrubs and tree saplings below 10 m, 102 species). Past extractions of large trees and incessant removal of small timber appear plausible reasons for the low height. Broadly, the climate is similar across sampled transects, but microhabitat conditions may vary and cause diversification of species in understory. Hence, rare species are the principal contributors.
Conclusions
The present study reveals that the ‘Khasi hill sal’ forests on northern slope of Meghalaya plateau bear close similarity with moist sal forests of eastern Himalayan foothills in Darjeeling and sal-dominated moist deciduous forests of Tripura in having high species richness, Shannon’s diversity and commonness of species. The number of species across transects (alpha diversity) did not vary greatly, but species composition among transects differed appreciably resulting into a fair compositional heterogeneity (beta diversity) and a high value of Shannon’s diversity (H′ = 3.395 nats). Unlike most other sal forests of India, the presence of Fagaceae with six species is remarkable. A pattern of mixed dominance of S. robusta and S. wallichii and co-dominance of P. kesiya and C. arborea is noteworthy as most sal forests reveal a very high dominance of S. robusta only. The high species richness was due to presence of many rare species. While the values of density are well within the range for sal forests of India, the values of basal area are considerably lower than other sal forests. The dispersion of many infrequent species was uniform and that of six out of ten most abundant species was clumped. Five species with moderate abundance showed uniform dispersion. The current disturbance in form of small timber extraction, fire and grazing is common and influencing regeneration of some species. As long as the levels of disturbance are in control, the ‘Khasi hill sal’ forests shall maintain a good demographic structure. A rise in disturbance levels shall expectedly endanger the robustness of forest structure and further threaten the relict sal forest ecosystem.
Authors’ information
AKT participated in this research as a doctoral student. US is a professor of botany and teaches biodiversity and ecology. He has been studying phytosociology and regeneration of forest ecosystems in northeast India under doctor of science programme at the North-Eastern Hill University, Shillong.
References
Anonymous:Climate: Nongpoh. 2014.
Aubréville A: La forêt coloniale: les forets de l'Afrique occidentale franҫaise. Ann Acad Sci Colon Paris 1938, 9: 1–245.
Balakrishnan NP (1981 & 1983) Flora of Jowai and Vicinity, Meghalaya, vol I & II. Botanical Survey of India, Howrah, India
Champion HG, Seth SK: A Revised Survey of the Forest Types of India. Government of India, New Delhi; 1968.
Curtis JT, McIntosh RP: The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31: 435–455.
Deka J, Tripathi OP, Khan ML: High dominance of Shorea robusta Gaertn. in Alluvial Plain Kamrup Sal Forest of Assam, N. E. India. Int J Ecosyst 2012, 2(4):67–73. 10.5923/j.ije.20120204.04
Dutta G, Devi A: Plant diversity and community structure in tropical moist deciduous sal ( Shorea robusta Gaertn.) forest of Assam, northeast India. J Environ Appl Biores 2013, 1(3):1–4.
Dutta G, Devi A: Plant diversity, population structure, and regeneration status in disturbed tropical forests in Assam, northeast India. J Forest Res 2013, 24(4):715–720. 10.1007/s11676-013-0409-y
Gautam KH, Devoe NN: Ecological and anthropogenic niches of sal ( Shorea robusta Gaertn. f.) forest and prospects for multiple-product forest management–a review. Forestry 2006, 79: 81–101. 10.1093/forestry/cpi063
Gnasser A: Geology of the Himalayas. John Wiley & Sons, New York; 1964.
Greig-Smith P: Quantitative Plant Ecology. 3rd edition. Blackwell Scientific, Oxford; 1983.
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontola Electron 4(1):9, http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Haridasan K, Rao RR (1985 & 1987) Forest flora of Meghalaya, vol I & II. Bishen Singh Mahendra Pal Singh, Dehra Dun, India
Hooker JD (1872–1897) Flora of British India, vol I through VII. L. Reeve and Company, London
Hubbell SP: Tree dispersion, abundance and diversity in a tropical dry forest. Science 1979, 203: 1299–1309. 10.1126/science.203.4387.1299
IUCN:The IUCN Red List of Threatened Species. Version 2014.1. 2014.
Jain SK, Rao RR: A Handbook of Field and Herbarium Methods. Today and Tomorrow Printers and Publishers, New Delhi; 1976.
Kanjilal UN, Kanjilal PC, Das A, De RN (1934–1940) Flora of Assam, vol I through IV. Government of Assam, India Kanjilal UN, Kanjilal PC, Das A, De RN (1934–1940) Flora of Assam, vol I through IV. Government of Assam, India
Kumar A, Marcot BG, Saxena A: Tree species diversity and distribution patterns in tropical forests of Garo Hills. Curr Sci 2006, 91(10):1370–1381.
Kushwaha SPS, Nandy S: Species diversity and community structure in sal ( Shorea robusta ) forests of two different rainfall regimes in West Bengal, India. Biodivers Conserv 2012, 21: 1215–1228. 10.1007/s10531-012-0264-8
Magurran AE: Ecological Diversity and its Measurement. Princeton University Press, New Jersey, USA; 1988.
Majumdar K, Shankar U, Dutta BK: Tree species diversity and stand structure along major community types in lowland primary and secondary moist deciduous forests in Tripura, Northeast India. J Forest Res 2012, 23(4):553–568. 10.1007/s11676-012-0295-8
Majumdar K, Dutta BK, Shankar U: Lower altitudinal variation in habitat associations, tree diversity and co-dominant population structures along moist deciduous forests dominated by sal ( Shorea robusta ) in northeast India. Forest Sci Technol 2014, 10: 1–12. 10.1080/21580103.2014.920732
Mueller-Dombois D, Ellenberg H: Aims and Methods of Vegetation Ecology. John Wiley and Sons, Inc., New York; 1974.
Murali KS, Shankar U, Uma Shaanker R, Ganeshaiah KN, Bawa KS: Extraction of non-timber forest products in the forest of Biligiri Rangan hills, India. 2. Impact of NTFP extraction on regeneration, population structure and species composition. Econ Bot 1996, 50: 252–269. 10.1007/BF02907329
Myers N: Biodiversity hotspots revisited. Bioscience 2003, 53: 916–917. 10.1641/0006-3568(2003)053[0676:LFTUN]2.0.CO;2
Olson DM, Dinerstein E: The Global 200: a representation approach to conserving the Earth’s most biologically valuable ecoregions. Conserv Biol 1998, 12: 502–515. 10.1046/j.1523-1739.1998.012003502.x
Pandey SK, Shukla RP: Plant diversity in managed sal ( Shorea robusta Gaertn.) forests of Gorakhpur, India: species composition, regeneration and conservation. Biodivers Conserv 2003, 12: 2295–2319. 10.1023/A:1024589230554
Pielou EC: Ecological Diversity. Wiley, New York; 1975.
Rahman MM, Nishat A, Vacik H: Anthropogenic disturbances and plant diversity of the Madhupur Sal forests ( Shorea robusta C.F. Gaertn) of Bangladesh. Int J Biodivers Sci, Ecosyst Serv Manage 2011, 5(3):162–173. 10.1080/17451590903236741
Richards PW: The Tropical Rain Forest: an Ecological Study. 1st edition. Cambridge University Press, Cambridge; 1952.
Shankar U: A case of high tree diversity in sal ( Shorea robusta )-dominated lowland forest of eastern Himalaya: floristic composition, regeneration and conservation. Curr Sci 2001, 81: 776–786.
Shankar U, Tripathi RS, Pandey HN: Structure and seasonal dynamics of humid tropical grasslands in Meghalaya, India. J Veg Sci 1991, 2: 711–714. 10.2307/3236181
Shankar U, Pandey HN, Tripathi RS: Phytomass dynamics and primary productivity in humid grasslands along altitudinal and rainfall gradients. Acta Oecol 1993, 14: 197–209.
Shankar U, Lama SD, Bawa KS: Ecosystem reconstruction through ‘taungya’ plantations following commercial logging of a dry, mixed deciduous forest in Darjeeling Himalaya. Forest Ecol Manage 1998, 102: 131–142. 10.1016/S0378-1127(97)00152-7
Shankar U, Murali KS, Uma Shaanker R, Ganeshaiah KN, Bawa KS: Extraction of non-timber forest products in the forest of Biligiri Rangan hills, India. 4. Impact on floristic diversity and population structure in a thorn scrub forest. Econ Bot 1998, 52: 302–315. 10.1007/BF02862149
Shannon CE: A mathematical theory of communication. Bell Syst Tech J 1948, 27(379–423):623–656. 10.1002/j.1538-7305.1948.tb00917.x
Simpson EH (1949) Measurement of diversity. Nature 163:688 Simpson EH (1949) Measurement of diversity. Nature 163:688
Singh A, Reddy VS, Singh JS: Analysis of woody vegetation of Corbett National Park. India Vegetatio 1995, 120: 69–79.
Tewari VP, Álvarez-gonzález JG, García O (2014) Developing a dynamic growth model for teak plantations in India. Forest Ecosyst 1:9, doi:10.1186/2197–5620–1-9
The Plant List:Version 1.1. 2013.
Tiwari DN: A Monograph on Sal (Shorea robusta). International Book Distributors, Dehra Dun, India; 1995.
Troup RS: The Silviculture of Indian Trees. Clarendon, Oxford; 1921.
Whittaker RH: Evolution and measurement of species diversity. Taxon 1972, 21: 213–251. 10.2307/1218190
Acknowledgements
The Department of Biotechnology (DBT), Government of India, New Delhi provided principal funding for this work through a project grant to US (grant number BT/PR7928/NDB/52/9/2006). AKT sincerely acknowledges a research fellowship from this project and from UGC’s meritorious fellowship programme. The authors are grateful to Prof. K.N. Ganeshaiah, UAS, Bangalore for project design and encouragement and thank the custodians of forests in Meghalaya to permit field work, the Botanical Survey of India (BSI), Shillong for access to herbarium, and the Head, Department of Botany, North-Eastern Hill University, Shillong for logistics. Invaluable help in the work rendered by D. Kumar, S. Borah and K. Nongrum is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AKT carried out the major field work, data collation and preliminary analysis. US designed the study, reviewed literature, analyzed data and developed the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tripathi, A.K., Shankar, U. Patterns of species dominance, diversity and dispersion in ‘Khasi hill sal’ forest ecosystem in northeast India. For. Ecosyst. 1, 23 (2014). https://doi.org/10.1186/s40663-014-0023-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40663-014-0023-2