Abstract
Background
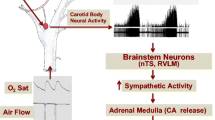
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of chronic intermittent hypoxia (CIH), which has been linked to the development of sympathoexcitation and hypertension. Furthermore, it has been shown that CIH induced inflammation and neuronal hyperactivation in the nucleus of the solitary tract (NTS), a key brainstem region involved in sympathetic and cardiovascular regulation. Since several studies have proposed that NTS astrocytes may mediate neuroinflammation, we aimed to determine the potential contribution of NTS-astrocytes on the pathogenesis of CIH-induced hypertension.
Results
Twenty-one days of CIH induced autonomic imbalance and hypertension in rats. Notably, acute chemogenetic inhibition (CNO) of medullary NTS astrocytes using Designer Receptors Exclusively Activated by Designers Drugs (DREADD) restored normal cardiac variability (LF/HF: 1.1 ± 0.2 vs. 2.4 ± 0.2 vs. 1.4 ± 0.3, Sham vs. CIH vs. CIH + CNO, respectively) and markedly reduced arterial blood pressure in rats exposed to CIH (MABP: 82.7 ± 1.2 vs. 104.8 ± 4.4 vs. 89.6 ± 0.9 mmHg, Sham vs. CIH vs. CIH + CNO, respectively). In addition, the potentiated sympathoexcitation elicit by acute hypoxic chemoreflex activation in rats exposed to CIH was also completely abolished by chemogenetic inhibition of NTS astrocytes using DREADDs.
Conclusion
Our results support a role for NTS astrocytes in the maintenance of heightened sympathetic drive and hypertension during chronic exposure to intermittent hypoxia mimicking OSA.
Similar content being viewed by others
Background
Obstructive sleep apnea (OSA) is a common sleep disorder that affects approximately 20% of the adult population worldwide [1]. It is characterized by recurrent episodes of partial or complete obstruction of the upper airway during sleep, resulting in chronic intermittent hypoxia (CIH) and sleep fragmentation [2]. OSA has been linked to a range of adverse health outcomes, including cardiovascular disease, neurocognitive impairment, and metabolic disorders [3, 4]. Thus, OSA is complex and involves interactions between various physiological systems, including the respiratory, autonomic, and central nervous systems.
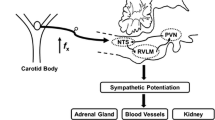
The evidence suggests that CIH, one main pathophysiological mechanism of OSA, enhances peripheral chemoafferent activity to the nucleus of the solitary tract (NTS), an integrative nucleus that communicate with main centers involve in autonomic control that participate in the increase sympathetic outflow and systemic hypertension [3, 5]. Indeed, CIH has been shown to increase the activity of neurons located in the caudal aspect of the NTS, leading to enhanced sympathetic activity, respiratory instability, and high blood pressures [6,7,8,9,10,11,12,13,14,15]. The precise mechanism(s) underpinning the effects of OSA on the neural control of hemodynamic function are still not completely understood. However, OSA-induced hypertension has been linked to systemic oxidative stress and inflammation [16,17,18,19]. Previously, we reported that increased peripheral chemoreceptor tonic discharges following exposure to CIH induced neuronal activation in the NTS [20]. Later, Oyarce & Iturriaga 2018 provided evidence supporting the presence of neuroinflammation in the NTS of rats exposed to CIH [21]. While the specific nature of NTS neuroinflammation following CIH has never been studied, the role of astrocytes in brain neuroinflammation is widely known [22]. Indeed, recent evidence suggested that astrocytes play a pivotal role in neuroinflammation in neurodegenerative and cardiovascular diseases [23,24,25,26,27]. It has been shown that astrocytes mediates the production of pro-inflammatory cytokines within the central nervous system which in turn modulate the activity of microglia contributing to the generation of a pro-inflammatory niche [28]. Despite the evidence, the possible role of astrocytes in the development of OSA-induced hypertension is not known. Therefore, we aimed to determine for the first time, the potential involvement of the NTS-astrocytes in the pathogenesis of CIH-induced hypertension. Particularly, we aimed to determine if selective chemogenetic inhibition of astrocytes residing within the caudal NTS using Designer Receptors Exclusively Activated by Designer Drugs (DREADD) improves cardiac autonomic control and reduces the hypertension in rats exposed to CIH.
Results
Twenty-one days of CIH results in systemic hypertension (MABP: 82.67 ± 1.21 vs. 104.80 ± 4.39 mmHg, Sham vs. CIH; Fig. 1D) and heart rate variability imbalance (LF/HF: 1.11 ± 0.16 vs. 2.36 ± 0.16, Sham vs. CIH; Fig. 2D) characterized by increases in sympathetic predominance (LF: 48.64 ± 4.2 vs. 67.70 ± 2.37, Sham vs. CIH; Fig. 2A). Acute chemogenetic inhibition of NTS astrocytes following 21 days of CIH results in a significant reduction in MABP (104.80 ± 4.39 vs. 89.63 ± 0.93 mmHg, CIH vs. CIH + CNO; Fig. 1D) and a complete restoration of autonomic balance. Indeed, heart rate variability values of CIH + CNO treated animals were undistinguishable compared to the ones obtained in Sham conditions (LF/HF: 1.11 ± 0.16 vs. 1.38 ± 0.28, Sham vs. CIH + CNO; Fig. 2D). Furthermore, we found that the exacerbated hemodynamic response to hypoxia (FiO2 10%) observed in rats exposed to CIH for 21 days (MABP: 95.85 ± 1.81 mmHg vs. 123.00 ± 3.95, Sham vs. CIH; Fig. 3D), was diminished by chemogenetic inhibition of NTS astrocytes (MABP: 123.00 ± 3.95 vs. 114.70 ± 3.20 mmHg, CIH vs. CIH + CNO; Fig. 3D). Hypoxia-induced sympatoexcitation was also found to be enhanced in rats exposed to 21 days of CIH (LF/HF: 1.72 ± 0.21 vs. 3.20 ± 0.29, Sham vs. CIH; Fig. 4D) and NTS astrocyte inhibition using DREADDs abolished the exaggerated sympathetic response to hypoxia (LF/HF: 3.20 ± 0.29 vs. 1.81 ± 0.34, CIH vs. CIH + CNO; Fig. 4D). We also performed experiments in Sham rats that received DREADD injections and CNO as well as in Control rats that only received CNO i.p. injections. We found that activation of DREADDs in Sham rats with CNO (1 mg/kg) has no effects on blood pressure nor in resting heart rate (Supplemental Fig. 1). Furthermore, we found that CNO (1 mg/kg i.p.) has no cardiac effects when injected i.p. in control rats. Indeed, heart systolic and diastolic function remains unaltered following CNO injection (Supplemental Fig. 1 and Supplemental Table 1).
Chemogenetic inhibition of NTS astrocytes abolished hypertension following CIH. (A) Representative blood pressure and heart rate recordings at normoxic conditions (FiO2 21%). Summary data showing (B) systolic (SBP) and (C) diastolic blood pressure (DBP), (D) mean arterial blood pressure (MABP), (E) and heart rate (HR). Data presented as mean ± standard error mean (SEM). *, p < 0.05; One-Way ANOVA for repeated measurements followed by Holm-Sidak pos hoc test. N = 4
CIH-induced sympatoexcitation is markedly reduced by acute inhibition of NTS astrocytes. (A) Representative power spectral density (PSD) spectrum of heart rate variability analysis and quantitative summary data showing cardiac autonomic balance through (B) low frequency (LF), (C) high frequency (HF) and (D) LF/HF ratio spectral components in normoxia (FiO2 21%). Data presented as mean ± standard error of the mean (SEM). *, p < 0.05; One-Way ANOVA for repeated measurements followed by Holm-Sidak pos hoc test. N = 4
Inhibition of NTS astrocytes blunted the exacerbated vascular response to hypoxia in rats exposed to CIH. (A) Representative blood pressure and heart rate recordings during hypoxia (FiO2 10%). Summary data showing (B) systolic (SBP) and (C) diastolic blood pressure (DBP), (D) mean arterial blood pressure (MABP), and (E) heart rate (HR). Data presented as mean ± standard error of the mean (SEM). *, p < 0.05; One-Way ANOVA for repeated measurements followed by Holm-Sidak pos hoc test. N = 4
Chemoreflex-induced sympatoexcitation is reduced by chemogenetic inhibition of NTS astrocytes in CIH rats. (A) Representative power spectral density (PSD) spectrum of heart rate variability analysis and quantitative summary data showing cardiac autonomic function through (B) low frequency (LF), (C) high frequency (HF) and (D) LF/HF ratio spectral components in Normoxia (FiO2 21%). Data presented as mean ± standard error of the mean (SEM). *, p < 0.05; One-Way ANOVA for repeated measurements followed by Holm-Sidak pos hoc test. N = 4
Discussion
Obstructive sleep apnea (OSA), a prevalent sleep disorder affecting millions of people worldwide, is associated with several cardiovascular and metabolic comorbidities, including hypertension [29,30,31]. The mechanisms involved in the development/maintenance of OSA-induced hypertension are multifactorial and involves at least, neuroinflammation, oxidative stress, and increased sympathetic drive [31,32,33]. In the present study, we investigated the role of astrocytes in the NTS, the primary site of integration of cardiovascular information in the brainstem, in the maintenance of high blood pressure during CIH, a main pathophysiological hallmark of OSA.
Our results showed for the first time that selective inhibition of astrocytes residing within the caudal NTS significantly reduced the CIH-induced hypertension in rats. Indeed, animals exposed for 21 days to CIH displayed resting sympathoexcitation and hypertension. Notably, selective inhibition of NTS astrocytes using a chemogenetic approached in freely moving animals results in a significant reduction in blood pressure and a complete restoration of normal heart rate variability in CIH animals. The later strongly supporting a role for NTS astrocytes in the maintenance of both sympathoexcitation and hypertension during CIH. In addition, we found that selective inhibition of NTS astrocytes reduce the exaggerated hemodynamic and sympathetic responses to hypoxia in CIH animals, suggesting that NTS astrocyte may participate in the regulation of the enhanced cardiovascular chemoreflex response observed in CIH. Further experiments are needed to fully determine que contribution of NTS astrocytes on chemoreflex function following CIH.
The precise mechanism by which NTS astrocytes contribute, in part, to the development of hypertension and potentiation of sympathetic drive during CIH remains unclear. However, it has been shown that sustained hypoxia induced NTS plasticity through activation of astrocytes within the NTS [34,35,36]. Interestingly, it is well-known that CIH increases carotid bodies peripheral chemoreceptor discharges to the NTS, increasing glutamate spillover [20]. Therefore, it is plausible to hypothesize that activation of peripheral chemoreceptors during CIH results in NTS astrocytes reactivity altering synaptic transmission at the level of NTS neurons being the outcome an exaggerated sympathetic drive and systemic hypertension. This interesting hypothesis deserves future investigations.
There are inherent limitations in the present study. We performed acute inhibition of NTS astrocytes in rats exposed to 21 days of CIH. While results showing that acute inhibition of NTS astrocytes offer salutary potential we cannot rule out the effects of chronic astrocyte inhibition on NTS-mediated cardiovascular regulation during CIH. Future studies are needed to fully determine the cardiovascular outcomes after chronic inhibition of NTS astrocytes following CIH. Although out of the scope of the present study, we did not provide any mechanistic insight into the alterations of NTS astrocytes during CIH. Nevertheless, we can speculate that during CIH astrocyte became reactive and promotes a pro-inflammatory niche within the NTS that increases neuronal firing. The later should increase sympathetic discharges being the outcome an increase in blood pressure. Further studies are needed to elucidate the mechanism(s) underlying astrocyte activation and its contribution to sympathoexcitation and hypertension in CIH mimicking OSA.
Conclusion
Our results showed that chemogenetic inhibition of astrocytes residing within the caudal region of the NTS, restores normal sympathetic drive and significantly reduces hypertension in rats exposed to 21 days of CIH mimicking OSA syndrome. Therefore, targeting medullary astrocytes may offer new avenues for the treatment of high blood pressure and other cardiovascular comorbidities in OSA patients.
Methods
Animals: Longitudinal studies were performed in unrestrained, freely moving 4-month-old male Sprague Dawley rats (n = 4) as we show in Fig. 5A. Animals were housed in individual chambers under sham-normoxic conditions first for one week. Then, chambers were flushed for 21 days with intermittent cycles of hypoxia (FiO2 5%,12 times/hour, 8 h/day). The O2 level inside the chambers was continuously monitored with an oxygen analyzer and the CO2 levels and humidity were maintained at low levels by continuous air extraction. At day 21 of CIH, hemodynamic parameters were recorded using indwelling blood pressure radiotelemetry before and after acute inhibition of NTS astrocytes (Fig. 5A). All experimental protocols were approved by the Ethics Committee for Animal Experiments of the Pontificia Universidad Católica de Chile (protocol ID 200,617,015) and were conducted according to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental design. (A) Adult male Sprague-Dawley rats were exposed to CIH for 21 days and monitored before CIH and pre/post-acute administration of clozapine N-Oxide (CNO). Animals were injected at 21d post CIH with AVV-GFAP-DREADDs(Gi)-mCherry bilaterally in commissural NTS (cNTS). (B) Representative immunostaining showing mCherry, GFAP and DAPI expression (left panel) at cNTS level, scale bar represent 170 μm (left panel) and 30 μm (middle panel). Arrowheads indicate co-localization of astrocytes and DREADDs in 2D and Z-plane. N = 4
Stereotaxic surgery and DREADD expression. Stereotaxic surgery was performed ketamine-xylazine (100 mg/Kg and 10 mg/Kg, respectively) anesthetized animals to delivered bilaterally into the NTS (-14.3 mm to bregma) an adeno-associated virus (450 nL/side, 1*1012 vg) containing an inhibitory (Gi) DREADD under the control of the GFAP promoter for selective astrocyte inhibition (Fig. 5B). One-week post-surgery, animals underwent physiological recordings (i.e. blood pressure, heart rate) before and 30 min after a single i.p. injection of clozapine N-oxide (CNO, 1 mg/kg).
Telemetry and Hypoxic stimulation. Arterial blood pressure was continuously monitored using radiotelemetry (DSI, USA). Acute cardiovascular response to hypoxia-induced chemoreflex activation was made by allowing the rats to breath hypoxic gas (FiO2 10%) for 10 min at rest.
Immunofluorescence and DREADDs expression. 4% paraformaldehyde fixed brainstem tissue was used for inmunohistochemical procedures in free-floating sections (25 μm) containing the NTS. Briefly, sections were first incubated in a PBS-buffered solution with 1% BSA, 0,5% Triton-X, and 2% gelatin from cold water fish skin followed by overnight primary anti-GFAP antibody incubation (rabbit anti-GFAP, 1:1000) at 4 °C. Then, a goat anti-rabbit Alexa 488 (1:1000) antibody was used to visualized GFAP under a confocal microscope.
Data Analysis
Data is presented as mean ± standard error of the mean (SEM) in the main text. Figures are presented as min to max box plots. Paired One-Way ANOVA was used to evaluate differences between longitudinal data following by Holm-Sidak post hoc test in GraphPad Prism 8.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Abbreviations
- CIH:
-
Chronic intermittent hypoxia
- OSA:
-
Obstructive sleep apnea
- MABP:
-
Mean arterial blood pressure
- SBP:
-
Systolic blood pressure
- DP:
-
Diastolic blood pressure
- HR:
-
Heart rate
- LF:
-
Low frequency
- HF:
-
High frequency
- NTS:
-
Nucleus of the solitary tract
- DREADDs:
-
Designer receptor exclusively activated by designer drugs
- PBS:
-
Phosphate buffer saline
- GFAP:
-
Glial fibrillar astrocyte protein
- CNO:
-
Clozapine N-oxide
- BSA:
-
Bovine serum albumin
References
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. https://doi.org/10.1093/aje/kws342.
Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147(1):266–74. https://doi.org/10.1378/chest.14-0500. PMID: 25560865; PMCID: PMC4285080.
Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(2):47–112. https://doi.org/10.1152/physrev.00043.2008. PMID: 20393191.
Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(24):569–76. https://doi.org/10.1016/j.jacc.2013.04.066. PMID: 23747759.
Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia augments acute hypoxic sensing via HIF-mediated ROS. Respir Physiol Neurobiol. 2016;226:27–30.
Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens. 2003;21(3):409–16. https://doi.org/10.1097/01.hjh.0000059026.06605.45.
Fletcher EC. Invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90(4):1600–5. https://doi.org/10.1152/jappl.2001.90.4.1600.
Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O’Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552(1):253–64. https://doi.org/10.1113/jphysiol.2003.047027.
Kubin L. Neural control of the Upper Airway: respiratory and state-dependent mechanisms. Compr Physiol. 2016;6(4):1801–50. https://doi.org/10.1002/cphy.c160002.
Zoccal DB, Furuya WI, Bassi M, Colombari DS, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol. 2014;5:238. https://doi.org/10.3389/fphys.2014.00238.
Iturriaga R, Alcayaga J, Chapleau MW, Somers VK. Carotid body chemoreceptors: physiology, pathology, and implications for health and disease. Physiol Rev. 2021;101(3):1177–235. https://doi.org/10.1152/physrev.00039.2019.
Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, Mehra R, Bozkurt B, Ndumele CE, Somers VK. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation. 2021;144(3):e56–e67. https://doi.org/10.1161/CIR.0000000000000988.
Kapa S, Sert Kuniyoshi FH, Somers VK. Sleep apnea and hypertension: interactions and implications for management. Hypertension. 2008;51(3):605–8. https://doi.org/10.1161/HYPERTENSIONAHA.106.076190.
Mitra AK, Bhuiyan AR, Jones EA. Association and Risk factors for Obstructive Sleep Apnea and Cardiovascular Diseases: a systematic review. Diseases. 2021;9(4):88. https://doi.org/10.3390/diseases9040088.
Okcay A, Somers VK, Caples SM. Obstructive sleep apnea and hypertension. J Clin Hypertens (Greenwich). 2008;10(7):549–55. https://doi.org/10.1111/j.1751-7176.2008.07811.x.
Venkataraman S, Vungarala S, Covassin N, Somers VK. Sleep apnea, hypertension and the sympathetic nervous system in the Adult Population. J Clin Med. 2020;9(2):591. https://doi.org/10.3390/jcm9020591.
Maniaci A, Iannella G, Cocuzza S, Vicini C, Magliulo G, Ferlito S, Cammaroto G, Meccariello G, De Vito A, Nicolai A, Pace A, Artico M, Taurone S. Oxidative stress and inflammation biomarker expression in obstructive sleep apnea patients. J Clin Med. 2021;10(2):277. https://doi.org/10.3390/jcm10020277.
Amorim MR, de Deus JL, Cazuza RA, et al. Neuroinflammation in the NTS is associated with changes in cardiovascular reflexes during systemic inflammation. J Neuroinflammation. 2019;16:125. https://doi.org/10.1186/s12974-019-1512-6.
Peracaula M, Torres D, Poyatos P, Luque N, Rojas E, Obrador A, Orriols R, Tura-Ceide O. Endothelial dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: a review article. Life (Basel). 2022;12(4):537. https://doi.org/10.3390/life12040537.
Del Rio R, Moya EA, Parga MJ, Madrid C, Iturriaga R. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur Respir J. 2012;39(6):1492–500. https://doi.org/10.1183/09031936.00141511.
Oyarce MP, Iturriaga R. Proinflammatory cytokines in the Nucleus of the solitary tract of hypertensive rats exposed to chronic intermittent hypoxia. Adv Exp Med Biol. 2018;1071:69–74. https://doi.org/10.1007/978-3-319-91137-3_8.
Giovannoni F, Quintana FJ. The role of astrocytes in CNS inflammation. Trends Immunol. 2020;41(9):805–19. https://doi.org/10.1016/j.it.2020.07.007.
Colonna M, Brioschi S. Neuroinflammation and neurodegeneration in human brain at single-cell resolution. Nat Rev Immunol. 2020;20(2):81–2. https://doi.org/10.1038/s41577-019-0262-0.
Ding ZB, Song LJ, Wang Q, Kumar G, Yan YQ, Ma CG. Astrocytes: a double-edged sword in neurodegenerative diseases. Neural Regen Res. 2021;16(9):1702–10. https://doi.org/10.4103/1673-5374.306064.
Siracusa R, Fusco R, Cuzzocrea S, Astrocytes. Role and functions in brain pathologies. Front Pharmacol. 2019;10:1114. https://doi.org/10.3389/fphar.2019.01114.
Wang Y, Meagher RB, Ambati S, Cheng H, Ma P, Phillips BG. Patients with obstructive sleep Apnea have altered levels of four Cytokines Associated with Cardiovascular and kidney disease, but Near normal levels with Airways Therapy. Nat Sci Sleep. 2021;13:457–66. https://doi.org/10.2147/NSS.S282869.
Müller MB, Stihl C, Schmid A, Hirschberger S, Mitsigiorgi R, Holzer M, Patscheider M, Weiss BG, Reichel C, Hübner M, Uhl B. A novel OSA-related model of intermittent hypoxia in endothelial cells under flow reveals pronounced inflammatory pathway activation. Front Physiol. 2023;14:1108966. https://doi.org/10.3389/fphys.2023.1108966.
Vainchtein ID, Molofsky AV. Astrocytes and microglia: in sickness and in Health. Trends Neurosci. 2020;43(3):144–54. https://doi.org/10.1016/j.tins.2020.01.003.
Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–8. https://doi.org/10.1164/ajrccm.157.1.9706079.
Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–13. https://doi.org/10.1164/ajrccm.163.3.9911064.
Cai A, Wang L, Zhou Y. Hypertension and obstructive sleep apnea. Hypertens Res. 2016;39(6):391–5. https://doi.org/10.1038/hr.2016.11.
Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126(2):313–23. https://doi.org/10.1016/j.neuroscience.2004.03.055.
Oyarce MP, Iturriaga R. Contribution of oxidative stress and inflammation to the Neurogenic Hypertension Induced by Intermittent Hypoxia. Front Physiol. 2018;9:893. https://doi.org/10.3389/fphys.2018.00893.
Accorsi-Mendonça D, Almado CE, Bonagamba LG, Castania JA, Moraes DJ, Machado BH. Enhanced firing in NTS Induced by Short-Term Sustained Hypoxia is modulated by Glia-Neuron Interaction. J Neurosci. 2015;35(17):6903–17. https://doi.org/10.1523/JNEUROSCI.4598-14.2015.
Tadmouri A, Champagnat J, Morin-Surun MP. Activation of microglia and astrocytes in the nucleus tractus solitarius during ventilatory acclimatization to 10% hypoxia in unanesthetized mice. J Neurosci Res. 2014;92(5):627–33. https://doi.org/10.1002/jnr.23336.
Accorsi-Mendonça D, Bonagamba LGH, Machado BH. Astrocytic modulation of glutamatergic synaptic transmission is reduced in NTS of rats submitted to short-term sustained hypoxia. J Neurophysiol. 2019;121(5):1822–30. https://doi.org/10.1152/jn.00279.2018.
Acknowledgements
We thank Mr. Fidel Flores for his help in managing the animal facility.
Funding
This work was supported by FONDECYT (#1211443; #1220950).
Author information
Authors and Affiliations
Contributions
R.D.R conceived and designed research; K.P., C.T., A.L.H., E.D.J, performed experiments, K.P., C.T., A.L.H., E.D.J, analyzed data; K.P, E.D.J prepared figures; K.P., E.D.J., C.T., A.L.H., R.I, drafted manuscript; R.D.R., edited and revised manuscript; K.P., E.D.J., C.T., A.L.H., R.I, and R.D.R., approved final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval
All experiments performed here were approved by the Ethical Committee of the Pontificia Universidad Católica de Chile (ID 170710022).
Consent for publication
Not applicable.
Competing interests
No conflict of interest is declared by authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pereyra, K., Las Heras, A., Toledo, C. et al. Chemogenetic inhibition of NTS astrocytes normalizes cardiac autonomic control and ameliorate hypertension during chronic intermittent hypoxia. Biol Res 56, 57 (2023). https://doi.org/10.1186/s40659-023-00463-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40659-023-00463-0