Abstract
Purpose
To investigate the optimal threshold for measuring thyroid volume in patients with Grave's hyperthyroidism (GH) by SPECT/CT.
Materials and methods
A 53 mL butterfly-shaped hollow container made of two 45-degree transparent elbows was put into a NEMA IEC phantom tank. The butterfly-shaped container and the tank were then filled with Na99mTcO4 of different radioactive concentrations, respectively, which could simulate thyroid gland with GH by different target-to-background ratios (T/B) (200:1, 600:1, 1000:1). The different T/B of planar imaging and SPECT/CT were acquired by a Discovery NM/CT 670 Pro SPECT/CT. With Thyroid software (Version 4.0) of GE-Xeleris workstation, the region of the thyroid gland in planar imaging was delineated. The thyroid area and average long diameter of both lobes were substituted into the Allen formula to calculate the thyroid volume. The calculation error was compared with the actual volume. Q-Metrix software was used to perform CT-based attenuation correction, scatter correction, resolution recovery. Ordered-subsets expectation maximization was used to reconstruct SPECT data. 20%, 25%, 30%, 40%, 50%, 60% thresholds were selected to automatically delineate the volume of interest and compared with the real volume, which determinated the optimal threshold. We measured the thyroid volume of 40 GH patients using the threshold and compared the volumes obtained by planar imaging and ultrasound three-dimensional. The differences of the volumes with different T/B and thresholds were compared by the ANOVA and least significant difference t test. The volumes delineated by SPECT/CT were evaluated using ANOVA, least significant difference t test, correlation analysis and, linear regression and Bland–Altman concordance test plot. The differences and consistency of thyroid volume were compared among the above three methods.
Results
There was no significant difference in the results between different T/B models (P > 0.05). The thyroid volume calculated by the planar imaging formula method was higher than the real volume, with an average overestimation of 22.81%. The volumes delineated by SPECT/CT threshold automatically decreased while the threshold increased. There were significant differences between groups with different thresholds (P < 0.001). With an average error of 3.73%, the thyroid volume analyzed by the threshold of 25% was close to the results of ultrasound measurement (P > 0.05). Thyroid volume measured by planar imaging method was significantly higher than ultrasound and SPECT/CT threshold automatic delineation method (P < 0.05). The agreement between the SPECT/CT 25% threshold and ultrasound (r = 0.956, b = 0.961) was better than that between the planar imaging and ultrasound (r = 0.590, b = 0.574). The Bland–Altman plot also showed that the thyroid volume measured by the 25% threshold automatic delineation method was in good agreement with the ultrasound measurement.
Conclusions
The T/B has no effect on the measurement of thyroid volume in GH patients; planar imaging method can significantly overestimate thyroid volume in GH patients, and 25% threshold automatic delineation method can obtain more accurate thyroid volume in GH patients.
Similar content being viewed by others
Introduction
131I has been used to treat hyperthyroidism since 1941 [1], it is one of the first-line treatment for Graves’ hyperthyroidism (GH) [2]. The dose of 131I is usually determined by the calculated dose method or the fixed dose method [3]. Compared with the fixed dose, the calculated method can reduce the dose of 131I, consequently reduce radiation damage of patients, conforming the concept of precise and individualized treatment. The dose of 131I is proportional to the volume of the thyroid gland [4,5,6] in the dosimetry method; therefore, the accurate determination of the thyroid volume is very important to determine the therapeutic dose of 131I. At present, the methods for thyroid volume mainly include: palpation, radionuclide imaging [7], ultrasound, CT, MRI [8] and PET/CT [9, 10]. Radionuclide imaging is a widely used method to obtain quantitative functional and volumetric information. Due to the lack of depth, the accuracy of planar imaging has been questioned [5]. The measurement of thyroid volume by planar imaging is greatly affected by the operator's experience. Even if the same operator repeats the operation, the repeatability is still suboptimal. Therefore, finding a more accurate and stable radionuclide imaging method has become a research hotspot.
With the continuous improvement of photon correction technology and iterative reconstruction algorithm of integrated SPECT/CT, quantitative technology has been achieved in SPECT/CT [11]. Previous studies [12,13,14,15,16] reported application of quantitative SPECT/CT in the diagnosis and treatment of thyroid diseases. Some researcher teams have stated that the thyroid volume of interest (VOI) can be delineated by automatically extracting the pixel points that are higher than the maximum uptake value and the threshold value and obtained the standardized uptake value (SUV) and volume of the thyroid in one step [13]. Although the feasibility of the method and the clinical significance of quantitative parameters were described, the selection of the threshold was not discussed in this study, which is an particularly important parameter [13]. In this research, a butterfly-shaped phantom is constructed to simulate the physiological characteristics of the GH. The optimal threshold for GH thyroid volume was determined using SPECT/CT quantitative method according to different T/B. Furthermore, the optimal threshold will be validated further in clinical data.
Materials and methods
Equipment and preparation of the simulated thyroid model
Discovery NM/CT 670 Pro of GE Company was used, equipped with 16-slice spiral CT, low-energy and high-resolution collimator. For radioisotope 99mTc, tomographic spatial resolution (without RR) of 10.59 mm and a system sensitivity (99mTc) of 73.4 cps/MBq, which were measured according to the NEMA guidelines. The software for quantitative analysis was Q.Metrix of GE-Xeleirs 4.0 workstation (GE Healthcare. USA). Na99mTcO4 was supplied by Wuhan Atomic Hi-Tech Co., Ltd. CRC-25R type radioactivity meter (CAPINTEC, USA) was applied to measure the radioactivity.
The thyroid model was made by bonding two 45-degree transparent elbows (Zaozhuang Yicai Trading Co., Ltd.), and the two ends of the elbows were sealed with waterproof tapes after filling with liquid. The inner diameter of a single elbow was 25 mm, the thickness of the wall was 3.5 mm, with 32 mm of the outer diameter and 50 mm of the height. The volume of the unilateral elbow in pure water was 26.5 mL, so the volume of the bilateral elbows was 53 mL, as shown in Fig. 1A, B. It was placed horizontally inside the NEMA IEC model (Data Spectrum, USA) from which the component and the ball were taken out. The volume of the model tank was 10,122 mL measured with pure water. Firstly, we kept the phantom dry. Then, we dissolved 0.6 mL of freshly rinsed 148 MBq Na99mTcO4 in 103 mL of water. 53 mL was taken out and filled into the bilateral lobes of the thyroid gland after well-mixed, with the radioactive concentration 142,857 Bq/mL. The remaining 50.6 mL of the solution was poured into the tank, and then, the tank was filled with water. The radioactive concentration of the tank was 714 Bq/mL. Regarding thyroid model as the target area and the tank as the background, a thyroid model with a tomographic T/B 200 was formed. The activity in the thyroid phantom was kept constant, and the background was diluted, the tomographic T/B 600 and 1000 models were prepared, as shown in Fig. 1C.
SPECT and SPECT/CT acquisition and image processing
The prepared thyroid model was placed on the examination bed, the bed height adjusted to coincide the cross light with the center of the model. Low-energy high-resolution (LEHR) collimator, energy peak (140 keV, window width 10%), matrix 256 × 256 and zoom 4.0 were selected for planar imaging. Different counts (300 Kcts/frame, 500 Kcts/frame and 800 Kcts/frame) were collected for each frame. The planar imaging data were analyzed by thyroid post-processing software (GE-Xeleirs workstation with a Version of 4.0). The GE ‘PHAGE PHASE’ blue boundary was used for manual delineation as the target and background, subsequently obtained the frontal projection area of the thyroid S (cm2), the average height of the thyroid in both lobes L (cm), and the planar imaging T/B (thyroid radioactivity count/background radioactivity count). According to the formula (1) [17], formula (2), we calculated the thyroid volume and compared it with the real volume (53 cm3).
SPECT/CT: main energy peak (140 keV, window width 10%), scatter window (120 keV, window width 5%), matrix 128 × 128, zoom 1.0, body contour, 6°/frame of 360° acquisition, three groups (12 s/frame, 15 s/frame and 18 s/frame, 60 frames in total) were collected for each image; 120 kV tube voltage, automatic exposure control, 1.25 mm slice thickness were selected for low-dose CT acquisition.
Additionally, SPECT images were reconstructed by using an iterative ordered subset expectation maximization (OSEM) algorithm (8 iterations and 10 subsets) with CT-based attenuation correction (CTAC), scatter correction (SC), and resolution recovery (RR), no post-processing filter. 20%, 25%, 30%, 40%, 50%, and 60% of the maximum uptake value of the target area were used as the thresholds to automatically delineate the VOI of the bilateral thyroid lobes in the Q.Metrix of GE-Xeleirs 4.0 workstation (GE Healthcare. USA), and the thyroid volume from each threshold was analyzed and compared with the true volume.
Patient information
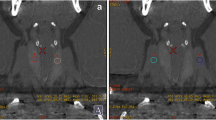
We retrospectively analyzed 40 patients with clinically diagnosed GH who underwent thyroid radionuclide (Na99mTcO4) planar imaging and tomographic imaging in our department from March 2021 to September 2021 who underwent neck ultrasound within one week in our hospital. There were 10 males and 30 females, aged (32 ± 13) years old. All patients stop antithyroid drugs for one week before scanning and did not receive iodine contrast agent and other factors that affect thyroid function within one month, with a low-iodine diet 1–2 weeks before the examination. The patient was placed in the supine position 30 min after intravenous injection of 185 MBq Na99mTcO4, and the thyroid planar imaging and SPECT/CT tomographic fusion imaging were performed. Planar imaging acquisition 500 Kcts, tomographic imaging 15 s/frame, other acquisition parameters and volume measurement methods were the same as the model. Thyroid volume measurement was estimated by 3D ultrasonography using a linear 7.5-MHz probe. During the ultrasound examination, subjects lay in a supine position with the neck hyperextended and the shoulders was supported by a pillow. The length (L1, L2), width (W1, W2) and thickness (T1, T2) of left and right thyroid lobes were measured, respectively. Thyroid volume by ultrasound was calculated using the formula (3) [18]. Using the optimal threshold determined by the model, the patient's thyroid volume was automatically delineated on SPECT/CT scan and compared with the results measured by ultrasound and planar imaging methods. Three methods of thyroid volume measurement were shown in Fig. 2.
Statistical processing
SPSS 20.0 software was used for statistical analysis, and data conforming to the normal distribution was expressed as mean ± standard deviation. ANOVA and t test were used to compare the differences in volume measurement of thyroid simulation model among different T/B and different thresholds. ANOVA, t test and Bland–Altman concordance test were used to evaluate the differences and consistency of SPECT/CT threshold automatic delineation, ultrasonography and planar imaging in measuring thyroid volume in patients with hyperthyroidism. P < 0.05 was considered to be statistically significant.
Results
Differences in volume measurement with three target-to-background ratio models by planar imaging formula method
To calculate the volume of the thyroid gland by using the planar imaging formula method, three groups were prepared according to their T/B. All three groups were higher than the real volume, which were 67.50 ± 0.26 mL, 62.98 ± 2.56 mL, 64.79 ± 2.79 mL, with an average error of 22.81%, showing no significant difference among the three groups (all P > 0.05), and the planar imaging results of different T/B models were shown in Table 1.
Differences in automatic delineation of the thyroid volumes with different thresholds and different T/B by quantitative SPECT/CT
There were no statistically significant differences in the volumes among three T/B groups with the same threshold (P > 0.05). As the threshold increased, the delineated volume decreased, showing significant differences between the groups with different thresholds (P < 0.001). 25% threshold was the closest to the real volume, with an average error of 3.73%. The results of automatic delineation of thyroid volume with different thresholds were shown in Table 2. Table 3 showed the error of automatic delineation of thyroid volume with different thresholds.
Difference and consistency of thyroid volume in GH patients measured by 25% threshold, planar imaging formula method and ultrasound method
The results of the thyroid volume of 40 patients with GH by three different methods demonstrated that automatic delineation method with 25% threshold was as accurate as the ultrasound results, showing no statistically significant differences (P > 0.05). Thyroid volume measured by planar imaging method was significantly higher than the other two methods (both P < 0.05). Compared with ultrasound, planar imaging showed an average overestimation of 25% [(39.64–31.68)/31.68]. The clinical data of 40 patients with GH and the thyroid volumes by three methods were shown in Table 4.
The correlation between SPECT/CT 25% threshold and ultrasound was well as shown in Table 5. The correlation between the SPECT/CT 25% threshold and ultrasound (r = 0.956, b = 0.961) was better than that between the planar imaging and ultrasound (r = 0.590, b = 0.574).
The correlation of volume measured by SPECT/CT 25% threshold and ultrasound was well, which was shown in Fig. 3. The Bland–Altman diagram shows that the thyroid volume measured by the 25% threshold automatic delineation method was in good agreement with the ultrasound measurement method. Bland–Altman consistency analysis was shown in Fig. 4.
Discussion
Among the common modalities for measuring thyroid volume in clinic, ultrasound, CT and MRI are three-dimensional imaging methods with difficulties in accurately reflecting the functional status of the thyroid glands. Although 124I-NaI PET/CT is an ideal imaging modality, the cost is too high [9, 10]. Planar imaging of thyroid radionuclide is simple and economical, which can simultaneously provide thyroid function status and thyroid volume. Therefore, it is a widely used thyroid mass assessment method in clinic. However, there are some factors affecting its accuracy in the measurement with planar imaging, such as thyroid size, geometry, gland thickness, and ratio of radioactive uptake between the gland and the surrounding background [7]. The SPECT/CT quantitative method can theoretically effectively compensate for these influencing factors. Nevertheless, the published studies are few, especially there is even no relevant literature about the threshold selection of relevant parameters. In this study, an self-made double-leaf butterfly-shaped container with a volume of 53 mL was placed in a NEMA IEC phantom tank to simulate the thyroid gland of patients with GH. The volume was compared with the real volume, and finally, the conclusions obtained were further verified in the clinical data. The methodology and conclusions were reasonable and reliable.
The model volume which was measured by the planar imaging formula method and the SPECT/CT automatic delineation threshold method was compared with the true value. Isselt et al [5] recommended to select a threshold value of 30% for 10–40 ml models to outline ROI, and we used 30% for 53 ml butterfly models. Our threshold delineation of the image had rougher boundaries and smaller measured volume, especially with inhomogeneous imaging agent, irregular thyroid gland or smaller T/B. It might be the special shape of our model, which also exceeded 40 ml in size, that caused the difference in results. Therefore, we chose the blue boundary of “PHAGE PHASE” as the standard manual delineation for delineating ROI (which was similar to the 20% threshold automatic delineation result, but the manual delineation boundary was smoother. We believed that manual delineation was more suitable for irregular shapes and thyroid glands with low T/B). The results showed that the planar imaging formula method significantly overestimated the thyroid volume, with an average overestimation of 22.81%, and the average error of SPECT/CT automatic delineation threshold to measure the thyroid volume was only 3.73%. The clinical data of GH patients also showed that the thyroid volume measured by the planar imaging formula was significantly higher than the thyroid volume measured by ultrasound and three-dimensional measurement of SPECT/CT. We have summarized at least 4 reasons: Firstly, the measurement of thyroid volume by ultrasound or radionuclide plane imaging is an empirical estimation formula based on regular mold (cylinder, ellipsoid). When the thyroid morphology of nodular goiter and multiple thyroid adenoma changes, the volume estimation error using the above method may increase. Secondly, regarding of the formula (1) for calculating the volume of planar imaging, K was a variable constant between 0.23 and 0.32 on the basis of specific and different instrument conditions. Allen and Goodwin [19] used a K factor of 0.128 when the frontal area of the gland was expressed in 0.25 inch squares and 0.323 when the area was expressed in square centimeters. We expressed the area in square centimeters, the K factor is fixed by 0.32 that may cause some degree of overestimation. Thirdly, there is still the influence from glandular isthmus. The larger area of the isthmus is, correspondingly the measuring volume is higher. But the isthmus is generally thinner than lobes, which may lead to overestimated results. Lastly, planar imaging was a two-dimensional imaging without depth information. Adipose tissue or tissues around the thyroid was inevitably included in the delineation of the target area, resulting in an overestimation of the frontal projection area of the 2 lobes and an overestimation of the thyroid volume.
GH is often manifested as an increased ratio of Na99mTcO4 uptake by the thyroid to the surrounding background. In this study, T/B was formulated at different of 200, 600, and 1000, and the corresponding T/B measured on planar imaging was 6, 16, and 24. There were no significant differences in the bilateral lobe area, average length and volume of the thyroid gland calculated by the planar imaging method among the three different T/B models. There was no significant difference in the thyroid volume measured by the SPECT/CT threshold automatic delineation method. It showed that there was no relation between the measurement of thyroid volume and T/B. In other words, the influence of T/B in the measurement of thyroid volume in patients with GH by radionuclide imaging was not significant.
Attenuation correction of SPECT data by low-dose CT was the basis of accurate quantification. However, compared with the increased radiation dose caused by overestimation of thyroid volume (increased dose caused from 131I), the increased radiation dose (less than 1 mSv) from low-dose CT is almost negligible. Quantitative SPECT/CT not only extraction of thyroid three-dimensional information and volume measurement but also quantification the thyroid function status, which is of great significance for the diagnosis and treatment of hyperthyroidism [13]. With this advanced processing method, the calculation of the target volume is simple and quick to achieve. Furthermore, since it is based on the threshold-based automatic segmentation, the segmentation results are only related to the distribution of radionuclides but not affected by thyroid morphology and operator. When the thyroid morphology of nodular goiter and multiple thyroid adenoma changes does not affect the volume measurement, but an underestimation of the volume may occur in the case of less hyperfunctioning nodules. It is particularly important that appropriate threshold selection was the premise of accurate measurement. Due to the existence of partial volume effect, the optimal threshold for target areas volume measurement of different sizes varies greatly. Pacilio et al. [20] obtained a Jaszczak model with a T/B of 14.4 by 99mTc perfusion showed that the optimal threshold decreased with the increase in the target volume studied. When the target volume was 19 mL, the optimal threshold was about 40%, and when the target volume was 0.5 mL, the optimal threshold was even over 90%. Another study [17] proposed that the optimal threshold for measuring thyroid volume by SPECT was 30% or 35%. In this study, different thresholds were used to measure the volume of the thyroid model, and the results showed that as the threshold increased, the measured volume decreased. In our study, the volume of the thyroid model measured by the automatic delineation of the 25% threshold is the closest to the real volume. At the same time, clinical data also show that the thyroid volume measured by the automatic delineation of the 25% threshold has a high consistency with the thyroid volume measured by ultrasound. It is consistent with the above study that the threshold changes with volume, but the optimal threshold is slightly different from them [17, 20], mainly because we selected a target volume that was more capable of simulation of the thyroid volume in GH patients. The target area was thyroid shaped, the target volume was more than 2 times Pacilio et al.’s study, and T/B was also higher [20]. Compared with the work of Pant et al. [17], we used low-dose CT to correct the SPECT data before measurement, which further restored the true distribution of intrathyroidal radionuclide. Therefore, the 25% threshold automatic delineation method of SPECT/CT can obtain more accurate thyroid volume in GH patients.
There are also some limitations in this study, such as the GH thyroid model volume setting is relatively simple and regular-shaped, which may not fully represent the real GH thyroid and the enrolled cases are limited. In further studies, we will set up a multi-group volume model to simulate GH thyroid and verify the accuracy of the 25% threshold in a larger group of GH patients.
Conclusions
In conclusion, different T/B had no significant effect on the measurement of thyroid volume in patients with hyperthyroidism; planar imaging method would significantly overestimate thyroid volume in patients with GH. 25% threshold automatic delineation method of quantitative SPECT/CT could obtain more accurate thyroid volume in patients with GH, providing accurate thyroid volume values for individualized doses of 131I therapy. While obtaining thyroid volume, quantitative SPECT/CT can also obtain quantitative parameters such as the standardized uptake value (SUV) of ROI in one stop. In the future, various models can be made to combine patient data. To study the quantitative thyroid parameters of SPECT/CT for the quantitative evaluation of different morphology and different functional states of thyroid, we can also try to quantify thyroid cancer 131I imaging, evaluate the efficacy, recurrence prediction etc., which has a broad application prospect.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GH:
-
Grave’s hyperthyroidism
- T/B:
-
Target-to-background ratios
- NEMA:
-
National Electrical Manufacturers Association
- IEC:
-
International Electrotechnical Commission
- CTAC:
-
CT-based attenuation correction
- SC:
-
Scatter correction
- RR:
-
Resolution recovery
- OSEM:
-
Ordered-subsets expectation maximization
- VOI:
-
Volume of interest
- SUV:
-
Standardized uptake value
References
Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018;7(4):167–86.
Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid: Off J Am Thyroid Assoc. 2016;26(10):1343–421.
de Rooij A, Vandenbroucke JP, Smit JW, Stokkel MP, Dekkers OM. Clinical outcomes after estimated versus calculated activity of radioiodine for the treatment of hyperthyroidism: systematic review and meta-analysis. Eur J Endocrinol. 2009;161(5):771–7.
Fujita N, Kato K, Abe S, Naganawa S. Variation in thyroid volumes due to differences in the measured length or area of the cross-sectional plane: a validation study of the ellipsoid approximation method using CT images. J Appl Clin Med Phys. 2021;22(4):15–25.
van Isselt JW, de Klerk JM, van Rijk PP, van Gils AP, Polman LJ, Kamphuis C, et al. Comparison of methods for thyroid volume estimation in patients with Graves’ disease. Eur J Nucl Med Mol Imaging. 2003;30(4):525–31.
Chen Y, Xie SP, He F, Chen J. Evaluation of the efficacy of standardized uptake value (SUV)-shape scheme for thyroid volume determination in Graves’ disease: a comparison with ultrasonography. Asia Ocean J Nucl Med Biol. 2017;5(1):44–8.
Wang H, Yu D, Tan Z, Hu R, Zhang B, Yu J. Estimation of thyroid volume from scintigraphy through 2D/3D registration of a statistical shape model. Phys Med Biol. 2019;64(9):095015.
Freesmeyer M, Wiegand S, Schierz JH, Winkens T, Licht K. Multimodal evaluation of 2-D and 3-D ultrasound, computed tomography and magnetic resonance imaging in measurements of the thyroid volume using universally applicable cross-sectional imaging software: a phantom study. Ultrasound Med Biol. 2014;40(7):1453–62.
Freesmeyer M, Kühnel C, Westphal JG. Time efficient 124I-PET volumetry in benign thyroid disorders by automatic isocontour procedures: mathematic adjustment using manual contoured measurements in low-dose CT. Ann Nucl Med. 2015;29(1):8–14.
Crawford DC, Flower MA, Pratt BE, Hill C, Zweit J, McCready VR, et al. Thyroid volume measurement in thyrotoxic patients: comparison between ultrasonography and iodine-124 positron emission tomography. Eur J Nucl Med. 1997;24(12):1470–8.
Bailey DL, Willowson KP. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med: Off Publ Soc Nucl Med. 2013;54(1):83–9.
Lee H, Kim JH, Kang YK, Moon JH, So Y, Lee WW. Quantitative single-photon emission computed tomography/computed tomography for technetium pertechnetate thyroid uptake measurement. Medicine. 2016;95(27):e4170.
Dong F, Li L, Bian Y, Li G, Han X, Li M, et al. Standardized uptake value using thyroid quantitative SPECT/CT for the diagnosis and evaluation of Graves’ disease: a prospective multicenter study. Biomed Res Int. 2019;2019:7589853.
Kim HJ, Bang JI, Kim JY, Moon JH, So Y, Lee WW. Novel application of quantitative single-photon emission computed tomography/computed tomography to predict early response to methimazole in Graves’ disease. Korean J Radiol. 2017;18(3):543–50.
Kim JY, Kim JH, Moon JH, Kim KM, Oh TJ, Lee DH, et al. Utility of quantitative parameters from single-photon emission computed tomography/computed tomography in patients with destructive thyroiditis. Korean J Radiol. 2018;19(3):470–80.
Lee R, So Y, Song YS, Lee WW. Evaluation of hot nodules of thyroid gland using Tc-99m pertechnetate: a novel approach using quantitative single-photon emission computed tomography/computed tomography. Nucl Med Mol Imaging. 2018;52(6):468–72.
Pant GS, Kumar R, Gupta AK, Sharma SK, Pandey AK. Estimation of thyroid mass in Graves’ disease by a scintigraphic method. Nucl Med Commun. 2003;24(7):743–8.
Turcios S, Lence-Anta JJ, Santana JL, Pereda CM, Velasco M, Chappe M, et al. Thyroid volume and its relation to anthropometric measures in a healthy Cuban population. Eur Thyroid J. 2015;4(1):55–61.
Allen HC Jr, Goodwin WE. The scintillation counter as an instrument for in vivo determination of thyroid weight. Radiology. 1952;58(1):68–79.
Pacilio M, Basile C, Shcherbinin S, et al. An innovative iterative thresholding algorithm for tumour segmentation and volumetric quantification on SPECT images: Monte Carlo-based methodology and validation. Med Phys. 2011;38(6):3050–61.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. The data were mainly acquired by CG, YZ and FF, and the data analysis was mainly performed by CG, FH and JH. All authors contributed to the interpretation of the data and to drafting and critically revising the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This retrospective study was approved by the Institutional Review Board of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gong, C., Zhang, Y., Feng, F. et al. The determination of the optimal threshold on measurement of thyroid volume using quantitative SPECT/CT for Graves' hyperthyroidism. EJNMMI Phys 11, 4 (2024). https://doi.org/10.1186/s40658-023-00608-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40658-023-00608-w