Abstract
Objective
To investigate the diagnostic value of diffusion kurtosis magnetic resonance imaging (DKI) and conventional diffusion-weighted imaging (DWI) for evaluating the response to first-line chemotherapy in unresectable pancreatic cancer.
Materials and methods
We retrospectively analyzed 21 patients with clinically and pathologically confirmed unresected pancreatic cancer who received palliative chemotherapy. Three-tesla MRI examinations containing DWI sequences with b values of 0, 100, 700, 1400, and 2100 s/mm2 were performed before and after chemotherapy. Parameters included the apparent diffusion coefficient (ADC), mean diffusion coefficient (MD), and mean diffusional kurtosis (MK). The performances of the DWI and DKI parameters in distinguishing the response to chemotherapy were evaluated by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. Overall survival (OS) was calculated from the date of first treatment to the date of death or the latest follow-up date.
Results
The ADCchange and MDchange were significantly higher in the responding group (PR group) than in the nonresponding group (non-PR group) (ADCchange: 0.21 ± 0.05 vs. 0.11 ± 0.09, P = 0.02; MDchange: 0.37 ± 0.24 vs. 0.10 ± 0.12, P = 0.002). No statistical significance was shown when comparing ADCpre, ADCpost, MKpre, MKpost, MKchange, MDpre, and MDpost between the PR and non-PR groups. The ROC curve analysis indicated that MDchange (AUC = 0.898, cutoff value = 0.7143) performed better than ADCchange (AUC = 0.806, cutoff value = 0.1369) in predicting the response to chemotherapy.
Conclusion
The ADCchange and MDchange demonstrated strong potential for evaluating the response to chemotherapy in unresectable pancreatic cancer. The MDchange showed higher specificity in the classification of PR and non-PR than the ADCchange. Other parameters, including ADCpre, ADCpost, MKpre, MKpost, MKchange, MDpre, and MDpost, are not suitable for response evaluation. The combined model SUMchange demonstrated superior performance compared to the individual DWI and DKI models. Further experiments are needed to evaluate the potential of DWI and DKI parameters in predicting the prognosis of patients with unresectable pancreatic cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
With the rapid development of real-time imaging technology, conventional magnetic resonance diffusion techniques have been widely used in pancreatic tissue imaging. The principle of DWI is based on the assumption that the diffusion motion of water molecules follows a normal distribution model in vivo [1]. In addition, the ADC value in the traditional DWI model is also affected by various b values [2].However, diffusion is restricted by the complex microstructure of living tissue and molecular barriers, leading to a non-Gaussian distribution [3, 4]. The DKI model, first proposed by Jensen et al., can be used to identify living tissue using the Gaussian distribution model [5].
As an extension of the diffusion tensor imaging (DTI) model, the DKI model can be used to assess the complexity of microstructures in non-Gaussian tissues [6]. DKI models typically employ high b-values and require at least 3 b-values and a diffusion-sensitive gradient field in 15 directions [7]. DKI uses the same pulse sequence as conventional DWI techniques and tends to adopt larger b values than DWI [8].
DKI technology has shown greater clinical value in tumor detection and staging than traditional DWI technology [1, 9,10,11,12].In terms of treatment response evaluation, Granata et al. found that, in locally advanced pancreatic cancer undergoing electrochemotherapy, changes in MD were statistically significant in different efficacy groups (Kruskal Wallis test, P = 0.01) [13]. These authors reported believed that the MDchange had excellent diagnostic performance for efficacy evaluation (sensitivity = 0.8, specificity = 1.0, AUC = 0.933) [13]. As is known to all, pancreatic cancer is a malignant digestive tumor with poor diagnosis [14]. For those patients who are diagnosed at advanced stage losing their opportunity to undergo radical surgery, first-line chemotherapy regimens recommended by NCCN guidelines are used to prolong the survival period and improve the life of quality [15, 16]. However, few articles have evaluated the efficacy of first-line chemotherapy among unresectable pancreatic cancer patients using DKI. The purpose of our study was to compare the application of DKI technology and traditional DWI technology in evaluating chemotherapy efficacy in patients with unresectable pancreatic cancer.
Materials and methods
Clinical data
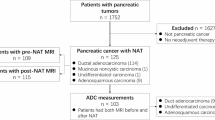
This retrospective study was approved by Fudan University Shanghai Cancer Center. We enrolled patients with pathologically confirmed PDAC by ultrasound-guided fine-needle aspiration biopsy from August 2021 to December 2022. Thirty-three patients fulfilling the following criteria were included in this study: (1) clinically diagnosed with unresectable pancreatic cancer; (2) without radiotherapy during the treatment phase; (3) three-tesla MRI with a complete DWI sequence before and after chemotherapy could be obtained; (4) the tumor size ≥ 2 cm; and (5) Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–1. Patients were excluded for incomplete standard treatment (severe adverse reaction, n = 3; cytoreductive surgery n = 1) or loss to follow-up (n = 4). According to these inclusions and exclusions, 21 patients were enrolled in this study. Detailed information about clinical characteristics is listed in Table 1.
Chemotherapy regimen
According to the recommendation of comprehensive guidelines for the diagnosis and treatment of pancreatic cancer [16], patients who underwent the following first-line chemotherapy regimens were selected: (1) gemcitabine combined with tegafur gimeracil oteracil potassium (GS): gemcitabine 1000 mg/m2 on Day 1 and Day 8, qd, intravenously; tegafur gimeracil oteracil potassium 60 to 100 mg, Day 1–15, bid, orally; every 3 weeks [17]; and (2) gemcitabine combined with nab-paclitaxel (AG): nab-paclitaxel 125 mg/m2 and gemcitabine 1000 g/m2 on Day 1, Day 8, and Day 15, qd, intravenously, every 4 weeks [18].
Magnetic resonance examination
Patients underwent scanning using a 3.0-T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) using a 16-channel phased-array volume coil as the receiving coil within 15 days before and after two courses of chemotherapy. MRI sequences included: T1-weighted breath-hold gradient-echo (repetition time (TR), 120 ms; echo time (TE), 1.4 ms; flip angle, 90°; field of view (FOV), 381 × 381 mm2; matrix, 320 × 198; number of slices, 42; thickness, 3.5 mm; acquisition time, 32 s) and T2-weighed breath-hold turbo spin‒echo (TR, 3,500 ms; TE, 83 ms; FOV, 381 × 381 mm2; matrix, 256 × 256; number of slices, 50; thickness, 4 mm; acquisition time, 3 min 15 s. The conventional DWI were obtained using a free-breathing single-shot echo-planar sequence (TR, 8,500 ms; TE, 56 ms; FOV, 381 × 309 mm2; matrix, 256 × 208; number of slices, 28; thickness, 5 mm; b values, 0, 50, 800 s/mm2, acquisition time, 2 min 40 s). The DKI sequence used the same scan parameters as the conventional DWI, except the b values (0, 100, 700, 1400, 2100 s/mm2), and acquisition time was 4 min 45 s. Big delta time of the mono-polar diffusion gradient is 32.87ms.
Response evaluation and follow-up
According to the Response Evaluation Criteria in Solid Tumor (RECIST) criteria [19], changes in the primary lesions after 2 cycles of first-line chemotherapy were assessed by an experienced abdominal tumor radiologist. All of the patients were divided into two groups: the PR group (≥ 30% decrease in the sum target lesion diameters, PR group) and the non-PR group (≤ 30% decrease in the sum target lesion diameters, non-PR group). The total number of measurable target lesions was no more than 5 per organ, and repeatable lesions were preferred. The response evaluation criteria are summarized in Table 2.
Measurement of DWI and DKI parameters
ADC values were calculated voxel-wise by fitting the DW images to a mono-exponential signal decay model [20, 21]:
ADC = ln(S0/Sb)/b,
in which Sb represents the MRI signal intensity with diffusion weighting b, S0 represents the intensity without a diffusion gradient, and ADC represents the apparent diffusion intensity.
The DKI parameters were generated voxel-wise by fitting the multi-b DWI to the diffusion kurtosis signal decay equation according to a two-variable linear least squares algorithm [1]:
S(b) = S0 × exp (−b·D + 1/6·b2D2K),
in which the D value represents the corrected diffusion coefficient, and it is different from the conventional ADC value since the D value equals the corrected ADC value in the non-Gaussian model. The K value represents the diffusion kurtosis coefficient, indicating the degree to which the molecular motion deviates from the ideal Gaussian distribution model. Conventional DWI parameters (ADC values) and DKI parameters (MD, MK) were obtained by analyzing multi-b-value DWI parameters using the Body Diffusion Toolbox postprocessing software (Siemens Healthcare GmbH, Erlangen, Germany).
Considering the tumor heterogeneity, the largest area of the tumor was manually selected on the ADC map with reference to the T2-weighted image, as in a previous study [22]. Three regions of interest (ROIs) were placed on the same slice, and the mean ADC, D, and K values were finally calculated, excluding pancreatic ducts, blood vessels, cysts and necrosis. The ROIs were drawn by consensus between two radiologists (with 5 and 10 years of clinical experience in abdominal MR imaging studies). Both radiologists were blinded to the final assessment of chemotherapy response. The corresponding DWI parameters could be obtained by the following equations: ADCchange = (ADCpost - ADCpre) /ADCpre, MKchange = (MKpre - MKpost) /MKpre; MDchange = (MDpost - MDpre) /MDpre; SUMchange = ((ADCpost+MDpost)-(ADCpre+MDpre)) / (ADCpre+MDpre). Among these values, ADCpre, MKpre, and MDpre represent the values before chemotherapy, while ADCpost, MKpost, and MDpost represent the values after two courses.
Statistical analysis
The nonparametric Shapiro-Wilk test and the independent t test were adopted to compare the DWI and DKI parameters among different efficacy groups before and after the treatment. Continuous variables are expressed as the mean ± standard deviation. All statistical analyses were performed using SPSS software (version 22.0, Chicago, IL, USA) and MedCalc software (version 17.5.5, MedCalc Software, Ostend, Belgium). Dependent variables (PR = 1,non-PR = 0) and independent variables (DWI and DKI parameters) were selected for the construction of ROC curves. P<0.05 was considered statistically significant. ROC curve analysis was used to evaluated potential variables. Internal validity was assessed by use of bootstrapping procedure. ROC analyses were further performed to evaluate the diagnostic efficacy of each parameter in predicting the chemotherapeutic response of unresectable pancreatic caner.
Results
Patient characteristics
The subjects enrolled numbered 21 patients, including 6 women and 15 men, with an average age of 64 ± 8 years old. Patients were classified into the PR group (n = 7, Fig. 1) and the non-PR group according to the treatment response (n = 14, Fig. 2).
A and E: Axial half-Fourier-acquired single-shot turbo spin echo (HASTE) T2-weighted images; B and F: Diffusion weighted images (DWI) with b-value 0 s/mm2; C and G: Apparent diffusion coefficient (ADC) maps; D and H: Diffusion kurtosis image (DKI) maps. Red arrow: the pancreatic lesion. The images in the first row represent the lesion before undergoing chemotherapy, and the images in the second row represent the lesion that underwent two courses of chemotherapy treatment. Before and after chemotherapy, the ADC and MD values increased significantly, while the MK value decreased slightly. The lesion noticeably shrank after treatment, evaluated as PR according to the RECIST criteria. This patient is a 69-year-old woman with a 4.2 × 3.6 cm mass in the tail of the pancreas and liver metastasis. The following parameters were used: ADCpre (1.06 × 10− 3 mm2/s), ADCpost (1.23 × 10− 3 mm2/s), ADCchange (0.16); MKpre (0.61), MKpost (0.60), MKchange (-0.01); MDpre (1.61 × 10− 3 mm2/s), MDpost (2.58 × 10− 3 mm2/s), MDchange (0.60)
A and E: T2-weighted images; B and F: Diffusion weighted image s(DWI) with b-value 0 s/mm2; C and G: Apparent diffusion coefficient (ADC) maps; D and H: Diffusion kurtosis imaging (DKI) maps. Images A to D represent the lesion before chemotherapy, while images E to H represent the lesion after 2 courses of chemotherapy. Red arrow: the pancreatic lesion. The ADC and MD values increased slightly, and the K value increased slightly after two courses of chemotherapy. The mass increased slightly after two courses of chemotherapy and was evaluated as non-PR. This patient is a 58-year-old male patient with a 4.8 × 3.5 cm mass in the tail of the pancreas accompanied by liver metastasis. Here are the related parameters: ADCpre=1.02 × 10− 3 mm2/s, ADCpost=1.12 × 10− 3 mm2/s, ADCchange = 0.10; MKpre=0.64, MKpost=0.65, MKchange=0.01; MDpre=1.83 × 10− 3 mm2/s, MDpost=2.07 × 10− 3 mm2/s, MDchange=0.13
Statistics of functional parameters
The ADCchange and MDchange in the PR group (0.21 ± 0.05, 0.37 ± 0.24) were significantly higher than those in the non-PR group (0.11 ± 0.09, 0.10 ± 0.12) (P = 0.02, 0.002) (Fig. 3). However, no statistically significant differences were shown between the PR group and non-PR group concerning certain aspects of ADCpre, ADCpost, MKpre, MKpost, MKchange, MDpre, and MDpost (P = 0.734, 0.09, 0.686, 0.289, 0.573, 0.153, 0.166) (Table 3). The ICCs ranged from 0.857 to 0.912 for intraobserver agreement. The AUC of DWI and DKI parameters were successfully validated by 1000 times bootstrapping. The results of ROC curve analysis showed that MDchange (AUC = 0.898) had greater diagnostic efficacy than ADCchange (AUC = 0.806) (Fig. 4). The MDchange had sensitivity of 85.7% and specificity of 85.7%, while the cutoff value was 0.1373. (Tadble 4). The combined model SUMchange showed higher AUC (0.912) than that of MDchange and ADCchange (Fig. 4).
Boxplot of apparent diffusion coefficient (ADC) and diffusion kurtosis imaging (DKI) parameter percentage change values between responders and nonresponders. A-C:ADC, MD, MK values before and after treatment between PR group and non-PR group. D-F: ADCchange, MDchange, MKchange values between PR group and non-PR group
Discussion
Currently, several studies have been reported distinguishing pancreatic ductal adenocarcinoma tissue from surrounding normal pancreatic tissue using DKI [23, 24]. In addition, many scholars have used DKI technology to evaluate the therapeutic effects on tumors in other parts. The purpose of our study was to compare the value of two diffusion techniques, DWI and DKI, in evaluating first-line chemotherapy in unresectable pancreatic cancer.
ADC values are affected by a variety of factors, including intracellular structures, cell membrane integrity, extracellular fiber composition, and more. In patients with unresectable pancreatic cancer, liquefaction, necrosis, fibrosis, loss of cellular structural integrity, and reduction of molecular barriers restricting the diffusion and movement of water molecules eventually lead to changes in corresponding diffusion parameters, providing a theoretical basis for our experimental design. Some scholars have claimed that a low ADC value before treatment often predicts poor prognosis of patients [25]. Conversely, Niwa et al. suggested that lower ADC values before chemotherapy were associated with shorter progression-free survival (PFS) after treatment of patients with resectable pancreatic cancer [26]. However, no significant differences were shown in ADCpre or ADCpost between the partial remission group (1.00 ± 0.55 × 10− 3 mm2/s, 1.21 ± 0.09 × 10− 3 mm2/s) and the nonpartial remission group (1.02 ± 0.14 × 10− 3 mm2/s, 1.13 ± 0.16 × 10− 3 mm2/s) in our study (P = 0.734, P = 0.09). This result is consistent with the findings of a previous study10. We found that the ADCchange in the PR group (0.21 ± 0.05) was significantly higher than that in the non-PR group (0.11 ± 0.09) (P = 0.02). Nishiofuku et al. also found that the ADCratio measured after four weeks of chemotherapy could predict chemotherapy sensitivity and was the most effective independent predictor of PFS (hazard ratio, 4.5; 95% confidence interval, 1.7–11.9; P = 0.002), which was not validated in our study [22].
Using the DKI model, we obtained two quantitative parameters, including the kurtosis value (K, indicating the degree of deviation from a Gaussian distribution) and diffusion coefficient (D, defined as the corrected ADC value from a non-Gaussian distribution)1. Compared with ADC, the mean kurtosis coefficient (MK) and mean diffusion coefficient (MD) could provide more information about tissue heterogeneity, vascularity and cellularity [27]. Philipp et al. suggested that the DKI-derived parameter D could be used as a noninvasive marker to assess the interstitial tissue component in pancreatic ductal adenocarcinoma, and it makes benefit for the diagnosis [23]. Among the diffusion parameters, the MD among the DKI parameters has been discovered to distinguish between among pancreatic parenchyma, peri-lesional inflammation and pancreatic tumors [24].
Currently, DKI technology has been widely used to evaluate the treatment efficacy in tumors of other parts of the body. In assessing the effect of induction chemotherapy (IC) on the treatment of locoregionally advanced nasopharyngeal carcinoma, Zhao et al. found that the mean MK (P < 0.001) values decreased dramatically, while MD (P < 0.001) significantly increased after treatment; additionally, ADCpre, MDpre and MKratio were not significantly different between the two groups [28]. In contrast to their outcomes, in our study, there were no significant differences in MKpre or MKpost between the PR group (0.67 ± 0.05, 0.58 ± 0.05) and the non-PR group (0.70 ± 0.11, 0.61 ± 0.09) (P = 0.686, P = 0.289). Some investigators have indicated that the influence of external factors on the K value is more obvious than that on the D value, such as breathing motion, noise, and artifacts1. Some studies have also shown that a decrease in the D value is not necessarily accompanied by an increase in the K value from normal tissues or tumor tissues [29]. In this trial, there was no significant difference in the alteration of the K value between the PR group and the non-PR group before and after chemotherapy. The reasons for the differences in the above conclusions could include: (1) different ROI segmentation; (2) various selections of multiple b values; 3)the diffusion time used in the single-shot EPI sequence; and 4) a variety of efficacy evaluation standards and postprocessing equipment and processing schemes.
Tumor tissue liquefaction necrosis and fibrosis were more obvious in the PR group than in the non-PR group, and the histological changes could be reflected by the ADCchange and MDchange. The MDchange in the PR group (0.37 ± 0.24) was significantly higher than that in the non-PR group (0.10 ± 0.12) (P = 0.002). The results of our study are consistent with those of Wu Rui etc [30], who claimed that the MDratio was significantly different between the PR group and the non-PR group in patients with cervical (neck) non-Hodgkin lymphoma (NHL), showing a significant, positive correlation and high agreement with the ADCratio (r = 0.776, P < 0.001). Similarly, we found that MDchange had a larger AUC (0.898 vs. 0.806) and higher specificity (0.857 vs. 0.643) than ADCchange, which can be explained by the difference between the DKI model and DWI model since MD should better reflect the motion state of free water. In addition, the SUMchange showed the highest AUC (0.912) as a combined model of DWI and DKI parameters, which indicated that the combination of DWI and DKI technique may contribute to further supplementation.
Our study has the following shortcomings. First, the sample size was relatively small, and a multicenter experiment with a large sample size is needed to confirm our conclusions. Second, we only evaluated the parameters after 2 courses, and more time points should be chosen to obtain the best time points for evaluating the treatment efficacy. Third, it may not be objective enough using treatment-response criteria to evaluate the prognostic ability. We will attempt to explore the potential relationship between DWI, DKI derived parameters and OS in the following study. Finally, we only summarized patients treated with two first-line chemotherapy regimens, which could have led to selection bias, and more treatment schemes should be included in future evaluations.
Summary
In conclusion, DWI and DKI parameters showed good diagnostic performance in differentiating PR and non-PR groups in patients with unresectable pancreatic cancer. Compared with DWI parameters, the MDchange among DKI parameters showed greater specificity in evaluating treatment response. Combined model SUMchange performed better than singe DWI and DKI model. DKI parameters could become new indicators for clinical efficacy evaluation while these results require further validation with a larger cohort.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DKI:
-
Diffusion kurtosis imaging
- DWI:
-
Diffusion weighted imaging
- ADC:
-
Apparent diffusion coefficient
- PDAC:
-
Pancreatic ductal adenocarcinoma
- ROI:
-
Region of interest
- ROC:
-
Receiver operator characteristic
- AUC:
-
Area under curve
- MD:
-
Mean diffusion
- RECIST:
-
Response evaluation criteria in solid tumours
- MK:
-
Mean kurtosis
- PR:
-
Partial response
- CR:
-
Complete response
- SD:
-
Stable disease
- PD:
-
Progressive disease
References
Jensen JH, Helpern JA. MRI quantification of non-gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698–710.
Brynolfsson P, et al. Haralick texture features from apparent diffusion coefficient (ADC) MRI images depend on imaging and pre-processing parameters. Sci Rep. 2017;7(1):4041.
Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 2010;23(7):836–48.
Christou A, et al. Accuracy of diffusion kurtosis imaging in characterization of breast lesions. Br J Radiol. 2017;90(1073):20160873.
Jensen JH, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432–40.
Poot DH, et al. Optimal experimental design for diffusion kurtosis imaging. IEEE Trans Med Imaging. 2010;29(3):819–29.
Hui ES, et al. Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. NeuroImage. 2008;42(1):122–34.
Huang Y, et al. MRI quantification of non-gaussian water diffusion in normal human kidney: a diffusional kurtosis imaging study. NMR Biomed. 2015;28(2):154–61.
Sun K, et al. Breast Cancer: Diffusion Kurtosis MR Imaging-Diagnostic accuracy and correlation with clinical-pathologic factors. Radiology. 2015;277(1):46–55.
Suo S, et al. Non-gaussian water diffusion kurtosis imaging of prostate cancer. Magn Reson Imaging. 2014;32(5):421–7.
Nogueira L, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol. 2014;24(6):1197–203.
Rosenkrantz AB, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging. 2012;30(10):1534–40.
Granata V, et al. Diffusion kurtosis imaging and conventional diffusion weighted imaging to assess electrochemotherapy response in locally advanced pancreatic cancer. Radiol Oncol. 2019;53(1):15–24.
Ke MJ, Ji LD, Li YX. Bioinformatics analysis combined with experiments to explore potential prognostic factors for pancreatic cancer. Cancer Cell Int. 2020;20:382.
Zhou L et al. YAP Inhibition by Nuciferine via AMPK-Mediated downregulation of HMGCR sensitizes pancreatic Cancer cells to Gemcitabine. Biomolecules, 2019. 9(10).
Tempero MA, Adenocarcinoma P, et al. Version 2.2017, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(8):1028–61.
Von Hoff DD, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Le Bihan D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–7.
Le Bihan D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505.
Nishiofuku H, et al. Increased tumour ADC value during chemotherapy predicts improved survival in unresectable pancreatic cancer. Eur Radiol. 2016;26(6):1835–42.
Mayer P et al. Diffusion Kurtosis Imaging-A Superior Approach to assess tumor-stroma ratio in pancreatic ductal adenocarcinoma. Cancers (Basel), 2020. 12(6).
Granata V, et al. Magnetic resonance imaging in the assessment of pancreatic cancer with quantitative parameter extraction by means of dynamic contrast-enhanced magnetic resonance imaging, diffusion kurtosis imaging and intravoxel incoherent motion diffusion-weighted imaging. Th Adv Gastroenterol. 2020;13:1756284819885052.
Chen BB, et al. Multiparametric PET/MR imaging biomarkers are associated with overall survival in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. 2018;45(7):1205–17.
Niwa T, et al. Advanced pancreatic cancer: the use of the apparent diffusion coefficient to predict response to chemotherapy. Br J Radiol. 2009;82(973):28–34.
Fujima N, et al. Prediction of the treatment outcome using intravoxel incoherent motion and diffusional kurtosis imaging in nasal or sinonasal squamous cell carcinoma patients. Eur Radiol. 2017;27(3):956–65.
Zhao DW, et al. Comparison of the pre-treatment functional MRI metrics’ efficacy in predicting Locoregionally advanced nasopharyngeal carcinoma response to induction chemotherapy. Cancer Imaging. 2021;21(1):59.
Wang P, et al. A study on diffusion and kurtosis features of cervical cancer based on non-gaussian diffusion weighted model. Magn Reson Imaging. 2018;47:60–6.
Wu R, et al. Assessment of chemotherapy response in non-hodgkin lymphoma involving the neck utilizing diffusion kurtosis imaging: a preliminary study. Diagn Interv Radiol. 2017;23(3):245–9.
Acknowledgements
On the completion of my thesis, I should like to express my deepest gratitude to all those whose suggestions and kindness have made this work possible. First and foremost, I’m greatly indebted to my Ph.D supervisor Zhengrong Zhou and who gave me valuable instructions, and urged me to begin my research work as soon as possible. His effective suggestions, shred comments and quick corrections have kept the thesis in the right direction. Additionally, I’m grateful to Ph.D Yuqin Zhang and Dr. Lei Chen and other team members who gave me a hand whenever i need.
Funding
Shanghai Minhang District Health Commission (2023MW28).
Author information
Authors and Affiliations
Contributions
1.Zehua Zhang: data curation, formal analysis, software, writing-original draft, funding acquisition.
2.Yuqin Zhang: data curation, formal analysis, software, writing-original draft.
3.Feixiang Hu: data curation, formal analysis.
4.Tiansong Xie: formal analysis, software.
5.Wei Liu: formal analysis, software.
6.Huijing Xiang: data curation, software.
7.Xiangxiang Li: data curation, software.
8.Lei Chen: investigation, writing-review&editing, funding acquisition.
9.Zhengrong Zhou: conceptualization, investigation, supervision, writing-review&editing.
Corresponding authors
Ethics declarations
Ethical approval
With approval from the institutional ethics committee of our hospital, the requirement for informed consent of this retrospective research was waived.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Z., Zhang, Y., Hu, F. et al. Value of diffusion kurtosis MR imaging and conventional diffusion weighed imaging for evaluating response to first-line chemotherapy in unresectable pancreatic cancer. Cancer Imaging 24, 29 (2024). https://doi.org/10.1186/s40644-024-00674-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-024-00674-y