Abstract
Background
The European Resuscitation Council 2021 guidelines for haemodynamic monitoring and management during post-resuscitation care from cardiac arrest call for an individualised approach to therapeutic interventions. Combining the cardiac function and venous return curves with the inclusion of the mean systemic filling pressure enables a physiological illustration of intravascular volume, vasoconstriction and inotropy. An analogue mean systemic filling pressure (Pmsa) may be calculated once cardiac output, mean arterial and central venous pressure are known. The NEUROPROTECT trial compared targeting a mean arterial pressure of 65 mmHg (standard) versus an early goal directed haemodynamic optimisation targeting 85 mmHg (high) in ICU for 36 h after cardiac arrest. The trial data were used in this study to calculate post hoc Pmsa and its expanded variables to comprehensively describe venous return physiology during post-cardiac arrest management. A general estimating equation model was used to analyse continuous variables split by standard and high mean arterial pressure groups.
Results
Data from 52 patients in each group were analysed. The driving pressure for venous return, and thus cardiac output, was higher in the high MAP group (p < 0.001) along with a numerically increased estimated stressed intravascular volume (mean difference 0.27 [− 0.014–0.55] L, p = 0.06). The heart efficiency was comparable (p = 0.43) in both the standard and high MAP target groups, suggesting that inotropy was similar despite increased arterial load in the high MAP group (p = 0.01). The efficiency of fluid boluses to increase cardiac output was increased in the higher MAP compared to standard MAP group (mean difference 0.26 [0.08–0.43] fraction units, p = 0.01).
Conclusions
Calculation of the analogue mean systemic filling pressure and expanded variables using haemodynamic data from the NEUROPROTECT trial demonstrated an increased venous return, and thus cardiac output, as well as increased volume responsiveness associated with targeting a higher MAP. Further studies of the analogue mean systemic filling pressure and its derived variables are warranted to individualise post-resuscitation care and evaluate any clinical benefit associated with this monitoring approach.
Similar content being viewed by others
Background
The European Resuscitation Council 2021 guidelines for haemodynamic monitoring and management during post-resuscitation care from cardiac arrest state that perfusion should be maintained with fluids, noradrenaline and/or dobutamine depending on individual patient needs for intravascular volume, vasoconstriction or inotropy [1]. Multiple concurrent factors affect systemic perfusion in the post-cardiac arrest setting, emphasising the need for an adequate monitoring approach to guide therapy in individual patients. The venous return physiology proposed by Guyton [2] provides a physiologically robust framework to evaluate the principal components of cardiac performance, combining the venous return and cardiac function curves [3]. The pressure gradient between the mean systemic filling pressure and the right atrial pressure represents the driving pressure for venous return, and thus determines the cardiac output [4]. The mean systemic filling pressure is the result of the elastic recoil pressure of the intravascular volume during no-flow conditions, or more precisely the stressed intravascular volume divided by the averaged systemic vascular compliance [5, 6]. Several methods exist to estimate the mean systemic filling pressure in the clinical setting [7]. The mathematical model developed by Parkin and Leaning to calculate an analogue mean systemic filling pressure (Pmsa) [8] has been experimentally validated [9,10,11] and is best aligned with measurements in humans [12,13,14]. Haemodynamic monitoring in post-cardiac arrest patients often include mean arterial pressure (MAP), central venous pressure (CVP) and cardiac output (CO) making the calculation of Pmsa feasible. Once Pmsa is known, several variables to evaluate venous return, cardiac function and the intravascular volume state may be derived. The application of measurable venous return physiology to evaluate haemodynamic post-resuscitation management has been very sparsely reported in the literature, with only one recent experimental study published [15]. In contrast, several studies of venous return physiology based on Pmsa have been reported in postoperative cardiac patients [16,17,18,19,20,21], during anaesthesia [22,23,24] and intensive care [25,26,27], and in a review of the passive leg raising test to assess volume responsiveness [28].
This exploratory study utilised data previously collected in a randomised study of goal-directed haemodynamic optimisation compared to standard care (the NEUROPROTECT trial [29, 30]) in post-cardiac arrest patients, applying Pmsa and its derived variables to illustrate the venous return physiology. The aim was to use these variables to comprehensively describe the effects of volume administration, vasopressor and inotropic support in two different strategies of post-resuscitation haemodynamic management.
Methods
The original data were captured in the randomised NEUROPROTECT clinical trial (NCT02541591). The protocol [30], cardiac arrest characteristics and primary outcomes [29] have been published earlier and this study is an exploratory, post hoc analysis of venous return physiology centred on Pmsa in the haemodynamic management of comatose survivors of cardiac arrest. The study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [31] (see Additional file 1).
The details of patient characteristics, the primary outcome of the extent of anoxic brain damage on diffusion-weighted magnetic resonance imaging, and the secondary outcomes of favourable neurological status at ICU discharge and at 180 days have been published [29]. In summary, comatose survivors of out-of-hospital cardiac arrest of a presumed cardiac cause were randomised to early goal directed haemodynamic optimisation (EGDHO) targeting a mean arterial pressure (MAP) of 85–100 mmHg and a mixed venous oxygen saturation (SvO2) of 65–75%, or to receive standard care with MAP maintained at 65 mmHg (MAP65) from admission to ICU and over the next 36 h. In the EGDHO group, fluids up to 3 L/24 h were administered as guided by stroke volume variation or a passive leg raising manoeuvre with blood transfusions used to maintain haemoglobin > 100 g/L, and infusions of dobutamine and/or norepinephrine added if necessary to achieve the haemodynamic targets. In the MAP65 group, haemodynamic support was provided at the discretion of the clinical team. Standard care in both groups included sedation (propofol, remifentanil), mechanical ventilation and targeted hypothermia at 33 °C for the first 24 h followed by rewarming at 0.3 °C/h until 36 °C.
Venous return physiological variables
The application of venous return physiological variables to interpret the haemodynamic status of patients admitted to ICU has been detailed in previous reports, for example [17, 25, 28], and the concepts are illustrated in the Additional File 2. Pmsa was calculated according to Parkin and Leaning [8]:
where the constant c incorporates the venoarterial compliance ratio and estimated venous resistance adjusted for age, height and weight [32]. The CO must equal the venous return (VR) to the heart which is regulated by the pressure gradient for venous return (VRdP) between Pmsa and the right atrial pressure, represented by CVP. Together with the resistance to venous return (RVR), the CO is determined by
The resistance to venous return (RVR) is hence calculated as the VRdP over CO. The pumping action of the heart maintains the VRdP, and the efficiency of the heart (Eh) may thus be calculated as [8, 33]
where a value ~ 1 reflects a normal heart function with CVP close to 0 and a value of 0 is seen in circulatory standstill when CVP and Pmsa are equilibrated. In addition to the static Eh, the degree to which a volume bolus changes the pumping capacity of the heart is defined by the ratio of the change in driving pressure for VR to the change in Pmsa, providing a dynamic measure of volume efficiency (Evol) [16, 17, 19]:
An integrative measure of hydraulic pumping ability of the heart is represented by the product of MAP and CO, referred to as cardiac power, which correlates with clinical outcomes in cardiac patients [34,35,36]. The cardiac power (CP) was scaled to the volume state represented by Pmsa as [16, 17, 19]:
and further analysed as a dynamic variable following a fluid bolus by the change in CP over the change in Pmsa, referred to as cardiac power efficiency (ECP) similar to Eh and Evol. In addition preload and pump function variables, it is important to consider the external opposition to ventricular ejection represented by the arterial load. An integrative measure of cardiac afterload that includes steady and pulsatile components is given by the effective arterial elastance (Ea) calculated as [37, 38]
Finally, the stressed volume was derived from extrapolating the slope of the line connecting Pmsa before and after a fluid bolus of 500 mL to the x-axis intercept corresponding to a Pmsa = 0 [17, 20], with the assumption that the administered volume remained in the intravascular compartment from the start of infusion to the next measurement (30–40 min).
The potential confounding introduced by mechanical circulatory support on the equations for venous return physiology is unclear, and these patients were excluded in the analyses.
Statistical analyses
Data are reported as means ± standard deviation or medians and interquartile ranges [IQR, 25th–75th percentile] as appropriate for variables with normal or non-normal distributions as judged by inspection of the Q–Q plots, Levene’s test of equality of variances, and the Shapiro–Wilk normality test. Categorical data are reported as counts and percentages. Longitudinal haemodynamic data were analysed using a generalised estimating equations (GEE) model that included factors for time, treatment and their interaction. The model included an exchangeable working correlation matrix to account for within-patient correlations. A Gaussian distribution with log-link function was used for all variables, except for Eh, Evol, ECP and Ea that were analysed using a gamma distribution since these variables were positive only and skewed to the right. Longitudinal haemodynamic data are reported as the marginal means and their 95% confidence intervals. The Wald test was applied to the robust standard errors in the GEE models and the robust z value reported as a p value from a normal distribution of the test statistic. In addition to the GEE model, data for the first 6 h were compared to the last 6 h using the Welch two sample t test with changes reported as mean difference including the 95% confidence interval. Correlations were assessed by Spearman’s correlation coefficient, rho, including the 95% confidence interval. Physiologically impossible data were deleted and similar to any missing data imputed using multiple imputation by chained equations with ten iterations, with the results from the calculated variables in each iteration pooled. Statistical analyses were performed using the R statistical software (v.4.0.3, R Foundation for Statistical Computing, Vienna, Austria) in RStudio using the ‘dplyr’, ‘mice’, ‘geepack’, ‘emmeans’ and ‘ggplot2’ packages. Statistical significance was set at a two-sided p < 0.05.
Results
A total of 104 patients were included in this post hoc study with 52 patients in the EGDHO group and 52 patients in the MAP65 group. Patient and characteristics are presented in Table 1. One patient in each group was supported with intra-aortic balloon counterpulsation and excluded in the analyses. There were no differences in the fluid boluses administered with 28 patients given 54 boluses in MAP65 group, and 21 patients given 46 boluses in the EGDHO group. A similar and small number of patients received blood transfusions. Patients in the EGDHO group received more noradrenaline and dobutamine, but less propofol and with no difference in the dose of remifentanil (Table 1).
The MAP was consistently higher in EGDHO compared to MAP65 (p < 0.01, Fig. 1A). There was no significant difference in CVP between groups (p = 0.77, Fig. 1B), while CVP decreased over time in both groups (mean difference 1.4 [0.4–2.3] mmHg, p = 0.004, in MAP65 and mean difference 1.9 [1.1–2.7] mmHg, p < 0.001, in EGDHO). The CO was higher in the EGDHO group (p = 0.02, Fig. 1C) with an increase over time in both groups (mean difference 0.60 [0.28–0.92] L/min, p < 0.001, in MAP65 and mean difference 1.13 [0.82–1.45] L/min, p < 0.001, in EGDHO).
Pmsa was not different between groups (p = 0.76) but decreased over time (mean difference 1.88 [1.11–2.66] mmHg, p = 0.005, in MAP65 and mean difference 1.30 [0.38–2.21] mmHg, p < 0.001, in EGDHO) (Fig. 2A). The VRdP was higher in the EGHDO group (p < 0.001), consistent with the higher CO, with no significant changes over time (mean difference 0.02 [− 0.14–0.11] mmHg, p = 0.80, in MAP65 and mean difference 0.00 [− 0.11–0.12] mmHg, p = 0.90, in EGDHO) (Fig. 2B). Eh was not different between groups (p = 0.43) and did not change over time (mean difference 0.03 [− 0.01–0.06] mmHg, p = 0.10, in MAP65 and mean difference 0.02 [− 0.02–0.05] mmHg, p = 0.30, in EGDHO) (Fig. 2C). An increased Evol (mean difference 0.26 [0.08–0.43], p = 0.01) for all bolus episodes was observed in the EGDHO group (0.42 [0.23–1.0]) compared to the MAP65 group (0.11 [0.02–0.89]).
Changes in analogue mean systemic filling pressure and derived variables. Values are shown for the study period of 36 h from admission to ICU as marginal means and the 95% confidence intervals. A Pmsa, analogue mean systemic filling pressure; B VRdP, driving pressure for venous return; C Eh, heart efficiency; D CP, cardiac power indexed by Pmsa; E Ea, effective arterial elastance; F RVR, resistance to venous return
The CP was greater in the EGDHO group (p < 0.001) with an increase over time observed in the EGDHO group (mean difference 0.012 [0.004–0.024) W/mmHg, p = 0.04] as well as in the MAP65 group (mean difference 0.015 [0.008–0.021 W/mmHg, p < 0.001) (Fig. 2D). ECP was greater in the EGDHO group for all fluid boluses (0.17 [0.10–0.39]) compared to MAPG65 (0.08 [0.01–0.17]), p = 0.04.
Ea was greater in the EGDHO group (p = 0.01) with a decrease over time only observed in the MAP65 group (mean difference 0.43 [0.11–0.75] mmHg/mL, p = 0.01) (Fig. 2E). Ea decreased after fluid bolus administration in all patients (mean difference 0.34 [0.23–0.45] mmHg/mL, p < 0.001) but with no significant difference between the treatment groups. Ea correlated with the RVR (0.81 [0.80–0.83], p < 0.001) and SVR (0.81 [0.80–0.83], p < 0.001) in all patients. The RVR was higher in the EGDHO group (p < 0.01) and decreased over time in both EGDHO (0.18 [0.09–0.28] mmHg/min/L, p < 0.001) and MAP65 (0.26 [0.16–0.36] mmHg/min/L, p < 0.001) groups (Fig. 2F).
The stressed volume estimated from all bolus episodes during the study period was numerically greater in the EGDHO group (1.75 [1.43–2.04] L) compared to the MAP65 group (1.43 [0.72–1.82] L), although this difference did not attain statistical significance (mean difference 0.27 [− 0.014–0.55], p = 0.06).
Discussion
The main findings of this explorative post-hoc study of the NEUROPROTECT haemodynamic data were: (1) the analogue mean systemic filling pressure reflecting the intravascular volume state was within a normal range, while decreasing over the study period up to 36 h after cardiac arrest; (2) the driving pressure for venous return, and thus cardiac output, was higher in the group targeting a higher MAP post-resuscitation along with an increased estimated stressed intravascular volume; (3) the heart efficiency was comparable in both the standard and high MAP target groups suggesting that inotropy was similar; (4) the cardiac power was higher in the group targeting a higher MAP as a result of maintaining both higher MAP and cardiac output; (5) the efficiency of fluid boluses to increase cardiac output and power was increased in the higher MAP compared to standard MAP group; and (6) both the arterial load and the resistance to venous return were increased in the higher MAP group while still sustaining an increased cardiac output.
Pmsa over the first few hours following admission to ICU was similar to Pmsa reported in postoperative cardiac patients [17] and patients with cardiogenic acute circulatory failure [25]. A decrease in Pmsa was subsequently observed that is likely to reflect the receding vasoconstrictive effects of acute resuscitation drugs including adrenaline and vasodilation from the institution of sedation in ICU. An absolute reduction in intravascular volume seems less likely given the similar incidence of fluid boluses in both groups. While Pmsa was not statistically significantly different between the two groups, it was numerically increased in the EGDHO with a less pronounced decrease over time.
A central finding of this study is the increased VRdP observed in the EGDHO group that sustained a greater cardiac output. This difference in VRdP was the combined result of the Pmsa trending higher and the CVP trending lower in the EGDHO group. A composite variable like VRdP contributes more information on the volume and compliance state of the cardiovascular system than the isolated values of Pmsa or CVP. The static nature of those latter variables limits any inference to vascular volume. This is often reported for CVP [39] but applies to Pmsa as well, while this acknowledgement should not be construed to dismiss their combined validity [33]. The VRdP around 3 mmHg in both groups was at the lower end of the normal range of 3–8 mmHg reported in humans [12, 40, 41], and corresponded to a cardiac output less than 4 L/min for most of the duration of the study. An increased VRdP in the EGDHO group is also supported by the increased, although statistically not significantly different, stressed volume in this group compared to MAP65. The estimated 1.7 L in the EGDHO group is similar to previous reports during hypothermic circulatory arrest [42] and in postoperative cardiac patients [17, 20]. It should be noted that the stressed volume was estimated by extrapolation of Pmsa changes before and after the administration of a fluid bolus. The number of bolus episodes were limited and dispersed over time. Thus caution is warranted when interpreting simple relationships between stressed volume estimates and Pmsa or VRdP. Supporting a greater stressed volume and VRdP in the EGDHO group overall is the corresponding increase in Evol, meaning that the 500 ml fluid boluses when given in this group more effectively increased VRdP and hence cardiac output. The increased dosing of noradrenaline in the EGDHO group plausibly mobilised volume from the unstressed compartment of predominantly large splanchnic veins [43] to the stressed portion of the intravascular volume, by reducing compliance in these capacitance vessels.
Eh was not significantly different between the two groups and hence the increased cardiac output in the EGDHO group would not appear to be related to an increased pump efficiency of the heart. Targeting a higher MAP could in theory have improved the coronary perfusion pressure with additional inotropic support from dobutamine, although low doses were used. The increased CP in the EGDHO group was proportionally driven more by the higher MAP than the CO, while the increased ECP reflected the increased cardiac output response to a volume bolus. The three efficiency variables are useful as they provide a scalar, continuous measure within the interval 0 to 1, as opposed to the frequently used dichotomous description of patients being ‘responsive’ or ‘non-responsive’ to haemodynamic interventions [19, 25]. Viewing Eh, Evol and ECP together, the main effects of the EGDHO interventions were an increased responsiveness of cardiac output to the volume state.
The arterial load was increased in the EGDHO group as indicated by Ea and RVR variables, while no adverse cardiac effects from high MAP were reported in the original study [29] or in a subsequent meta-analysis [44]. The increased Ea is not unexpected given a higher MAP target in the EGDHO and it still represents a relatively schematic measurement of a complex cardiovascular phenomenon [37]. The greater RVR in the EGDHO group targeting a higher MAP was associated with vasoconstriction by the increased dose of noradrenaline and reduced vasodilation associated with the decreased dose of propofol. The decreased dose of propofol in the EGDHO group may also have facilitated reaching the target variables by reducing the effects of decreased cardiac output, myocardial depression and venodilation as reflected by increased VRdP in this group. The correlation between Ea and RVR arguably incorporates a degree of mathematical coupling from the inclusion of cardiac output or stroke volume in both variables. The arterial load represented by Ea is different from the average resistance to a blood corpuscular element represented by RVR.
This study has some important strengths and limitations. The detailed haemodynamic evaluation using venous return physiology adds novel insights into post-resuscitation management. The individualised approach to fluids, vasopressors and inotropes called for in the ERC 2021 guidelines [1] may be facilitated by the comprehensive set of continuous variables reported in this study, allowing an analysis of the main cardiovascular domains relating to intravascular volume, vascular resistance and heart pump function. The consecutive and granular data over 36 h of post-resuscitation management allowed cardiovascular dynamics to be assessed in detail. The study is limited by its post-hoc nature with potential residual confounding and the results should be viewed as exploratory and hypothesis generating. The precision of cardiac output monitoring in the MAP65 (Vigileo, Edwards Life Sciences) and the EGDHO (pulmonary artery catheter thermodilution) groups may vary but is unlikely to have had a major impact given the scaling of CO in the Pmsa equation. Two tertiary-level hospitals contributed data to the original study, but the cohort is relatively small and its external validity may be challenged. Most data were obtained during hypothermia that may affect vascular tone and compliance and observations might be different in normothermic conditions.
In conclusion, applying venous return physiology to the analysis of post-resuscitation haemodynamic data in the NEUROPROTECT trial demonstrated an increased venous return and volume responsiveness associated with targeting a higher MAP improved systemic perfusion. Further studies of the analogue mean systemic filling pressure and its derived variables are warranted to individualise post-resuscitation care and evaluate any clinical benefit associated with this monitoring approach.
Availability of data and materials
The data analysed for this post hoc analysis are not publicly available but are available from Dr. K Ameloot on reasonable request and pending approval by relevant ethics committees.
Abbreviations
- CO:
-
Cardiac output
- CVP:
-
Central venous pressure
- CP:
-
Cardiac power
- E a :
-
Effective arterial elastance
- E CP :
-
Cardiac power efficiency
- EGDHO:
-
Early goal directed haemodynamic optimisation targeting MAP 85 mmHg
- E h :
-
Heart efficiency
- E vol :
-
Volume efficiency
- MAP:
-
Mean arterial pressure
- MAP65:
-
Standard haemodynamic therapy targeting MAP 65 mmHg
- P msa :
-
Analogue mean systemic filling pressure
- RVR:
-
Resistance to venous return
- VRdP:
-
Venous return pressure gradient
References
Nolan JP, Sandroni C, Bottiger BW, Cariou A, Cronberg T, Friberg H, Genbrugge C, Haywood K, Lilja G, Moulaert VRM, Nikolaou N, Mariero Olasveengen T, Skrifvars MB, Taccone F, Soar J (2021) European Resuscitation Council and European Society of intensive care medicine guidelines 2021: post-resuscitation care. Resuscitation 161:220–269
Guyton AC (1968) Regulation of cardiac output. Anesthesiology 29:314–326
Guyton AC (1955) Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev 35:123–129
Guyton AC, Lindsey AW, Abernathy B, Richardson T (1957) Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol 189:609–615
Henderson WR, Griesdale DE, Walley KR, Sheel AW (2010) Clinical review: guyton–the role of mean circulatory filling pressure and right atrial pressure in controlling cardiac output. Crit Care 14:243
Guyton AC, Polizo D, Armstrong GG (1954) Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am J Physiol 179:261–267
Wijnberge M, Sindhunata DP, Pinsky MR, Vlaar AP, Ouweneel E, Jansen JR, Veelo DP, Geerts BF (2018) Estimating mean circulatory filling pressure in clinical practice: a systematic review comparing three bedside methods in the critically ill. Ann Intensive Care 8:73
Parkin WG, Leaning MS (2008) Therapeutic control of the circulation. J Clin Monit Comput 22:391–400
Werner-Moller P, Heinisch PP, Hana A, Bachmann KF, Sondergaard S, Jakob SM, Takala J, Berger D (1985) (2022) Experimental validation of a mean systemic pressure analog against zero-flow measurements in porcine VA-ECMO. J Appl Physiol 132:726–736
Werner-Moller P, Sondergaard S, Jakob SM, Takala J, Berger D (1985) (2019) Effect of volume status on the estimation of mean systemic filling pressure. J Appl Physiol 126:1503–1513
Moller PW, Winkler B, Hurni S, Heinisch PP, Bloch A, Sondergaard S, Jakob SM, Takala J, Berger D (2017) Right atrial pressure and venous return during cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 313:H408–H420
Jellinek H, Krenn H, Oczenski W, Veit F, Schwarz S, Fitzgerald RD (1985) (2000) Influence of positive airway pressure on the pressure gradient for venous return in humans. J Appl Physiol 88:926–932
Repesse X, Charron C, Fink J, Beauchet A, Deleu F, Slama M, Belliard G, Vieillard-Baron A (2015) Value and determinants of the mean systemic filling pressure in critically ill patients. Am J Physiol Heart Circ Physiol 309:H1003-1007
Wijnberge M, Schuurmans J, de Wilde RBP, Kerstens MK, Vlaar AP, Hollmann MW, Veelo DP, Pinsky MR, Jansen JRC, Geerts BF (2020) Defining human mean circulatory filling pressure in the intensive care unit. J Appl Physiol (1985) 129:311–316
Chalkias A, Laou E, Papagiannakis N, Varvarousi G, Ragias D, Koutsovasilis A, Makris D, Varvarousis D, Iacovidou N, Pantazopoulos I, Xanthos T (2022) Determinants of venous return in steady-state physiology and asphyxia-induced circulatory shock and arrest: an experimental study. Intensive Care Med Exp 10:13
Gupta K, Sondergaard S, Parkin G, Leaning M, Aneman A (2015) Applying mean systemic filling pressure to assess the response to fluid boluses in cardiac post-surgical patients. Intensive Care Med 41:265–272
Schulz L, Geri G, Vieillard-Baron A, Vignon P, Parkin G, Aneman A (2021) Volume status and volume responsiveness in postoperative cardiac surgical patients: an observational, multicentre cohort study. Acta Anaesthesiol Scand 65:320–328
Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, Rhodes A (2013) Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med 39:1299–1305
Sondergaard S, Larsson JS, Moller PW (2019) The haemodynamic effects of crystalloid and colloid volume resuscitation on primary, derived and efficiency variables in post-CABG patients. Intensive Care Med Exp 7:13
Maas JJ, Geerts BF, van den Berg PC, Pinsky MR, Jansen JR (2009) Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med 37:912–918
Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR (2013) Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med 41:143–150
Hahn R, He R, Li Y (2022) Mean systemic filling pressure indicates fluid responsiveness and anaesthesia-induced unstressed blood volume. Anaesthesiol Intensive Ther 54:369–377
Zucker M, Kagan G, Adi N, Ronel I, Matot I, Zac L, Goren O (2022) Changes in mean systemic filling pressure as an estimate of hemodynamic response to anesthesia induction using propofol. BMC Anesthesiol 22:234
Vos JJ, Kalmar AF, Hendriks HGD, Bakker J, Scheeren TWL (2018) The effect of fluid resuscitation on the effective circulating volume in patients undergoing liver surgery: a post-hoc analysis of a randomized controlled trial. J Clin Monit Comput 32:73–80
Aneman A, Schulz L, Prat G, Slama M, Vignon P, Vieillard-Baron A (2023) Volume responsiveness revisited: an observational multicenter study of continuous versus binary outcomes combining echocardiography and venous return physiology. Am J Physiol Heart Circ Physiol 325:H1069–H1080
Persichini R, Lai C, Teboul JL, Adda I, Guerin L, Monnet X (2022) Venous return and mean systemic filling pressure: physiology and clinical applications. Crit Care 26:150
Persichini R, Silva S, Teboul JL, Jozwiak M, Chemla D, Richard C, Monnet X (2012) Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 40:3146–3153
Cooke K, Sharvill R, Sondergaard S, Aneman A (2018) Volume responsiveness assessed by passive leg raising and a fluid challenge: a critical review focused on mean systemic filling pressure. Anaesthesia 73:313–322
Ameloot K, De Deyne C, Eertmans W, Ferdinande B, Dupont M, Palmers PJ, Petit T, Nuyens P, Maeremans J, Vundelinckx J, Vanhaverbeke M, Belmans A, Peeters R, Demaerel P, Lemmens R, Dens J, Janssens S (2019) Early goal-directed haemodynamic optimization of cerebral oxygenation in comatose survivors after cardiac arrest: the Neuroprotect post-cardiac arrest trial. Eur Heart J 40:1804–1814
Ameloot K, De Deyne C, Ferdinande B, Dupont M, Palmers PJ, Petit T, Eertmans W, Moonen C, Belmans A, Lemmens R, Dens J, Janssens S (2017) Mean arterial pressure of 65 mm Hg versus 85–100 mm Hg in comatose survivors after cardiac arrest: rationale and study design of the Neuroprotect post-cardiac arrest trial. Am Heart J 191:91–98
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349
Moller PW, Parkin WG (2022) Correct calculation of the mean systemic pressure analogue. Intensive Care Med 48:1679–1680
Sondergaard S, Parkin G, Aneman A (2016) Central venous pressure: soon an outcome-associated matter. Curr Opin Anaesthesiol 29:179–185
Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G, Investigators S (2004) Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 44:340–348
Mendoza DD, Cooper HA, Panza JA (2007) Cardiac power output predicts mortality across a broad spectrum of patients with acute cardiac disease. Am Heart J 153:366–370
Popovic B, Fay R, Cravoisy-Popovic A, Levy B (2014) Cardiac power index, mean arterial pressure, and Simplified Acute Physiology Score II are strong predictors of survival and response to revascularization in cardiogenic shock. Shock 42:22–26
Jozwiak M, Millasseau S, Richard C, Monnet X, Mercado P, Depret F, Alphonsine JE, Teboul JL, Chemla D (2019) Validation and critical evaluation of the effective arterial elastance in critically ill patients. Crit Care Med 47:e317–e324
Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA (1992) Effective arterial elastance as index of arterial vascular load in humans. Circulation 86:513–521
Marik PE, Baram M, Vahid B (2008) Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 134:172–178
Nanas S, Magder S (1992) Adaptations of the peripheral circulation to PEEP. Am Rev Respir Dis 146:688–693
Magder S (2016) Volume and its relationship to cardiac output and venous return. Crit Care 20:271
Magder S, De Varennes B (1998) Clinical death and the measurement of stressed vascular volume. Crit Care Med 26:1061–1064
Greenway CV (1983) Role of splanchnic venous system in overall cardiovascular homeostasis. Fed Proc 42:1678–1684
Niemela V, Siddiqui F, Ameloot K, Reinikainen M, Grand J, Hastbacka J, Hassager C, Kjaergaard J, Aneman A, Tiainen M, Nielsen N, Harboe Olsen M, Jorgensen CK, Juul Petersen J, Dankiewicz J, Saxena M, Jakobsen JC, Skrifvars MB (2023) Higher versus lower blood pressure targets after cardiac arrest: systematic review with individual patient data meta-analysis. Resuscitation 189:109862
Acknowledgements
Not applicable.
Funding
The original trial data collection was funded by a non-commercial TBM grant from the Flemish government (IWT Flanders, Belgium).
Author information
Authors and Affiliations
Contributions
AA: concept, data analysis, manuscript draft. KA: original trial conduct and data collection, critical revision of the manuscript draft. MS: critical revision of the manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The data used in this study were collected after ethics approval at the participating centres UZ Leuven and ZOL-Genk (s58017). Written informed consent was obtained from a next of kin or, if unavailable, a procedure for inclusion in emergency situations was applied. A definitive post hoc consent was ultimately obtained from patients who recovered sufficiently to make independent decisions.
Consent for publication
Not applicable.
Competing interests
Markus Skrifvars has received speaker’s fees from BARD Medical (Ireland). The other authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
40635_2024_657_MOESM2_ESM.pdf
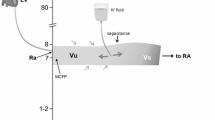
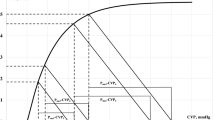
Additional file 2. Illustration of haemodynamic physiology concepts. A Cardiac output is determined by the intersection of the venous return and cardiac function curves, indicated in the graph for the positions pre and post the administration of a volume bolus. The corresponding change in venous return pressure gradient (VRdP) over the change in the analogue mean systemic filling pressure (Pmsa) describes the efficiency of the volume bolus to increase cardiac output (Evol). B Volume pressure curve of the left ventricle indicating the slope of the effective arterial elastance given by the end-systolic blood pressure (ESP) over the stroke volume (SV). C Analogue mean systemic filling pressure before (Pre Pmsa) administration of a known quantity of volume (500 mL) and the resulting increase in filling pressure (Post Pmsa). The line connecting these two observations was extrapolated to a Pmsa of zero, indicating the stressed volume, i.e., the volume that starts to generate an elastic recoil pressure within the vasculature
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aneman, A., Skrifvars, M.B. & Ameloot, K. Venous return physiology applied to post-cardiac arrest haemodynamic management: a post hoc analysis of the NEUROPROTECT trial. ICMx 12, 70 (2024). https://doi.org/10.1186/s40635-024-00657-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-024-00657-0