Abstract
There is a need to monitor tidal volume in critically ill patients with acute respiratory failure, given its relation with adverse clinical outcome. However, quantification of tidal volume in non-intubated patients is challenging. In this proof-of-concept study, we evaluated whether ultrasound measurements of diaphragm excursion could be a valid surrogate for tidal volume in patients with respiratory failure. Diaphragm excursions and tidal volumes were simultaneously measured in invasively ventilated patients (N = 21) and healthy volunteers (N = 20). Linear mixed models were used to estimate the ratio between tidal volume and diaphragm excursion. The tidal volume–diaphragm excursion ratio was 201 mL/cm in ICU patients [95% confidence interval (CI) 161–240 mL/cm], and 361 (294–428) mL/cm in healthy volunteers. An excellent association was shown within participants (R2 = 0.96 in ICU patients, R2 = 0.90 in healthy volunteers). However, the differences between observed tidal volume and tidal volume as predicted by the linear mixed models were considerable: the 95% limits of agreement in Bland–Altman plots were ± 91 mL in ICU patients and ± 396 mL in healthy volunteers. Likewise, the variability in tidal volume estimation between participants was large. This study shows that diaphragm excursions measured with ultrasound correlate with tidal volume, yet quantification of absolute tidal volume from diaphragm excursion is unreliable.
Similar content being viewed by others
Background
High respiratory effort and tidal volume (TV) have been linked to aggravation of lung injury in patients with acute respiratory failure, also referred to as patient self-inflicted lung injury (P-SILI) [1, 2]. Timely identification of patients with respiratory deterioration may be of clinical relevance [3, 4]. The ROX-index (SpO2/FiO2/respiratory rate) has been validated to identify patients on high-flow nasal oxygen at risk for requiring endotracheal intubation [5]. Yet, upon increased respiratory loading, changes in TV precede increases in respiratory rate [6]. Indeed, replacing respiratory rate by TV in the ROX-index significantly improved predicting requirement of invasive mechanical ventilation in patients with respiratory failure [7]. However, TV measurement in non-intubated critically ill patients is challenging given the need for accurate airflow measurements.
Ultrasound assessment of diaphragm excursion is reproducible and fair correlations with TV were reported in non-clinical studies [8,9,10]. Therefore, we hypothesized that bedside measurement of diaphragm excursion could be a valid surrogate for TV. Studies evaluating diaphragm motion have been performed earlier in the context of weaning from mechanical ventilation [11]. The aim of the current proof-of-concept study was to determine the relationship between TV and diaphragm excursions in ICU patients and healthy volunteers. In addition, we investigated correlations between changes in TV and diaphragm excursion within patients.
Methods
In this prospective study two groups were studied: healthy volunteers and patients on invasive mechanical ventilation, enrolled between August and December 2022. The ethics board approved the study (MEC-2022-0451) and written informed consent was obtained, through legal representatives whenever necessary. Patients with tracheostomy, Body Mass Index (BMI) > 35 kg/m2, exacerbation of obstructive lung disease, large pleural effusions (> 1.5 cm), neuromuscular disease or diaphragm paralysis (defined as known paralysis in medical history or having paradoxal diaphragm movement on ultrasound) were excluded.
Simultaneous measurements of TV and diaphragm excursion were obtained in at least 3 breaths per participant. The right hemi-diaphragm was visualized in semi-recumbent position (30 degrees) using subcostal view in M-mode (Sparq, Philips; 2–4 MHz probe) by a single observer experienced in diaphragm ultrasound, as previously described[12]. Images were stored for offline analysis (Sante DICOM Viewer). In patients on invasive mechanical ventilation (Servo-U, Getinge, Sweden) measurements were performed during the first few minutes of a spontaneous breathing trial with positive end-expiratory pressure (PEEP) of 5 cmH2O and no inspiratory pressure support. Healthy volunteers were breathing through a mouthpiece with flow sensor connected to a signal acquisition system (BIOPAC Systems, USA), while wearing a nose clip to prevent air leakage. They were instructed to perform tidal breathing as well as deep breathing at non-maximal levels to generate a range of TV. To maximize precision of the measurements, both TV and diaphragm excursions were determined offline and thus were not read from the ventilator or ultrasound machine directly during the imaging procedure. Diaphragm excursions were determined by measurement of the amplitude of the M-line, while being blinded for the corresponding TV values. TV were extracted from the integral of the inspiratory flow-time curve as exported from the ventilator monitor in ICU patients or as measured with a dedicated transducer in healthy subjects.
Using intra-class correlation coefficient (ICC) analysis, single observer test–retest reliability was assessed for diaphragm excursion in a random sample of 3 healthy volunteers and 3 patients (n = 74 breaths) using a two-way mixed model with single measures of agreement. Furthermore, we assessed the stability of the ratio between diaphragm excursion and TV within a subject as a surrogate of measurement reliability, considering that this ratio should not change within the short time interval. To this end, we used 3–5 breaths for all subjects and employed a two-way mixed model with single measures of consistency.
Statistical analysis was performed with R (RStudio, version 4.2.2). A linear mixed model with a random intercept per participant and fixed effect of diaphragm excursion was used to estimate the TV-diaphragm excursion ratio, thereby taking multiple and variable measurements per subject into account. The agreement between the observed TV and the TV as predicted by the linear mixed model was evaluated using a Bland–Altman plot. In addition, to test if the relationship between TV and diaphragm excursion was affected by any participant characteristics, such as age, BMI and chest circumference, these characteristics were individually added to the linear mixed model as fixed effects. For all analyses, a p value < 0.05 was considered statistically significant.
Results
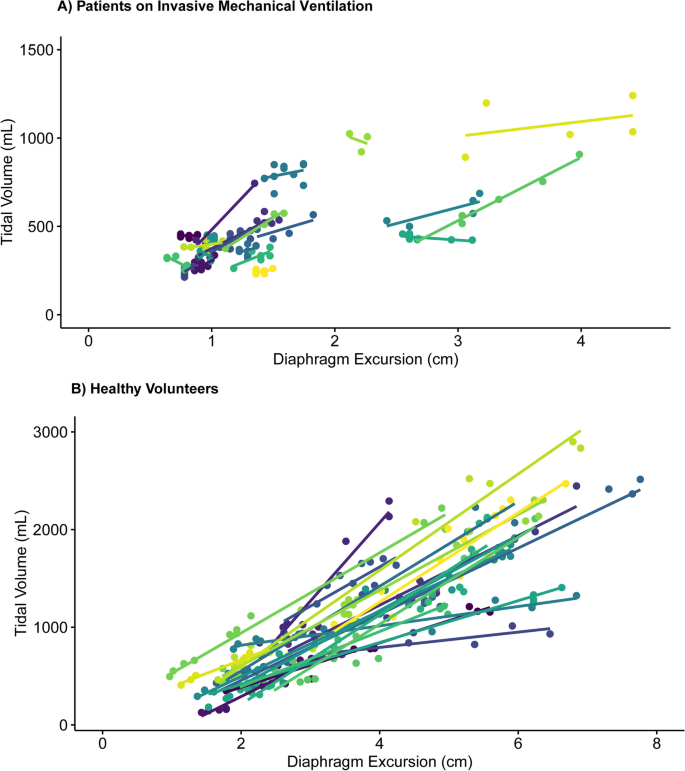
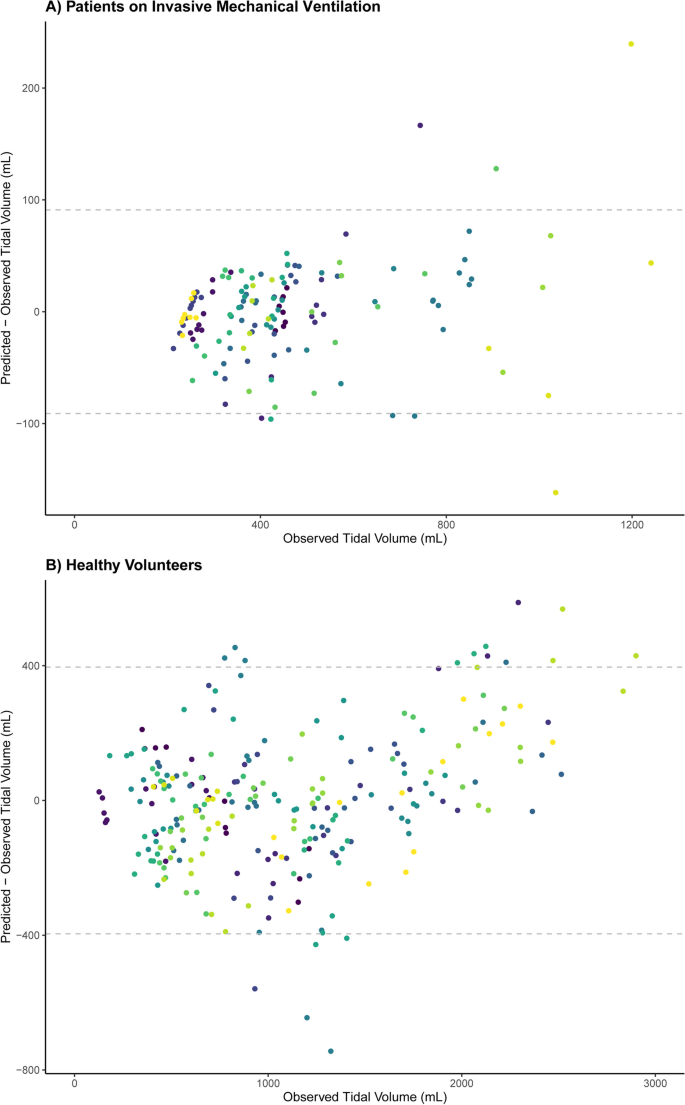
ICU patients (N = 21) and healthy volunteers (N = 20) (Table 1) yielded 139 (median 6, IQR 5–8) and 255 (median 13, IQR 11–14) analyzable breaths, respectively. A good stability of the TV-diaphragm excursion ratio over consecutive breaths within one subject was shown (ICC: 0.86). The mean (± standard deviation) difference between first and second diaphragm excursion measurement was 0.035 ± 0.20 cm. The ICC for intra-observer variability between the first and second measurement of diaphragm excursion was 0.99. The models indicated an excellent association within participants (R2 = 0.96 in invasively ventilated patients, R2 = 0.90 in healthy volunteers), as illustrated in Fig. 1. The TV-diaphragm excursion ratio was 201 mL/cm in ICU patients (95% confidence interval (CI) 161–240 mL/cm), p < 0.001, and 361 (95% CI 294–428) mL/cm, p < 0.001 in healthy volunteers. The mean (± standard deviation) value of the per-patient intercept of the model was 0 ± 157 mL in ICU patients, and 0 ± 267 mL for healthy volunteers. The variability in TV estimation between participants was large: e.g., a diaphragm excursion of 1.5 cm could correspond to a TV between 250 and 750 mL in ICU patients (Fig. 1). Participant characteristics (age, sex, height, Ideal Body Weight, BMI, chest circumference and days on invasive mechanical ventilation) did not affect the relationship between diaphragm excursion and TV (Table 1). Bland–Altman plots (Fig. 2) showed considerable differences between observed and predicted TV (95% limits of agreement: ± 91 mL in ICU patients and ± 396 mL in healthy volunteers).
Bland–Altman plot showing the association between observed and predicted Tidal Volumes based on the linear mixed model, separated for ICU patients on invasive mechanical ventilation and healthy volunteers. Every color represents a different participant. The dashed lines indicate 95% limits of agreement: ± 91 mL in ICU patients (A) and ± 396 mL in healthy volunteers (B)
Discussion
Our study demonstrates a correlation between ultrasound measurement of diaphragm excursion and TV in healthy volunteers, but the large variability in the data in ICU patients indicates a less obvious association. This precludes a reliable estimation of the absolute value of TV from diaphragm excursion measurement in the clinical setting.
The difference in TV-diaphragm excursion ratio between ICU patients and healthy volunteers may be explained by a smaller distribution of TV in ICU patients. Indeed, an additional (sensitivity) analysis of the TV-diaphragm excursion relationship in healthy volunteers when including only breaths in the same TV range as ICU patients (TV ≤ 1250 mL) indicated that the TV-diaphragm excursion ratio is comparable to ICU patients [231 (209–255) mL/cm]. In addition, altered respiratory mechanics and potential effects of PEEP on diaphragm efficiency [13] have likely played a role. An earlier study showed that the application of PEEP resulted in caudal displacement of the diaphragm and decreased the diaphragm contractile efficiency. Possibly, the decrease in TV-diaphragm excursion ratio in ICU patients compared to healthy volunteers is explained by PEEP.
Our results contrast with earlier studies that observed a fair correlation between TV and diaphragm excursion [9, 10]. However, these studies were performed in non-clinical settings, and used simple linear regression analysis without accounting for multiple measurements per participant. Furthermore, their larger TV-diaphragm excursion ratios (555 and 625 mL/cm, respectively) may be explained by recruitment of accessory muscles, since participants in earlier studies were instructed to inhale up to total lung capacity.
There are limitations of our study to acknowledge. First, we did not quantify accessory muscle use, although the association between diaphragm excursion and TV is affected by these muscles. Occult recruitment of accessory muscles may, therefore, have distorted the TV-diaphragm excursion ratio especially in ICU patients. However, we evaluated the potential of diaphragm excursion as bedside tool to monitor TV. Simultaneous evaluation of accessory muscle recruitment might have improved the understanding of the association between diaphragm excursion and TV, but would also complicate its clinical applicability. Second, the average time on invasive mechanical ventilation in the studied ICU patients was rather short. We recognize that the association between diaphragm excursion and TV may differ in patients with prolonged invasive mechanical ventilation due to diaphragm muscle dysfunction [14]. However, the targeted population to use diaphragm excursion as proxy for TV would concern non-intubated patients rather than those with prolonged invasive mechanical ventilation. Third, we excluded patients with high BMI due to difficulty of imaging the diaphragm, and also patients with exacerbation of obstructive lung disease due to flattening of their diaphragm resulting from pulmonary hyperinflation. This may affect the generalizability of our results as these are common comorbidities in the ICU population. Fourth, images from multiple breaths were obtained once in each participant. The use of a single ultrasonographer may imply that if this method were to be translated to clinical practice more variability from different observers may be introduced. However, the reproducibility of diaphragm excursion measurements via ultrasound has already been substantiated in a large study [8]. Consequently, we reasoned that imaging performed by multiple observers was deemed unnecessary in this study. Finally, TV was derived from the flow tracings but under different gas conditions (body temperature, pressure, water vapor saturated in ventilated patients and ambient temperature and pressure in healthy subjects); this will not affect the primary conclusion and between-subject variability but may result in a slightly higher absolute ratio (mL/cm) for healthy volunteers compared to ventilated patients.
Adequate diaphragm imaging is pivotal to establish the TV-diaphragm excursion ratio in the critical care setting. M-mode ultrasound measures unidimensional diaphragm movement and requires diaphragm motion perfectly aligned with the M-mode line. Even then, commonly employed one-dimensional measures of diaphragm excursion cannot capture the complete diaphragm motion, which is multidimensional. Our study emphasizes the complexity of the resultant relationship between a single measurement of diaphragm excursion and TV. Hence, a one-dimensional measure is unsuitable to determine absolute values or a safe cutoff for TV. Advanced techniques such as speckle tracking may have superior performance by quantifying diaphragm motion in multiple dimensions [15, 16]. Prior studies were often hampered by the application of inspiratory pressure support during ultrasound measurements. However, it should be stressed that such measurements of excursion should be performed in patients without inspiratory ventilator support [12, 17] to reliably reflect the patient’s own contribution to generating TV, such as done in our study. The relationship between diaphragm excursion as measured with speckle tracking and TV and its possible role in predicting the need for intubation in non-intubated patients with acute respiratory failure requires further study.
Conclusion
To conclude, in this proof-of-concept study in critically ill patients and healthy volunteers, single measurement of diaphragm excursion is not a clinically feasible surrogate for absolute values of TV. Consecutive measurements of diaphragm excursion may indicate changes in TV within patients with respiratory failure, yet its margin of error is too large to use the measurement for monitoring clinical deterioration. Therefore, diaphragm excursions measured with ultrasound should not be used to identify patients at risk for P-SILI.
Take home message
Monitoring tidal volume in patients with respiratory failure is necessary, but its measurement in non-intubated patients is challenging. This study shows that diaphragm excursions measured with ultrasound correlate with tidal volume, yet quantification of absolute values for tidal volume from diaphragm excursion is unreliable.
Tweet
Measurements of diaphragm excursions with ultrasound correlate with tidal volume, but should not be used to determine tidal volume.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICU:
-
Intensive care unit
- IMV:
-
Invasive mechanical ventilation
- P-SILI:
-
Patient self-inflicted lung injury
- PEEP:
-
Positive end-expiratory pressure
- TV:
-
Tidal volume
- BMI:
-
Body Mass Index
- IBW:
-
Ideal body weight
References
Grieco DL, Menga LS, Eleuteri D, Antonelli M (2019) Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol 85(9):1014–1023
Yoshida T, Grieco DL, Brochard L, Fujino Y (2020) Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr Opin Crit Care 26(1):59–65
Brochard L, Slutsky A, Pesenti A (2017) Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 195(4):438–442
Kang BJ, Koh Y, Lim CM, Huh JW, Baek S, Han M et al (2015) Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med 41(4):623–632
Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernandez G et al (2019) An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med 199(11):1368–1376
Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D (2020) Respiratory drive in critically ill patients. Pathophysiology and clinical implications. Am J Respir Crit Care Med 201(1):20–32
Chen D, Heunks L, Pan C, Xie J, Qiu H, Yang Y et al (2022) A novel index to predict the failure of high-flow nasal cannula in patients with acute hypoxemic respiratory failure: a pilot study. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202203-0561LE
Boussuges A, Gole Y, Blanc P (2009) Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest 135(2):391–400
Cohen E, Mier A, Heywood P, Murphy K, Boultbee J, Guz A (1994) Excursion-volume relation of the right hemidiaphragm measured by ultrasonography and respiratory airflow measurements. Thorax 49(9):885–889
Houston JG, Angus RM, Cowan MD, McMillan NC, Thomson NC (1994) Ultrasound assessment of normal hemidiaphragmatic movement: relation to inspiratory volume. Thorax 49(5):500–503
Parada-Gereda HM, Tibaduiza AL, Rico-Mendoza A, Molano-Franco D, Nieto VH, Arias-Ortiz WA et al (2023) Effectiveness of diaphragmatic ultrasound as a predictor of successful weaning from mechanical ventilation: a systematic review and meta-analysis. Crit Care (London, England) 27(1):174
Tuinman PR, Jonkman AH, Dres M, Shi ZH, Goligher EC, Goffi A, et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Med. 2020;46(4):594–605.
Jansen D, Jonkman AH, Vries HJ, Wennen M, Elshof J, Hoofs MA et al (2021) Positive end-expiratory pressure affects geometry and function of the human diaphragm. J Appl Physiol (1985) 131(4):1328–1339
Vassilakopoulos T, Petrof BJ (2004) Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 169(3):336–341
Oppersma E, Hatam N, Doorduin J, van der Hoeven JG, Marx G, Goetzenich A et al (2017) Functional assessment of the diaphragm by speckle tracking ultrasound during inspiratory loading. J Appl Physiol (1985) 123(5):1063–1070
Huang D, Song F, Luo B, Wang S, Qin T, Lin Z et al (2023) Using automatic speckle tracking imaging to measure diaphragm excursion and predict the outcome of mechanical ventilation weaning. Critical Care (London, England) 27(1):18
Sabourin E, Carpentier C, Lai C, Monnet X, Pham T (2023) “Under pressure”: should we use diaphragm excursion to predict weaning success in patients receiving pressure support ventilation? Critical Care (London, England) 27(1):238
World Medical Association Declaration of Helsinki (2013) ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Acknowledgements
We thank all study participants for making the study possible.
Funding
This study was partly funded by an unrestricted grant by Fisher & Paykel Healthcare (Auckland, New Zealand).
Author information
Authors and Affiliations
Contributions
MLJ: made substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of data; and drafted the work. AHJ: made substantial contributions to the conception and design of the work, analysis, and interpretation of data; and drafted the work. MW: made substantial contributions to the analysis and interpretation of data and substantively revised the manuscript. HE and EJW: made substantial contributions the conception and design of the work, interpretation of data and substantively revised the manuscript. LH: made substantial contributions to the conception and design of the work, interpretation of data, substantively revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics board approved the study (MEC-2022-0451) and informed consent was obtained. The study has been carried out in accordance with the Helsinki declaration for medical research involving humans [18].
Consent for publication
Not applicable.
Competing interests
AHJ has received personal fees from Liberate Medical (Crestwood, Kentucky). HE has received unrestricted research grants from Fisher & Paykel Healthcare (Auckland, New Zealand), La Roche Ltd. (Bazel, Switzerland) and Ventinova Medical B.V. (Eindhoven, the Netherlands). LH has received speakers fee from Getinge (Sweden), research support from Liberate Medical (Crestwood, Kentucky), ZonMw (Netherlands), and the European Respiratory Society, and personal fees from American Thoracic Society.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janssen, M.L., Jonkman, A.H., Wennen, M. et al. Diaphragm excursions as proxy for tidal volume during spontaneous breathing in invasively ventilated ICU patients. ICMx 11, 73 (2023). https://doi.org/10.1186/s40635-023-00553-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00553-z