Abstract
Background
Volume expansion and vasopressors for the treatment of shock is an intensive process that requires frequent assessments and adjustments. Strict blood pressure goals in multiple physiologic states of shock (traumatic brain injury, sepsis, and hemorrhagic) have been associated with improved outcomes. The availability of continuous physiologic data is amenable to closed-loop automated critical care to improve goal-directed resuscitation.
Methods
Five adult swine were anesthetized and subjected to a controlled 30% estimated total blood volume hemorrhage followed by 30 min of complete supra-celiac aortic occlusion and then autotransfusion back to euvolemia with removal of aortic balloon. The animals underwent closed-loop critical care for 255 min after removal of the endovascular aortic balloon. The closed-loop critical care algorithm used proximal aortic pressure and central venous pressure as physiologic input data. The algorithm had the option to provide programmatic control of pumps for titration of vasopressors and weight-based crystalloid boluses (5 ml/kg) to maintain a mean arterial pressure between 60 and 70 mmHg.
Results
During the 255 min of critical care the animals experienced hypotension (< 60 mmHg) 15.3% (interquartile range: 8.6–16.9%), hypertension (> 70 mmHg) 7.7% (interquartile range: 6.7–9.4%), and normotension (60–70 mmHg) 76.9% (interquartile range: 76.5–81.2%) of the time. Excluding the first 60 min of the critical care phase the animals experienced hypotension 1.0% (interquartile range: 0.5–6.7%) of the time. Median intervention rate was 8.47 interventions per hour (interquartile range: 7.8–9.2 interventions per hour). The proportion of interventions was 61.5% (interquartile range: 61.1–66.7%) weight-based crystalloid boluses and 38.5% (interquartile range: 33.3–38.9%) titration of vasopressors.
Conclusion
This autonomous critical care platform uses critical care adjuncts in an ischemia–reperfusion injury model, utilizing goal-directed closed-loop critical care algorithm and device actuation. This description highlights the potential for this approach to deliver nuanced critical care in the ICU environment, thereby optimizing resuscitative efforts and expanding capabilities through cognitive offloading. Future efforts will focus on optimizing this platform through comparative studies of inputs, therapies, and comparison to manual critical care.
Similar content being viewed by others
Introduction
The cornerstone of shock management has relied upon support of hemodynamics to maintain tissue perfusion and resulting oxygen delivery. This has primarily relied upon vasopressors and volume optimization, outside of the needed intervention for source control in the patient with septic shock, arrest of hemorrhage in the bleeding patient, or coronary artery bypass/percutaneous coronary intervention in the patient with ischemic cardiogenic shock. The vital signs of a patient in shock are labile and require intensive care unit (ICU) admission for invasive hemodynamic monitoring. Additionally, close attention by healthcare personnel is necessary to provide frequent assessments and adjustments in response to a patient's changing condition. This involves manual titration of fluid and vasopressor infusions for the treatment of a patient with severe shock, which can be a time consuming process [1]. Also, the excessive cognitive load associated with these tasks, amplified across an ICU with numerous critically ill patients, can lead to errors, burnout, job dissatisfaction, and increased patient mortality [2,3,4].
Hemodynamic support in shock, centers around the goal of strict blood pressure control, which has been suggested to confer benefit in many shock etiologies [5,6,7,8]. While establishing target hemodynamic endpoints is straightforward, either based on standard practice or individualized goals, the real challenge in the context of a critically ill patient on numerous infusions and life support devices is to achieve and maintain these endpoints over time. Prior studies in ICU and operating room patients on vasopressors report 6.0–11.2% of time spent with a mean arterial pressure (MAP) < 60 mmHg [9, 10]. While in one study 60% of the time patients in an ICU on vasopressors had a MAP > 70 mmHg and in another, 40% of the time was spent with a MAP > 80 mmHg [9, 10]. This bias towards the hypertensive state suggests an intentional effort on the part of providers. This in part reflects an intentional permissive stance, where hypertension is deemed preferable over hypotension both from clinical outcomes perspective and from the realization that tight blood pressure targeting is inherently more labor intensive and more difficult to achieve. However, SEPSIS-PAM suggests that a bias towards achieving or allowing hypertension through the use of exogenous vasopressor administration does not improve overall (exception of patients with chronic hypertension and association with renal failure) and a meta-analysis did not identify any benefits to supratherapeutic use of vasopressors with increased risk of new-onset supraventricular tachycardia [11, 12]. In addition, there are small case series reporting on the rare but limb threatening complication of symmetric peripheral gangrene due to excessive vasopressor use [13].
It is certainly true that failure to achieve or maintain target hemodynamic goals is unavoidable in certain situations due to a host of factors. However, optimization of hemodynamics, specifically achieving target hemodynamic goals, can undoubtedly be achieved through improvements in clinical care. How to do so is a matter of debate, but it is increasingly recognized that automation utilizing closed-loop fluid and drug delivery is a viable and rational approach to solving this clinical dilemma [14, 15]. By combining ubiquitous continuous hemodynamic monitoring platforms, embedded microsystems to process these data using established rule sets or intelligent algorithms, and output devices that can administer or titrate fluids and drugs, many aspects of critical care management can be either partially or fully automated.
There are several benefits to an automated critical care platform to administer and titrate fluids and drugs. First, the time required to complete the cycle of recognition of clinical change, intervention, and clinical effect would be dramatically shorter. This allows for a strategy where smaller changes are made more frequently, leading to tighter control of hemodynamics. Second, the programming of a closed-loop control system effectively leads to standardization of care protocols, which allows best practices and safeguards to be implemented regardless of the ability or immediate availability of the bedside provider. Lastly, a closed-loop control system would likely decrease task load for bedside providers and improve provider bandwidth. In the setting of staff shortages or in austere environments this may allow expanded patient care capabilities.
There is recent interest using translational models for testing of closed-loop resuscitation paradigms for the titration of vasopressors during anesthesia and resuscitation of hemorrhagic shock [16,17,18]. Closed-loop automation of fluid and drug delivery has been examined in humans for treatment of burn resuscitation and blood pressure control under general anesthesia [19, 20]. Additionally, there have been feasibility studies exploring fully automated life support in healthy swine [21]. However, the development of an automated critical care platform for the management of shock states for use in the ICU environment has not been extensively explored. Our group is developing such a platform, specifically for use in vasodilatory shock [22]. We hypothesize that in a swine model of ischemia–reperfusion injury, an automated critical care platform can be developed that provides fully autonomous crystalloid and vasopressor titration based on a simple heuristic model of critical care. This would provide a testing platform for precise measurements and reproducible treatment for the study of various critical care strategies.
Methods
Overview
The Institutional Animal Care and Use Committee at Wake Forest Baptist Medical Center approved this study. All animal care and use were in strict compliance with the Guide for the Care and Use of Laboratory Animals in a facility accredited by AAALAC.
Animal preparation
Healthy adult, castrate male and non-pregnant female, Yorkshire-cross swine (Oak Hill Genetics, Ewing, IL, USA) were fasted for 12 h before experimentation, then premedicated with 5–7 mg/kg intramuscular tiletamine/zolazepam (Telazol, Zoetic Inc. Olot, Spain). After isoflurane induction and endotracheal intubation, animals were maintained with ~ 2% isoflurane and mechanically ventilated to maintain end-tidal CO2 at 35–45 mmHg. All animals received a 1-L bolus of balanced electrolyte solution, followed by maintenance of intravenous fluids of 10 mL/kg/h (PLASMA-LYTE A, Baxter Healthcare Corporation, Deerfield, IL, USA). To offset the vasodilatory effects of isoflurane, an intravenous infusion of norepinephrine (0.02 mcg/kg/min) was instituted upon venous access and titrated before experimentation to achieve a target MAP > 60 mmHg. An underbody warmer was used to maintain core body temperature between 37 and 39 ºC. A laparotomy was undertaken for placement of a cystostomy tube and a splenectomy was performed to minimize hemodynamic variation from autotransfusion. The maintenance fluid rate was decreased to 5 mL/kg/h after abdominal wall closure.
Instrumentation
A surgical cut-down approach was used to place a 7 Fr arterial sheath in the right common femoral artery and a 9 Fr arterial sheath in the left common femoral artery. A dual-lumen 9 Fr venous resuscitation line was placed in the left femoral vein for blood transfusion and resuscitation fluids. Left external jugular vein was surgically exposed and cannulated with a 9 Fr sheath, respectively, to allow for maintenance fluid, vasoactive medication administration, and central venous pressure measurements. A 7 Fr arterial sheath was placed in the left axillary artery after surgical exposure for proximal blood pressure measurements. The right brachial artery was exposed and cannulated with a 5 Fr sheath to facilitate frequent lab draws. A 7 Fr custom compliant aortic balloon catheter was introduced through the left femoral 9 Fr arterial sheath and positioned at the level of the diaphragm. The position of the aortic balloon catheter was confirmed in the distal descending thoracic aorta by manual palpation. Finally, the abdomen was closed with cable ties and plastic sheet to minimize insensible fluid losses. Intravenous heparin (50 units/kg bolus and 10 unit/kg/h continuous rate) was administered to achieve an activated clotting time of ~ 100 s based on a pre-calculated rate heuristically developed from prior studies to prevent complications of intra-aortic balloon placement (Additional file 1: Fig. S1).
Data collection
Physiologic measurements of proximal blood pressure, distal blood pressure, and central venous pressure, were collected in real time with a multi-channel data acquisition system at a rate of 1000 Hz (Powerlab, AD Instruments, Colorado Springs, CO, USA).
Experimental flow
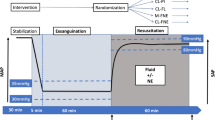
After instrumentation, the animals underwent an automated computer-controlled 30% estimated total body blood volume controlled hemorrhage over 30 min and the shed blood was collected in gently agitated citrated bags (Fenwell Blood Collection, Fresenius Kabi, Bad Homburg, Germany) and stored in a warm water bath at 39 ºC. Estimated total blood volume in milliliters was calculated as 60 ml/kg. After hemorrhage, the prepositioned aortic occlusion balloon was autonomously inflated to complete occlusion as measured by the complete absence of distal arterial pulse pressure for 30 min. At T45 (during occlusion phase), the automated drug delivery system infused 200 mg of calcium gluconate over 20 min to counteract the anticipated transfusion of citrate from the autologous blood transfusion. At T55, the animals were returned to 95% of total blood volume over 18 min. Starting at T60 the aortic balloon was programmatically weaned over 15 min during transfusion to prevent complete hemodynamic collapse from reperfusion injury and then the aortic balloon was removed. The critical care phase lasted for a predetermined 255 min (5.5 h from start of experiment).
Automated critical care system
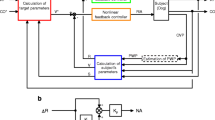
A custom-designed central processing unit was built using an Arduino embedded microsystem (Arduino LLC, Boston, MA, USA) for monitoring continuous analog data output from the physiologic recording system (Power Lab, AD Instruments, Colorado Springs, CO, USA). A custom graphical user interface was developed using C#.NET (Windows Corporation, Redmond, Washington, USA) to display real-time blood pressure measurements and to execute logic for control of peripheral devices (step-by-step pump controls). Additionally, a novel builder functionality was developed to carefully choreograph events throughout the experiment, enabling replication of experimentation with millisecond precision. Using three wireless transceivers (Pololu Corporation, Las Vegas, NV, USA), bidirectional communication was established to control a multi-channel peristaltic pump (Isamatec, Antylia Scientific Company, Vernon Hills, IL, USA), high-volume peristaltic pump (Masterflex, Antylia Scientific, Vernon Hills, IL, USA), and custom-built balloon syringe pump [22]. This created a closed-loop physiology monitoring, injury creation, interpretation, and intervention system. See Fig. 1 for schematic representation of the hardware setup and Fig. 2 for an example of the system in use during an experiment.
A picture of the setup in use for a critical care experiment. Data Acquisition platform (Powerlab, AD Instruments, Colorado Springs, CO, USA), Central Controlled (Arduino, Arduino LLC, Boston, MA, USA), Wixel Wireless Transceiver, Pololu Corporation, Las Vegas Nevada (Wireless receiver), Lab Grade multi-channel infusion pump (Isamatec, Cole-Palmer an Antylia Scientific Company, Vernon Hills, IL, USA), and Micro bolus Infusion Pump (Masterflex an Antylia Scientific Company, Antylia Scientific, Vernon Hills, IL, USA)
Standardized critical care algorithm
Algorithms for drug and fluid delivery and titration were designed based on observations from multiple prior swine studies and heuristics of critical care for the management of shock. This iteration is an improvement on the original algorithm used for development of combined resuscitation with endovascular perfusion augmentation study [23]. The main inputs include arterial blood pressure, central venous pressure, current norepinephrine dose, and number of crystalloid boluses. Crystalloid boluses were given as weight-based (5 mL/kg, i.e., for a 70-kg pig corresponds to 350 mL over 2 min which corresponds to an infusion rate of approximately 10.5 L/h). This combined automated system and algorithm has been termed Precision Automated Critical Care Management (PACC-MAN). See Additional file 1: Fig. S2 for a complete diagram of the critical care algorithm.
Continuous proximal mean aortic blood pressure is measured and the average for the last 1 min is continuously calculated. If the animal has an average mean arterial blood pressure of 60–70 mmHg (inclusive), then unless the animal is on a large dose of norepinephrine (> 0.5 mcg/kg/min) and a low central venous pressure (< 6 mmHg) the animal does not receive an intervention. If the animal has an average mean arterial blood pressure over 1 min > 70 mmHg, then this is an opportunity to decrease vasopressors.
Vasopressor titration is performed using an algorithm that factors in the absolute difference between current MAP and target MAP of 65 mmHg, square root of current norepinephrine rate, scaling factor (determined based on branch in algorithm, integers like 1 or 2), and scaling constant (0.0072). The function is still relatively linear in the normal ranges of mild hypotension and mild hypertension.
The majority of the complexity is borne out in the hypotension algorithm with two main branches. The first branch, denoted “Severe Hypotension” (MAP < 50 mmHg), triggers a weight-based fluid bolus (5 mL/kg) and a simultaneous vasopressor dose increase. The second branch, denoted “Moderate Hypotension” (MAP 50–59 mmHg, inclusive) is more nuanced. This involves an algorithm for the estimation of “fluid responsiveness”. “Fluid responsiveness” is generally defined as a cardiac output augmentation of 10–15% in response to crystalloid therapy and a change in blood pressure is commonly used to assess a hypotensive patient's cardiac output response to a fluid challenge [24, 25]. While the efficacy of blood pressure change’s correlation to cardiac output augmentation is debated, it is a reliable input for building a closed-loop critical care algorithm. Therefore, a preload-dependent state was defined as a change in blood pressure in response to a weight-based test bolus (5 mL/kg) over 2 min. A “responsive” state was defined as an increase in MAP of at least 5 mmHg. Additional interventions are based on this binary classifier. In general, the “fluid responsive” state involves a more fluid-avid resuscitation, whereas the “fluid non-responsive” state involves a more vasopressor-avid resuscitation. There are specific corner cases for the “fluid non-responsive” states to (1) avoid cyclic increases in norepinephrine (“CVP-based Fluid Bolus”) and (2) attempt a fluid bolus for animals on maximum pressors, hypotensive, and not volume responsive (“Pressor Resistance Algorithm”). This algorithm was based on heuristics from numerous animals that have undergone this specific injury.
Every four interventions or after an hour since the last fluid responsiveness test bolus, the algorithm resets to “unknown” and an additional test bolus is administered in response to the next episode of hypotension.
Data analysis
Data analysis was performed with Python Version 3.7 (Python Software Foundation, Wilmington, Delaware, USA), Excel 2016 (Microsoft Corporation, Redmond, Washington, USA), and R statistical software (R Foundation for Statistical Computing, Vienna, Austria). Due to anticipated small sample size values are presented as medians with interquartile range. Based on our review of the literature and severity of this injury model the goal of the algorithm was to avoid hypotension of 90% while having < 20% of the time spent at hypertension.
Hypotension was defined as MAP of < 60 mmHg, hypertension as MAP of > 70 mmHg, and normotension as MAP of ≥ 60 mmHg and ≤ 70 mmHg. 1000-Hz data were binned into 1-min blocks of time.
A priori exclusion criterion was a white blood cell count > 25 109/L or expiration before the end of study as defined by a MAP < 20 mmHg for 5 min during any time point (including instrumentation). Also if norepinephrine at a dose rate of > 0.1 mcg/kg/min was required for more than 10 min cumulative duration during setup or if norepinephrine at a dose rate of > 0.06 mcg/kg/min was required to maintain normotension immediately prior to the beginning of the experiment (time zero).
Results
There were 8 animals available for analysis and underwent standardized critical care after ischemic perfusion injury. One animal was excluded due to protocol violation (error with pump rates), one animal had injury protocol violations and then died early in experiment, and one animal died later during the critical care phase. A complete list of baseline characteristics can be seen in Table 1. The median peak lactate after injury was 9.77 mmol/L (interquartile range: 8.86–10.17 mmol/L) and the median lactate at end of experiment was 4.91 mmol/L (interquartile range: 4.51–5.04 mmol/L). See Fig. 3 for lactate levels throughout the experimental protocol.
During the 255 min of the critical care phase, the animals experienced hypotension (< 60 mmHg) 15.29% (interquartile range: 8.63–16.47%) of the time, hypertension (> 70 mmHg) 7.84% (interquartile range: 6.67–9.02%) of the time, and normotension (60–70 mmHg) 76.86% (interquartile range: 76.86–81.18%) of the time. During the first 60 min of the critical care phase (immediately following the injury phase) the proportion of time spent at normotension was 56.67% (interquartile range: 35.00–56.67%), while time at hypotension was 46.33% (interquartile range: 35.00–65.00%) with negligible time at hypertension 0% (interquartile range: 0–0%). When examining the critical care phase beyond the first 60 min (the latter part including 155 min of critical care), the animals experienced normotension 83.08% (interquartile range: 83.08–87.18%) of the time, hypotension 1.03% (interquartile range: 0.51–7.69%) of the time, and hypertension 10.00% (interquartile range: 8.72–11.79%) of the time. Figure 4 is a visual representation of the mean arterial blood pressure throughout the experiment. Table 2 provides a breakdown for each animal and their proportion of time at specific blood pressure ranges.
The median intervention rate was 8.47 interventions per hour (interquartile range: 7.76–9.18 interventions per hour). The interventions were proportioned as 61.54% (interquartile range: 61.11–66.67%) weight-based crystalloid boluses and 38.46% (interquartile range: 33.33–38.89%) titration of vasopressors (increase or decrease). Figure 4 provides a plot of the norepinephrine dose and number of weight-based crystalloid boluses throughout the course of the experiment.
Discussion
In a swine model of ischemia–reperfusion injury causing vasodilatory shock, this autonomous critical care algorithm using closed-loop control of vasopressor titration and crystalloid boluses was able to provide hemodynamic support using mean arterial pressure and central venous pressure as inputs to avoid hypotension 84.72% (normotension 76.86% and hypertension 7.84%) of the time. In addition, this algorithm using a rule-based heuristic model of critical care was able to provide frequent interventions during the first hour after injury for the “active resuscitation” and then transition to long-term critical care with goal-directed blood pressure management, avoiding hypotension 93.08% of the time.
This translational model is severe and one animal did die early in the experiment, but that animal also had protocol violations during the injury model potentially making the injury more severe and another animal died later in the experiment and unclear the cause as it was sudden. The initial severe hypotension after removal of endovascular balloon may be unavoidable with traditional critical care adjuncts (pressors and crystalloid). The main application of this system at this time would be to have a form of “standardized critical care” from which to test hypotheses in translational models of differing novel adjunctive critical care interventions. Although this model of critical care could provide the groundwork for developing clinically testable algorithms and hardware solutions to assist with off-loading cognitive load for the clinician and nursing staff. The potential for closed-loop critical care in the operating room and in the intensive care unit as a clinical support tool for monitoring and intervening is certainly far away at this point, but the field is progressing quickly with the ever increasing availability of real-time digital healthcare information.
The main limitations of this model are sample size (5 swine) and duration of critical care (255 min). This was not a survival study and therefore long-term implications of this injury model and interventions are unknown. Also this algorithm did not have a direct comparison to a control group. This algorithm also may suffer from overfitting to this injury model and needs additional testing in a wider variety of shock models to verify its generalizability. Additionally, the potential instability of closed-loop inputs (blood pressures) in potential real-world scenarios was not tested. Finally, the granularity of “fluid responsiveness” is overly simplistic in this iteration (although blood pressure response is still widely used in intensive care settings). Our group is currently iterating refinements of our methodology to assess fluid responsiveness based around the concept of augmentation of cardiac output: a machine learning model, near-infrared spectroscopy, continuous cardiac thermistor, and mixed central venous spectroscopy to potentially more accurately measure the gradations of fluid responsiveness as opposed to a simple binary classifier based on blood pressure [24, 26, 27].
Autonomous control systems for use in medical applications has gained significant interest in the broad literature, the potential for closed-loop control is quickly becoming technically feasible, but not without reasons for concern. Among them are control system failure, automation bias, skill degradation, lack of operational transparency, and increased risk arising from system complexity [28]. Additionally, there is legitimate concern from a medico-legal perspective regarding liability in the context of adverse outcomes attributable to failures involving automated interventions. There are additional regulatory hurdles to developing autonomous platforms, particularly those that involve actuation of devices or control drug and fluid delivery. Furthermore, developing clinically relevant algorithms that can be generalized to the entire patient population and across disease states represents an obstacle, particularly given the protracted pace of clinical research. Ultimately, many hurdles still need to be crossed to deliver these types of autonomous therapies from bench to bedside. But, our ongoing federally funded research aims to develop a commercially viable prototype which is slated to occur over the next 3 years.
The next steps in refinement of the PACC-MAN platform will center around additional inputs to the decision tree, to include metabolic measures such as lactate and urine output. Additionally, we plan to augment the complement of interventions to include additional vasopressor agents (i.e., vasopressin) and to refine the implementation of existing interventions, ultimately to improve the fidelity of the model and to optimize physiologic endpoints of resuscitation. Finally, future work will directly compare this platform to human control alone, as well as assessing its efficacy in longer term experiments and survival models.
Conclusion
This autonomous critical care platform, utilizing volume expansion and vasopressor titration in a model of ischemia–reperfusion injury, using a goal-directed closed-loop algorithm based on heuristics of critical care provides a platform for prospective testing of critical care adjuncts in the treatment of circulatory failure. This descriptive technique can provide the framework for a patient-specific and “just enough” approach to critical care. Future directions include testing against manual critical care, variations of diagnostic/treatment paradigms, and injury models.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICU:
-
Intensive care unit
- MAP:
-
Mean arterial blood pressure
- PACC-MAN:
-
Precision automated critical care management
- CVP:
-
Central venous pressure
References
Butler R, Monsalve M, Thomas GW, Herman T, Segre AM, Polgreen PM, Suneja M (2018) Estimating time physicians and other health care workers spend with patients in an intensive care unit using a sensor network. Am J Med 131(8):972.e9-972.e15
Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH (2000) Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA 288(16):1987–1993
Tubbs-Cooley HL, Mara CA, Carle AC, Mark BA, Pickler RH (2019) Association of nurse workload with missed nursing care in the neonatal intensive care unit. JAMA Pediatr 173(1):44–51
Rasooly IR, Kern-Goldberger AS, Xiao R, Ponnala S, Ruppel H, Luo B, Craig S, Khan A, McLoone M, Ferro D, Muthu N, Won J, Bonafide CP (2021) Physiologic monitor alarm burden and nurses’ subjective workload in a Children’s Hospital. Hosp Pediatr 11(7):703–710
ATLS Subcommittee; American College of Surgeons’ Committee on Trauma; International ATLS working group. Advanced trauma life support Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg. 2013;74(5):1363–6.
Butcher I, Maas AI, Lu J, Marmarou A, Murray GD, Mushkudiani NA, McHugh GS, Steyerberg EW (2007) Prognostic value of admission blood pressure in traumatic brain injury: results from the IMPACT study. J Neurotrauma 24(2):294–302
Brain Trauma Foundation. Guidelines for the Management of Severe Traumatic Brain Injury. 4th edition. (2016). Available from: https://braintrauma.org/uploads/03/12/Guidelines_for_Management_of_Severe_TBI_4th_Edition.pdf. Accessed 14 Aug 21.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 45(3):486–552
Rinehart J, Ma M, Calderon MD, Bardaji A, Hafiane R, Van der Linden P, Joosten A (2019) Blood pressure variability in surgical and intensive care patients: Is there a potential for closed-loop vasopressor administration? Anaesth Crit Care Pain Med 38(1):69–71
Lamontagne F, Cook DJ, Meade MO, Seely A, Day AG, Charbonney E, Serri K, Skrobik Y, Hebert P, St-Arnaud C, Quiroz-Martinez H, Mayette M, Heyland DK (2017) Vasopressor use for severe hypotension—a multicentre prospective observational study. PLoS ONE 12(1):e0167840
Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Hervé F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P, SEPSISPAM Investigators (2014) High versus low blood-pressure target in patients with septic shock. N Engl J Med 370(17):1583–1593
Hylands M, Moller MH, Asfar P, Toma A, Frenette AJ, Beaudoin N, Belley-Côté É, D’Aragon F, Laake JH, Siemieniuk RA, Charbonney E, Lauzier F, Kwong J, Rochwerg B, Vandvik PO, Guyatt G, Lamontagne F (2017) A systematic review of vasopressor blood pressure targets in critically ill adults with hypotension. Can J Anaesth 64(7):703–715
Livesey M, Jauregui JJ, Hamaker MC, Pensy RA, Langhammer CG, Eglseder WA (2020) Management of vasopressor induced ischemia. J Orthop 16(22):497–502
Rinehart J, Liu N, Alexander B, Cannesson M (2012) Review article: closed-loop systems in anesthesia: is there a potential for closed-loop fluid management and hemodynamic optimization? Anesth Analg 114(1):130–143
Bighamian R, Rubbo C, Thorsen JE, Hahn J-O, Reisner AT (2014) Is there opportunity for automated decision-support and closed-loop control in ICU patients receiving vasopressor infusion? Annu Int Conf IEEE Eng Med Biol Soc. 2014:1949–1952
Joosten A, Delaporte A, Alexander B, Su F, Creteur J, Vincent JL, Cannesson M, Rinehart J (2019) Automated titration of vasopressor infusion using a closed-loop controller: in vivo feasibility study using a swine model. Anesthesiology 130(3):394–403
Chaisson NF, Kirschner RA, Deyo DJ, Lopez JA, Prough DS, Kramer GC (2003) Near-infrared spectroscopy-guided closed-loop resuscitation of hemorrhage. J Trauma 54(5 Suppl):S183–S192
Libert N, Chenegros G, Harrois A, Baudry N, Cordurie G, Benosman R, Vicaut E, Duranteau J (2018) Performance of closed-loop resuscitation of haemorrhagic shock with fluid alone or in combination with norepinephrine: an experimental study. Ann Intensive Care 8(1):89
Salinas J, Chung KK, Mann EA, Cancio LC, Kramer GC, Serio-Melvin ML, Renz EM, Wade CE, Wolf SE (2011) Computerized decision support system improves fluid resuscitation following severe burns: an original study. Crit Care Med 39(9):2031–2038
Joosten A, Chirnoaga D, Van der Linden P, Barvais L, Alexander B, Duranteau J, Vincent JL, Cannesson M, Rinehart J (2021) Automated closed-loop versus manually controlled norepinephrine infusion in patients undergoing intermediate- to high-risk abdominal surgery: a randomised controlled trial. Br J Anaesth 126(1):210–218
Klingert W, Peter J, Thiel C, Thiel K, Rosenstiel W, Klingert K, Grasshoff C, Königsrainer A, Schenk M (2018) Fully automated life support: an implementation and feasibility pilot study in healthy pigs. Intensive Care Med Exp 6(1):2
Williams TK, Neff LP, Tibbits EM, Hoareau GL, Simon MA, Davidson AJ, Desoucy ES, Faulconer ER, Johnson MA (2019) A novel automated endovascular variable aortic control device to expand function of standard reboa catheters. J Endovasc Resuscit Trauma Manage 3(1):3–10
Johnson MA, Tibbits EM, Hoareau GL, Simon MA, Davidson AJ, DeSoucy ES, Faulconer ER, Grayson JK, Neff LP, Williams TK (2019) Endovascular perfusion augmentation for critical care: partial aortic occlusion for treatment of severe ischemia-reperfusion shock. Shock 51(5):659–666
Monnet X, Marik PE, Teboul JL (2016) Prediction of fluid responsiveness: an update. Ann Intensive Care 6(1):111. https://doi.org/10.1186/s13613-016-0216-7
Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, Sander M, Spies C, Lefrant JY, De Backer D, FENICE Investigators; ESICM Trial Group (2015) Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med 41(9):1529–1537
Arya VK, Al-Moustadi W, Dutta V (2022) Cardiac output monitoring—invasive and noninvasive. Curr Opin Crit Care 28(3):340–347
Butler E, Nguyen J, Mahendran S, Aneman A (2018) Fluid responsiveness after cardiac surgery (FRACAS): a prospective observational study using peripheral near-infrared spectroscopy. J Cardiothorac Vasc Anesth 32(1):197–204
Parvinian B, Scully C, Wiyor H, Kumar A, Weininger S (2018) Regulatory considerations for physiological closed-loop controlled medical devices used for automated critical care: food and drug administration workshop discussion topics. Anesth Analg 126(6):1916–1925
Acknowledgments
We would like to acknowledge the Animal Resource Program at Wake Forest Baptist Medical Center.
Funding
Funding for this study was provided by the US Army Medical Research and Development Command. Award Number: W81XWH-18-0072.
Author information
Authors and Affiliations
Contributions
All authors contributed to the literature search and study design. TKW, LPN and MRL collected the data. TKW, LPN, MAJ, EGD and NTPP performed the data analysis and interpretation. TKW, LPN, MAJ, EGD and NTPP wrote the article, which all authors critically revised. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Animal studies were approved through the Institutional Animal Care and Use Committee at Wake Forest Baptist Medical Center (A21-092).
Consent for publication
Not applicable
Competing interests
L.P.N., T.K.W., M.A.J. are co-founders of Certus Critical Care, Incorporated. N.T.P.P. is a paid consultant for Certus Critical Care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
. Instrumentation anatomy. Figure S2. Flow diagram of the algorithmic approach to critical care.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patel, N.T.P., Goenaga-Diaz, E.J., Lane, M.R. et al. Closed-loop automated critical care as proof-of-concept study for resuscitation in a swine model of ischemia–reperfusion injury. ICMx 10, 30 (2022). https://doi.org/10.1186/s40635-022-00459-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-022-00459-2