Abstract
The COVID-19 outbreak caused by SARS-CoV-2 in late 2019 has spread rapidly across the world to form a global epidemic of respiratory infectious diseases. Increased investigations on diagnostic tools are currently implemented to assist rapid identification of the virus because mass and rapid diagnosis might be the best way to prevent the outbreak of the virus. This critical review discusses the detection principles, fabrication techniques, and applications on the rapid detection of SARS-CoV-2 with three categories: rapid nuclear acid augmentation test, rapid immunoassay test and biosensors. Special efforts were put on enhancement of nanomaterials on biosensors for rapid, sensitive, and low-cost diagnostics of SARS-CoV-2 virus. Future developments are suggested regarding potential candidates in hospitals, clinics and laboratories for control and prevention of large-scale epidemic.
Graphical Abstract

Similar content being viewed by others
1 Introduction
COVID-19 is an infectious disease caused by the SARS-CoV-2 coronavirus. The global excess mortality associated with COVID-19 was estimated to be 14.91 million, suggesting 9.49 million more deaths than those globally reported as directly attributable to COVID-19 [1]. Public health and social measures have been implemented across the world to reduce SARS-CoV-2 transmission, morbidity, and mortality from COVID-19 and to prevent the overburdening of the health systems and other critical social functions. SARS-CoV-2 primarily affects the respiratory system [2] with associated symptoms such as fever, cough, expectoration, headache, myalgia, or fatigue. Individuals with asymptomatic and atypical clinical manifestations contribute factors to complicate disease transmission [3]. SARS-CoV-2 also may cause severe pneumonia and acute respiratory distress syndrome [4]. It is worth noting that in addition to the respiratory system, SARS-CoV-2 damages the cardiovascular system, the endocrine system, and the reproductive system [5]. Previous investigations have suggested that manifestations of cardiovascular disease are a significant cause of mortality [6]. In the reproductive system, extensive studies have shown that SARS-CoV-2 can affect male serum testosterone, fertility, sexual function [7,8,9] and female ovarian function as well as pregnancy [10,11,12]. Recent evidence supported that SARS-CoV-2 could also affect the urinary tract [13], and neuropsychiatric symptoms [14]. Other reports also implied association of COVID-19 with digestive disorders [15] and Alzheimer’s disease [16]. Moreover, patients with COVID-19 may also experience eye symptoms such as dry eyes, conjunctival hyperemia, and conjunctival congestion [17]. At the same time, the COVID-19 stigmatization also brought various long-term complications and sequelae [18], even additional pain to patients [19]. It was also observed that psychological symptoms including anxiety, depression, and post-traumatic stress disorder have an association with post-COVID-19 [20, 21]. Despite worldwide efforts to contain the spread of SARS-CoV-2, the COVID-19 pandemic continued as the virus evolved into several variants and mutants [22]. When it comes to SARS-CoV-2 detection, SARS-CoV-2 in wastewater poses a high health risk to human beings [23], and wastewater surveillance becomes a vital part of the assessment and detection of SARS-CoV-2 [24, 25]. Hence, it could be of great significance to detect SARS-CoV-2 for assessment of risks and epidemiology of infectious diseases as well as the development of new responses to combat pathogens in the future [26].
To date, there are two general types of rapid tests available for COVID-19, namely, serological tests and nucleic acid-based tests. While serological detection has the advantages of being easier to conduct without need for sophisticated instruments, they highly depend on antibody detection, which requires seroconversion to occur in patients prior to administration of the test. Amongst nucleic acid-based tests, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) is still the golden standard for the detection of SARS-CoV-2 with limitations such as being time-consuming and causing false negatives. Recent evidence suggested that individuals tested with typical symptoms but showed negative in RT-qPCR results had a high likelihood of actually being infected with COVID-19 [27,28,29]. Countless factors influence the detection of SARS-CoV-2 using RT-qPCR, such as disease staging, sample collection methodology and sample storage, RNA extraction methodologies, choice of different SARS-CoV-2 targets, maximum Cycle Threshold (Ct), primer–probe dimerization occurrence, etc. [30] Furthermore, having a point mutation in the SARS-CoV-2 N gene (e.g., G29195T) may result in false-negative SARS-CoV-2 RT-qPCR results [31]. Therefore, diagnostic tools that could rapidly detect COVID-19 play critical roles in combating SARS-CoV-2.
We conducted a biometric analysis of articles related to the rapid detection of COVID-19 since its emergence and searched for articles in the “Web of Science” database using the search formula “(TS = (COVID-19) OR TS = (SARS-CoV-2)) AND TS = (rapid detection)”. The result was 3566 articles, and 3409 articles were retrieved after searching directly for scientific papers. A statistical analysis based on the timing of these articles revealed (Fig. 1) that the number of articles published in 2020 due to the emergence of the COVID-19 shortly after was only 13.79% of the total, after which the amount of research exploded in 2021 (39.69%) and 2022 (39.31%). Since April of 2023, its relevant research reached only less than 1/5 (7.22%) of that in 2022. In addition, the titles and keywords of these articles were carefully analyzed (Fig. 2). When studying the words included in the titles, the words “antigen” and “evaluation” appear more frequently, followed by “amplification”, “Point-of-Care”, “lateral flow”, and “biosensor”, indicating the importance of Point-of-Care, nucleic acid amplification, lateral flow, and biosensor in rapid detection of COVID-19. Furthermore, the high frequency of the phrases “nanomaterials” and “gold nanoparticles” is eye-catching, probably due to the use of nanomaterials in rapid detection kits as well as biosensors. From the perspective of this review, nanomaterials are driving the development of rapid detection and their position in the field of detection is gaining ground with each passing day. The keywords “PCR”, “biosensor”, and “nanomaterials” were used and analyzed for all the retrieved articles. Figure 1 shows that the reports on “nanomaterials” had an increasing trend, unlike the other two keywords “biosensors” or “PCR”, indicating that nanomaterials with good performance are favored by more researchers.
Since the outbreak of COVID-19, many researchers have developed plenty of methods for rapid detection of SARS-CoV-2 and its variants, most of which rely on the development of nanotechnology that makes it possible to go beyond traditional RT-qPCR. In this article, we summarized recent reported methods so far for the rapid detection of SARS-CoV-2 based on nucleic acid amplification technology (NAAT) and lateral flow assay (LFA), and biosensor (Fig. 3). We compared these methods according to targets, testing principles and analytical performance, and provided an outlook on those methods for the rapid detection of SARS-CoV-2. The goal of this review is to explore recent rapid detection developments that are designed for specific detection of the full virus, viral protein, or antibodies against viral antigens from viruses. Given the large number of publications in this field, each section focuses on different techniques associated with rapid detection of specific viruses that mostly emerged in the last four years, especially related to SARS-CoV-2. This review will improve the management of the COVID-19 pandemic by encouraging people to self-quarantine, by preventing the spread of the virus, and by helping all prepare for future pandemics by allowing for faster response times.
2 Testing principles
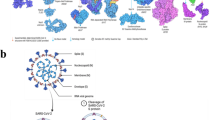
Based on the biological structure of the SARS-CoV-2 (Fig. 4), there are three major methods to detect SARS-CoV-2: RNA, antigen (Ag) and antibody (Ab) [32]. Antigens include the spike protein (S), the envelope protein (E), the membrane protein (M), and the nucleoside protein (N). Methods that detect the RNA are mostly NAAT such as RT-qPCR and reverse transcriptase loop-mediated isothermal amplification (RT-LAMP), both of which have excellent sensitivity and selectivity. In comparison to RT-qPCR, RT-LAMP, proposed by many researchers, does not require use of expensive equipment and an RNA extraction step while reducing overall costs by speeding up the detection time in about 30–45 min [33,34,35,36]. However, RT-LAMP may produce false negatives due to improper sampling, transport, or handling. In addition, it may not be suitable for detection of mutated viruses. Notably, the false negative rate can be reduced by optimizing the NAAT process, such as adding a nucleic acid enrichment step, multiplex RT-qPCR, or creating a one-pot cyclic probe-mediated isothermal amplification protocol that combines the amplification and detection processes [37,38,39,40]. The rapid antigen test (RAT) targeting viral proteins has been shown to be used for the detection or monitoring of close contacts and high-risk groups with advantages of being easier, faster, and less costly, and disadvantages of being less sensitive than nucleic acid-based molecular tests [41, 42]. The sensitivity of RAT depends on the viral load of the sample based on data from a study suggesting that the sensitivity is only achieved when the viral load of the sample is high: the sensitivity is 90% for the cycle of quantification (Cq) range of 20–25 for RT-qPCR, and only 10% for the Cq range of 25–30 [43]. Abs test differs from other tests because it not only detects whether a person is infected but also reflects responses of the host after being vaccinated [32]. In summary, highly sensitive RNA assays and time-saving antigen assays are used to diagnose viruses, while Ab assays are used to aid in diagnosis and response to vaccine response.

Copyright 2022 Elsevier. b Reprinted with permission from ref. 48. Copyright 2022 American Chemical Society. c Reprinted with permission from ref. 49. Copyright 2022 Royal Society of Chemistry. d Reprinted with permission from ref. 50. Copyright 2022 John Wiley and Sons. e Reprinted with permission from ref. 51. Copyright 2022 American Chemical Society. f Reprinted with permission from ref. 52. Copyright 2022 Multidisciplinary Digital Publishing Institute. g Reprinted with permission from ref 53. Copyright 2022 American Chemical Society. h Reprinted with permission from ref 54. Copyright 2022 Elsevier. i Reprinted with permission from ref. 55. Copyright 2022 Elsevier. j Reprinted with permission from ref 56. Copyright 2022 Elsevier. k Reprinted with permission from ref 57. Copyright 2021 Springer Link. l Reprinted with permission from ref 58. Copyright 2022 Elsevier
Biological structure and of the SARS-CoV-2 and enhanced biosensor by nanomaterials. a Reprinted with permission from ref. 47.
Besides the above three principles for detection, there are other new but not mature testing strategies. For example, because patients infected with COVID-19 could exhale characteristic volatile organic compounds (VOCs), including 2,4-octadiene, 1-chloroheptane, nonanal(1a) and methylpent-2-enal (1b), a colorimetric method could be used to detect VOCs to determine the infection of COVID-19 with the advantages of being rapid, painless for asymptomatic infected patients [44]. Another way is to detect the main protease (Mpro) because it is specific to SARS-CoV-2 during replication and transcription. Jin et al. [45] created a label-free peptide (ZY7) with a net neutral charge that could decompose into positively charged fragments in the presence of Mpro, causing color changes in aggregation of negatively charged bis (psulfonatophenyl) phenylphosphine-modified gold nanoparticles (AuNPs), which is fast and convenient. Gut microbiota-Fusicatenibacter, as a very sensitive biomarker during SARS-CoV-2, may also become a new diagnostic tool. Hence, there is no relevant report available [46]. RNA, Ag, and Ab were used as target detectors in the method described in this paper.
3 Rapid detection methods
3.1 Methods based on NAAT
The gold standard method of NAATs, RT-qPCR, has evolved towards rapid, convenient, or simple techniques. Naranbat et al. [59] proposed a method characterized by the absence of viral (universal) transport medium and RNA extraction steps, which could greatly simplify the entire process such that test results could be available within only 1 to 2 h. Lee et al. [60] developed a deep learning model using the fluorescence values in each cycle of RT-qPCR, making sensitive predictions before the RT-qPCR results were available. Delpuech et al. [61] proposed to heat and inactivate SARS-CoV-2 samples prior to laboratory processing to reduce the overall cost, testing time, as well as safety hazard issues with less than 1 Cq loss in sensitivity compared to standard RT-qPCR. Chen et al. [62] developed a water-bath PCR that can quickly achieve thermal cycling and simultaneously detect SARS-CoV-2 with fluorescent LFA to make the whole process both faster and more sensitive. As an emerging detection technique, Digital PCR (dPCR) does not rely on a standard curve for the quantification of nucleic acid molecules and is highly sensitive for absolute quantification of RNA. It is even more reliable than RT-qPCR for the detection of SARS-CoV-2 in low viral load specimens or in wastewater [63,64,65]. Yolda-Carr et al. [66] developed a portable, real-time PCR device for the detection of SARS-CoV-2 in saliva samples, which consists of the SalivaDirect protocol [67] combined with the Ubiquitome Liberty16 system. This device could be connected to a smartphone to generate real-time test reports, which is more convenient, faster with improved sensitivity. For the detection of SARS-CoV-2 variants, one common method is to sequence the whole-genome. However, sequencing an entire genome requires relatively high costs. To address this problem, researchers [68, 69] established an RT-qPCR assay using the receptor-binding domain RNA of the spike protein of the SARS-CoV-2 variant as specific primers and probes. Xiong et al. [70] found two mutations, C1709A and C56G, that are specific to the genomes of Alpha and Delta variants. They established an amplification refractory mutation system combined with quantitative reverse transcription-qPCR based on these mutations, being able to complete full detection within 2.5 h. Dächert et al. [71] reported that the combination of variant‑specific PCR and nanopore-based full-length genome sequencing enabled not only rapid detection of the Omicron but also sensitive identification of newly emerging variants. Nucleic acid amplification on a chip is a highly viable potential technique for simplifying PT-qPCR while maintaining high sensitivity, which increases the possibility of rapid and accurate molecular diagnostics at home [72]. Another research work by Lee’s group [73] designed a multiplex RT-qPCR capable of simultaneously detecting SARS-CoV-2 and partial variants and integrated a microfluidic chip-based as a platform to reduce the detection time by more than half. For the RT-qPCR to be further improved, (1) optimization in the thermal cycling with precise temperature control and removal or reduction in the RNA extraction process are two important ways to shorten the overall detection time, (2) updates to the readout method is crucial to make RT-qPCR more portable, and (3) incorporation of multiplex RT-qPCR is an important means to detect mutant strains.
In addition to RT-qPCR, other methods of NAATs were also used for detection of SARS-CoV-2 (Fig. 5), among which RT-LAMP is widely used. Compared to RT-qPCR, the RT-LAMP assay process is faster in detection time, simpler in operation, and lower in overall cost. Many researchers have developed convenient and visualized assays that utilize RT-LAMP, making the whole process from sample to results less time-consuming. Several studies have reported LFA for RT-LAMP combined with CRISPR-Cas12 for SARS-CoV-2, which does not require thermocycling steps for amplification of the specific targeted nucleic acid while maintaining the selectivity and sensitivity levels [74, 75]. The advantage of this method is less time-consuming and could be visually detected by naked eyes. Colbert et al. [76] paired RT-LAMP with particle diffusometry, a particle imaging technique, to detect SARS-CoV-2, which means that just one smartphone device can be used for on-site testing. Iijima et al. [77] presented for the first time the detection of the L452R spike mutation by RT-LAMP coupled with a bioluminescent assay in real-time, which implies that RT-LAMP-based detection of mutant viruses is possible. In short, RT-LAMP for isothermal amplification of nucleic acids greatly compensates for the time-consuming problem of RT-qPCR.
The development of isothermal amplification technology has diversified the methods for NAAT-based detection of RNA. Shanmugakani and Wu [78] developed a reverse transcription helicase-dependent amplification (RT-HDA)-coupled dipstick technique, which does not require thermal cycling or expensive equipment while saves time. Researchers also reported detection of SARS-CoV-2 based on a rapidly integrated recombinase polymerase amplification (RPA), which is a novel isothermal amplification technique to complete amplification in 15–20 min [79,80,81]. Li et al. [82] used primer exchange reaction (PER) to amplify nucleic acids, which was combined with CRISPR-Cas12 for rapid detection of SARS-CoV-2. Since PER is performed by automatic extension of short primers to sequence-specific single-stranded DNA after a target-catalyzed hairpin template in the presence of a strand displacing polymerase, it is faster and easier than reverse transcription-mediated amplification. However, expensive and heavyweight equipment on nucleic acid amplification is still a common problem for NAAT assays.
3.2 Rapid diagnostic test kit
3.2.1 Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) is often used for the detection of viral antibodies and has been developed as a rapid diagnostic test kit due to its ease of operation and use of inexpensive equipment. In ELISA of SARS-CoV-2, different structural proteins could be used as Ag to detect the corresponding antibodies and researchers have developed ELISAs with good sensitivity and specificity [83,84,85,86]. Using microfluidic technology, González-González et al. [87] developed an automated ELISA chip for detecting antibodies to SARS-CoV-2, enabling on-site testing that may only require a smartphone with a camera. Kasetsirikul et al. [88] invented a paper-based ELISA for detection of antibodies to SARS-CoV-2, which could significantly reduce costs and make the test faster than conventional ELISAs. It could be completed within 30 min. Due to the increase in vaccination and cured patients, the SARS-CoV-2 Ab test cannot be used as a diagnostic tool but only as a diagnostic aid or a way for post-vaccination evaluation. Therefore, researchers have developed ELISA-based RAT. Domenico et al. [89] prepared a rapid test kit for simultaneous detection of two antigens using a double antibody sandwich method, which has the advantage of being fast at about 30 min, simple, and directly observable with the naked eye, but it could not detect Ag at low concentrations. To some extent, ELISA is able to characterize viruses in a more time-efficient and portable way than the gold standard while it might not specifically detect the RNA of viruses.
3.2.2 Lateral flow immunoassay
Compared to ELISA, lateral flow immunoassay (LFIA) is more stable because of labels, such as AuNPs and fluorescein isothiocyanate on a paper-based diagnostic platform, which makes it more suitable for commercialization. The utilization of nanoparticles as labels has gained attention in developing rapid diagnostic test kits for improved diagnosis and treatment. The conventional LFIA device are generally composed of three major parts, i.e., substrate based on papers, antibodies or antigens as detection element and reporters as signal-transforming element (Fig. 6). The fabricated structure, principle and detection mechanism of LFIA are also shown in Fig. 6. The structure of LFIA generally consists of sample pad, conjugated pad, test pad, absorbent pad and backing pad. When an assay is carried out on a LFIA, a small volume of sample is dropped onto the pad, migrates on the conjugated pad, then carries conjugated particles to the test pad. Target such as in the given sample are recognized and bonded with detection antibodies on reporter surface in conjugated pad, where complexes interact with capture antibodies on test line and free reporters bound on control line. LFIA with flow-through immunoreactivity on a Nitrocellulose membrane specifically recognizes SARS-CoV-2 antigens and antibodies and produces an optical signal visible to the naked eye. The control line is designed to improve the specificity of the assay and thus avoid false negatives.
Schematics of a typical LIFA for COVID-19 diagnostics. a Components of a LIFA test kit. b Detection principles of Ag test and Ab tests. c Mechanisms for the functioning of LFIA. Reprinted with permission from ref 181.
The key issue of LFIA is the relatively lower detection sensitivity and efficiency that still needs further improvement. Notably, nanomaterials are a decisive factor and a significant contributor to improve the performance of COVID-19 rapid diagnostic kits [90]. Peng et al. [91] enhanced the sensitivity by depositing copper on AuNPs-labeled LFIA test papers, resulting in a detection limit of 10 pg/mL for this RAT. Szekely et al. [92] combined carboxy gold nanoshells with antibodies to form stable conjugates to target low mutation rate ‘N’ to obtain a rapid diagnostic test kit with sensitivity comparable to RT-PCR. Lee et al. [93] developed an LFIA-based sandwich immunoassay for determination of antibodies to SARS-CoV-2, using colored cellulose nanobeads to label secondary antibodies in the sandwich structure to reflect the presence of antibodies to neo-coronavirus. Chen et al. [94] successfully labeled ‘N’ with selenium nanoparticles and developed a rapid LFIA-based test for detecting antibodies to SARS-CoV-2 with results readable within 10 min. Zhang et al. [95] reported two highly sensitive LFIAs for the detection of SARS-CoV-2 receptor binding domain (RBD) and ‘N’ using AIE luminophores with good optical properties and less susceptibility as a fluorescent label. Duan et al. [96] applied ratiometric fluorescent analysis for dual-detection LFIA for the first time, using carboxyl-functionalized Europium chelate nanoparticles to label the RBD and set up human angiotensin-converting enzyme 2 (hACE2), which can bind to RBD as a calibration line. Their results showed higher precision and sensitivity with a wider dynamic linear range for Ab detection. Dighe et al. [97] used antisense oligonucleotides labeled with 6-carboxyfluorescein and biotin to specifically identify SARS-CoV-2 genes as probes using AuNPs capped with cysteamine as control signals to improve the sensitivity of this LFA-based detection of SARS-CoV-2 RNA. The possible downside of this approach is lower sensitivity and false positives [98,99,100]. Therefore, a top priority is to develop highly sensitive LFIA-based rapid detection kits. LFIA test strips are considered the economical alternative for the instant diagnosis of COVID-19 in public health centre [198]. The compositions and properties of these components are closely related to the performance of paper-based POC immunoassays. (e.g., traditional paper and emerging paper materials) and principles (e.g., interface). More research is focused on promoting high-throughput immune-analyzers for mass screening. The sensing principle involves detecting analytes (could be an Ag or Ab) with the help of secondary antibodies conjugated with labels such as gold nanoparticles, fluorescent molecules and quantum dots, promoting visual sensing of color changes.
The key issue of LFIA is the relatively lower detection sensitivity and efficiency that still needs further improvement. The biosensor for SARS-CoV-2 virus or other targets based on LFIA with lower prices, better stability and lower detection limit. There is no doubt about LFIA will bring a huge effect to the present POCT market.
LFIA-based rapid diagnostic test kits are now commercially available with the form of rapid diagnostic kits on the market, most of which are LFIA-based RATs. Compared to RATs, rapid antibody test kits are inferior as a diagnostic tool but they could be used as a screening tool [101, 102]. Some investigators evaluated the limits of detection (LOD) of different kits and showed that most of them are sensitive to detect SARS-CoV-2 [103,104,105,106]. Many researchers have compared and evaluated these kits with PCR and found that almost all kits on the market have 100% specificity, but sensitivity varies widely, with only a very few having relatively higher sensitivity than others [107]. The sensitivity of RAT highly depends on the viral load and high viral loads leading a sensitivity of over 90% for RAT [108]. Table 1 indicates that several antigen detection kits are currently available on the market: STANDARD Q COVID-19 Ag tests (SD Biosensor Inc., Korea), Panbio™ COVID-19 Ag Rapid Test Device (Abbott, Germany), etc. Table 1 also shows that the target of the RATs is ‘N’ and the detection time is mostly around 15 min. It has high sensitivity and meets WHO requirements (at least 80% sensitivity and 97% specificity compared to RT-qPCR) in symptomatic patients or exposed individuals. However, for asymptomatic populations or areas with low infection rates, the sensitivity is not comparable to that of RT-qPCR [109]. It is worth noting that although RAT sensitivity is lower compared to RT-qPCR, the use of RAT will reduce much of the burden. In the near future, RAT may shorten the isolation time in the face of a huge number of people needs to be tested [110, 111]. The collection of samples from different sites (nasopharyngeal swabs, nasal swabs, and saliva) also had a greater effect on sensitivity, with nasopharyngeal swabs having the best sensitivity and saliva having the worst sensitivity. Even though the sensitivity of saliva is poor, it can reduce the pain of the subject during sampling, so it makes sense to develop a highly sensitive saliva test kit. In addition, combinatorial antigen test kits enable the combination of two antigen test kits, which have been shown to increase sensitivity [112]. The sensitivity of the available RATs has been reduced for mutant strains, but they are still an irreplaceable screening tool because of their high efficiency [113]. In general, commercially available RATs are highly variable for various reasons including viral load, variant strains, target population, etc. despite their outstanding high efficiency.
In the critical context LFIA has been commonly used for POCT of SARS-CoV-2 due to its low cost and portability, which could be an instrumental factor in the successful fight against the outbreak [182]. Although LFIA-based rapid test kits can fulfill the criteria for POCT, it still confronts some challenges such as improved sensitivity, poor stability and inability to detect nucleic acids [29]. Similarly, NAAT-based SARS-CoV-2 POCT tends to be used in healthcare settings rather than for self-testing [183]. Accordingly, novel ideas for better use in SARS-CoV-2 POCT have been proposed. For example, Ran Liu et al. [184] combined a CRISPR-Cas12-based assay for nucleic acids and a portable meter, and finally have refined the detection of N in the system, which enables patients to quantitatively test for a wide range of SARS-CoV-2 markers at home with a portable device. It is worth noting that SARS-CoV-2 POCT, centered on biosensing that is free from laboratory dependence, is being developed with great enthusiasm and has the potential to be generalized in the future. The structural design using biosensing and the introduction of smartphones can satisfy the demands of POCT even more, but its commercialization needs to take into account cost, biosafety, data security, and stability [185]. In addition, microfluidic chip-based POCT for SARS-CoV-2 is considered an ideal diagnostic tool for pandemic response. Its small dimensions and portability, high detection efficiency and considerable commercialization value have increased its popularity, which has led to the development of a variety of microfluidic platform-based designs and assays including nucleic acid amplification, immunosensors and biosensors [186,187,188]. Overall, POCT has significant implications for pandemics similar to SARS-CoV-2, and despite the development of multiple portable devices for POCT in addition to LFIA, there are serious challenges in actual commercial development.
3.3 Biosensors
3.3.1 Electrochemical biosensor
Electrochemical biosensors are capable of rapidly converting biological signals into electrical signals. They can provide enhanced selectivity and sensitivity and are widely used in virus detection because of shorter reaction times and use of less sample volume. Even so, there is still a huge challenge regarding its signal amplification, stability, and commercialization [26, 126]. Among the electrochemical biosensors to detect SARS-CoV-2, immunoimpedance biosensors for Ag and Ab detection are preferred (Fig. 7a). Its sensitivity could be enhanced by modifying the electrodes using nanoscale materials with good conductivity such as AuNPs and single-walled carbon nanotubes [127, 128]. Screen-printed carbon electrode (SPCE)-based biosensors are compact, fast, and low cost with the value of potential commercialization [129, 130]. Haghayegh et al. [53] modified buffer-based zinc oxide/reduced graphene oxide on the SPCE surface to increase the electrical signal, and this RAT was able to detect ‘N’ within 15 min. Soto and Orozco [131] developed an immunoimpedance biosensor by modifying functionalized processed peptides capable of specifically recognizing ‘S’ on screen-printed gold electrodes. Polypyrrole is favored by researchers for its better surface area, high electrical conductivity and electrochemical activity, and its synthesized nanotubular form with better properties than the spherical form as a substrate [47]. Mehmandoust et al. [55] synthesized SiO2@UiO-66 nanocomposite as a metal–organic framework and modified it on SPCE to greatly improve the conductivity of the electrode, which is capable of sensitively detecting SARS-CoV-2 Ag. The road to commercialization is not far away with the increasing development of screen-printed, electrode-based impedance immunosensors.
For current and voltage biosensors, biosensor sensitivity and convenience can also be improved by modifying materials with excellent properties or selecting materials with better structural properties as substrates. Zhao et al. [54] modified colloidal quantum dots with increased surface area and dangling bonds on the electrode to firmly adsorb the SARS-CoV-2 Ag, and the electrochemical biosensor was able to detect SARS-CoV-2 antibodies in less than one minute. Liv and Kayaba [50] prepared a gold cluster and Ag modified on a glassy carbon electrode (GCE) for the detection of antibodies to SARS-CoV-2. Kim et al. [132] developed a RAT with a LOD of 1.17 fg/mL by immobilizing an Ab on a dual-gate oxide semiconductor thin-film transistor that amplifies electrical signals as a substrate. Whether voltage biosensors, current biosensors or impedance biosensors, most of them use antigen–antibody specific binding to detect Ag or Ab.
However, the electrochemical biosensor-based approach to detect SARS-CoV-2 is not limited to Ag and Ab detection and can directly detect RNA without relying on NAAT. Heo et al. [133] immobilized the reporter RNA labeled with methylene blue and biotin labeled ends on a SPCE modified with nanocomposites and gold nanoflowers to form an electrochemical aptamer biosensor for the detection of SARS-CoV-2 RNA (Fig. 7b). The biosensor incorporated CRISPR-Cas13 and formed a complex of the target RNA with Cas13a-crRNA, which cleaved the reporter RNA immobilized on the electrode to produce a change in the electrical signal.
To date, an increasing number of electrochemical biosensors emerged for the detection of SARS-CoV-2. Park et al. [134] applied multiple vertically paired electrodes to develop a capacitive biosensor for detection of SARS-CoV-2 Ag with higher sensitivity than conventional capacitive biosensors based on interdigitated electrode. Jiang et al. [135] modified a magnetic capture probe on a screen-printed gold electrode and then hybridized Ru(bpy)32+-labeled signal probe with electrochemiluminescence (ECL) signal to the SARS-CoV-2 RNA. The obtained biosensor could specifically identify the RNA and generate a highly sensitive ECL signal with the detection range of 0.1 fM to 10 µM. McClements et al. [48] used molecularly imprinted polymer nanoparticles to create an imprint of SARS-CoV-2 Ag and modified it on a screen-printed electrode to detect the Ag, a molecularly imprinted biosensor that is more stable and reliable and can deliver results within 15 min. Yet these biosensors were apparently developed without attention to their commercialization possibilities despite their outstanding sensitivity and innovation.
Field-effect transistor-based biosensors (BioFETs) are highly sensitive, have a wide detection range, and can be made ultra-sensitive with high electron mobility transistors. However, they are often limited by their high cost, poor reproducibility, and lack of portability [136,137,138]. Researchers have combined BioFET with enzymatic reactions to collect electrical signals generated by changes in pH of the solution to detect SARS-CoV-2 Ag or antibodies, and have found that the application of phosphatase is more stable than the application of urease [139]. Chen et al. [140] have developed a portable biosensor for in situ detection of N in saliva based on an electrical double-layer gated BioFET system, which can be read on an iPhone through a portable reader. Electromechanical biosensors formed by the combination of microelectromechanical systems and field-effect transistors have ultra-high sensitivity. Researchers have developed BioFET for the detection of SARS-CoV-2 RNA using carbon nanotubes as the substrate and RNA hybridization as the signal generator [141]. Wang et al. [142] created an ultra-fast and portable electromechanical aptamer biosensor for ultra-fast detection of SARS-CoV-2 RNA that does not require nucleic acid amplification by using flexible single-stranded DNA linked by rigid tetrahedral double-stranded DNA as a probe. Because of its important entry level among all electrochemical biosensors, development of portable and low-cost commercially available BioFETs have attracted a lot of researchers.
3.3.2 Optical biosensors
The optical biosensors currently used for rapid detection of SARS-CoV-2 are colorimetric and immunofluorescent biosensors as well as biosensors based on spectroscopic techniques. The biggest advantage of colorimetric and immunofluorescence biosensors is that the results are usually visible to the naked eye (Fig. 7c). Mohamad Mahani et al. [143] reported the FRET-based aptasensor for interleukin-6 as a biomarker for COVID-19 progression using nitrogen-doped carbon quantum dots and gold nanoparticles. Alhadrami et al. [144] reported a colorimetric biosensor of using a cotton swab as a substrate to collect the detected S through the lactoferrin general capture agent, it could specifically bind an orange nanopolymer-modified Ab to produce an optical signature visible to the naked eye, which is available in 5 min and suitable for field detection. The label-free detection of SARS-CoV-2 spike protein is demonstrated by using slightly tapered no-core fiber (ST-NCF) functionalized with ACE2.The ACE2-immobilized ST-NCF sensor head was exposed to the samples of SARS-CoV-2 spike protein with concentrations ranging from 1 to 104 ng/mL [145]. Kang et al. [146] developed a hairpin structure of hACE2 mimetic peptide beacon, which has only a weak fluorescence signal due to the fluorescence resonance energy transfer effect in the normal state, and the hairpin structure is opened to generate a fluorescence signal when affected by S. The whole process could be completed within 3 h. These two types of biosensors are usually combined with LFIA to form the rapid detection kits mentioned in this paper. Feng Long constructed a new all-fiber Fresnel reflection microfluidic biosensor which was constructed through combining all-fiber optical system, microfluidic chip, and multimode fiber bio-probe. The limits of detection of SARS-CoV-2IgM and SARS-CoV-2 IgG were 0.82 ng/mL and 0.45 ng/mL, respectively [147].
The use of spectroscopic techniques such as dynamic light scattering and surface-enhanced Raman scattering (SERS), which are faster, more economical, and more accurate than traditional detection methods, for the detection of SARS-CoV-2 is just around the corner [148,149,150]. Kawasaki et al. [151] used an imprinted photonic crystal film as a substrate and immobilized antibodies on its surface to identify Ag with high sensitivity, and finally performed simple reflectance measurements by an optical device equipped with a spectrometer in a smartphone. Hadi et al. [152] combined U-Bent plastic optical fiber with nanogold to immobilize the Ab and used it as a probe to build a fiber optic biosensor, and finally diagnosed the presence of N by the change of the biosensor optical intensity. Optical biosensors based on surface plasmon resonance are increasingly used for the detection of new coronaviruses due to their high sensitivity [153]. Rahmati et al. [154] reported a new detection strategy which was used to improve the sensitivity of SARS-CoV-2 spike receptor-binding domain based on a lateral flow immunoassay platform utilizing a delayed hydrophobic barrier fabricated. Zheng et al. [52] developed a localized surface plasmon resonance (LSPR) biosensor based on a vertical microcavity with nano-porous gold modified on its surface and immobilized with antibodies to the SARS-CoV-2, which generates an optical signal when the target Ag is captured by the biosensor. Liang et al. [155] combined the LSPR biosensor with optical imaging and artificial intelligence methods to be able to detect new coronaviruses within 12 min. These types of optical biosensors are moving towards portability and commercialization because of their high sensitivity, possibility to save time, and low cost compared to electrochemical biosensors.
3.3.3 Nanomaterials for biosensors
3.3.3.1 Variety of nanomaterials
Biosensors tremendously advanced for the detection of virus, pathogens and microorganisms [156]. Nanomaterials commonly used in the fabrication of biosensors to improve their performance [157], where they can improve the true positive rate of biosensors and in the green synthesis to make biosensors sustainable and environmentally compatible [158, 159]. Figure 4 shows various nanomaterials used as biosensors for the detection of SARS-CoV-2. Noble metal and inorganic metal oxides nanoparticles assume an important role in improving sensitivity and accuracy, such as nano-gold, nano-silver, and nano-zinc oxide [160,161,162], but bimetallic nanomaterials can greatly increase the performance of biosensors compared to the monometallic as excellent signal amplifiers, and some are even called nanozyme because of their catalytic properties [163]. The stability and high activity of such nanozyme also make it convenient to store them for a long time, which makes it possible to use them as specific identifiers for the preparation of biosensors [164]. Nevertheless, low biocompatibility is a fatal drawback for metallic materials, the use of carbon-based materials can overcome this limitation and it has been shown that the use of carbon materials as substrates is a potential area of rapid development for biosensors [160, 165, 166].
When it comes to carbon-based materials, carbon nanotubes with high specific surface area, good electrical and thermal conductivity have been widely used for biosensors [167], especially, graphene nanomaterials with excellent biocompatibility have become a new buzz with vast attention, and graphene-based electrochemical biosensors with ultra-precise detection capabilities have been heavily worked on [168,169,170,171,172]. Graphene and its derivatives are undoubtedly desirable materials for the construction of efficient virus detection biosensors. Wei Li Ang et al. [56] discussed the use of graphene nanocolloids as electroactive materials to develop an electrochemical biosensor for the detection of SARS-CoV-2 RNA in the range of 10–10 M to 10–5 M. Furthermore, MXenes with unique two-dimensional structure and good electrical conductivity and ductility, have become attractive materials in the development of biosensors, but they have synthetic material waste disposal and mass production stability problems still to be solved [173, 174].
3.3.3.2 Application of nanomaterials
Nanomaterials are applied in the diagnosis of COVID-19 through portable colorimetric devices and biosensors with various transduction mechanisms. AuNPs and its complexes have been widely used in rapid test kits and colorimetric sensors to make the test results visible to the naked eye. Precious metals, carbon-based nanomaterials and conductive polymers with favorable electrical conductivity as well as the limitations and accuracy of detection enhance the electrochemical activity of electrochemical sensors such as voltammetric and impedance biosensors [201]. For optical sensors such as FRET, SERS and LSPR sensors nanomaterials with excellent optical properties have great applications [52, 143, 148]. Besides, nanomaterials have been continuously utilized in sensors such as ECL sensors, FET sensors, and so on [203]. Notably, magnetic nanomaterials were successfully applied to amplifier of genome. S B. Somvanshi et al. [206] report the fabrication and application of surface-functionalized magnetic zinc ferrate nanoparticles for the rapid detection of SARS-CoV-2 RNA, and the proposed model allows RNA extraction from multiple samples. It has to be recognized that nanomaterials are being applied to a wider category of biosensors.
3.3.3.3 Function of nanomaterials
Five kinds of nanomaterials including nanoparticles, nanowires and nanorods, carbon nanotubes, and quantum dots have been currently well utilized in biosensors to improve sensing efficiency and diagnostic sensitivity [167]. Nanomaterials in biosensors mainly take advantage of structural, conductive, and optical properties to obtain a larger specific surface area as well as to increase the rate of electron transfer, similarly, nanoparticles such as AuNPs are often used as signal transducers or nano-lanterns to become an important component in electrochemical and optical sensors [189,190,191,192].
With the advancement of nanotechnology, more and more functions of nanomaterials are being developed for the construction of biosensors and play an even more irreplaceable function in the diagnosis of COVID-19. Nanomaterials with excellent construction can contain and modify biomolecules in a superior manner. Nanomembrane graphene was synthesized by binding AuNPs and nano-islands on reduced graphene oxide, which is capable of remarkable binding of S and Ab with an affinity constant of 0.93 × 109 M−1 [193]. Andrei Pligovka et al. synthesized complexly structured two-level 3D cylindrical nanomembranes by stepwise oxidation, and the optical properties of this material may have great potential for application in label-free optical biosensors [194]. Secondly, nanomaterials possessing specificity can serve as receptors instead of less stable biomolecules as core members of the sensing mechanism. Biosensors based on biomimetic nanomaterials as well as molecularly imprinted nano-polymers as recognition systems for the detection of SARS-CoV-2 have been developed, which opens a new chapter in the integration of nanomaterials into biosensors [195, 196]. In addition, nanofiber membranes fabricated based on electrostatic textile technology are fine flexible substrates for wearable sensors for diagnosis of COVID-19 [197]. Altogether, nanomaterials in future research may act as substrates, receptors, signal transducers and powerful bio-binders thus becoming an integral part of biosensors.
4 Conclusion and future perspectives
To date, methods for rapid detection of SARS-CoV-2 fall into three broad categories: NAATs, rapid diagnostic test kits, and biosensors. NAAT based methods were mainly used to detect SARS-CoV-2 RNA by reducing the required time to improve PCR or using more rapid nucleic acid amplification techniques such as LAMP, RT-HDA, and RPA. There are two types of rapid diagnostic test kits, one that utilizes ELISA (which usually requires labeling with enzymes) and another that uses LFIA (which tends to use more stable chemical labels). While ELISA is only suitable for detection of SARS-CoV-2 antigens and antibodies, LFIA is dominated by RAT although it is also proposed for RNA detection. For detection of variants, in contrast, researchers tend to use less mutable N as the target of RAT thus avoiding false negatives. Biosensors are the preferred detection platform for researchers due to their good sensitivity and selectivity as well as the short detection period. They could be divided into electrochemical biosensors and optical biosensors depending on the output signal. Their targets could be Ag, antibodies, and RNA. With the continuous development of biosensors, a variety of biosensor platforms have emerged for the detection of SARS-CoV-2, among which BioFET, screen-printed sensors, and optical biosensors based on SPR are more favorable and generally used.
The performance of biosensors could be affected by different sensing methods and modification materials. First of all, receptors such as antigens, antibodies, aptamers, and molecularly imprinted polymers as recognition systems targeting SARS-CoV-2 play a decisive role in the performance of the sensors. Although immune response-based sensors have been developed and are well established for the diagnosis of COVID-19, the inability to detect RNA as well as poorly stabilized antigens and antibodies has been a hindrance to development. In contrast, more stable aptamer sensors are robust in detecting different types of SARS-CoV-2 biomarkers (RNA and Ag) and have excellent specificity to reduce false positives [199]. Table 2 shows the advantages and disadvantages of the two bioreceptors. The advantages of aptamers as bioreceptors are the ability to target a variety of biomarkers, easily synthesized in large quantities and great stability, whereas high specificity and short time-consumption are the benefits of recognition based on immune response [198]. Different from the above bioreceptors, easily synthesized polymers containing specific molecular imprints for targeting SARS-CoV-2 are less susceptible to environmental factors and thus greatly improve sensor stability, yet such asynchronous sensors have not been extensively investigated [200]. The biosensors need to compensate for the disadvantages and develop further research directions to develop cost-effective POCT for the diagnosis of COVID-19. For another, nanotechnology is a common means to improve biosensor performance. For example, AuNPs are often used as labels in optical biosensors to improve biosensor sensitivity [176,177,178,179]. Nanomaterials in future research may function as substrates, receptors, signal transducers and powerful bio-binders thus becoming an integral part of biosensors. For portability, the combination of microfluidics and screen-printing technology make the biosensor detection platform smaller and more reliable even using a smartphone, making it more suitable for rapid detection in the field.
We have compared the above rapid detection methods for SARS-CoV-2 and the performance of the different biosensors (Table 3). For the detection of RNA, we found that NAAT-based methods were more sensitive with lower detection limits, but the biosensor-based method has the shortest time of 4 min, while for the detection of Ag and antibodies, the biosensor platform has both higher sensitivity and much shorter time (1 min). Therefore, the biosensor platform is far ahead of other methods in terms of time. In addition, many of the biosensor-based assays are capable of quantitative analysis of COVID-19, which is impossible with rapid diagnostic test kits.
Among the methods for rapid detection of SARS-CoV-2, the biosensor platform is a promising diagnostic method for future applications because it can accurately detect SARS-CoV-2 and variants in very short time and does not require expensive instrumentation and specialized technicians. Despite superior performance of the biosensor in all aspects, the problem of commercialization still exists. The cost of biosensor fabrication, the difficulty of mass production, and stability in use all need to be considered for widespread commercialization. Advances in nanotechnology within the last few decades have resulted in major improvements in electrochemical biosensing making them simple and efficient tools to measure the concentration of analytes and the detection of pathogens. Moreover, further work has been performed to miniaturize biosensors and make them portable, cost-effective, and reduce the sample size. These improvements have made electrochemical biosensors more and more attractive for developing POC tools with the help of development of nanotechnology [180]. More efforts have been put on improving sensitivity, enhancing portability, and reducing costs.
Compared with related categories [201,202,203], we focus on biosensors for rapid detection of SARS-CoV-2 based on nucleic acid amplification and LFIA to find novel directions from already established diagnostic technologies. The innovation of this review is the application, development and perspectives of nanomaterials in the rapid detection of biosensors. In particular, our perspective is more oriented towards the pragmatic application in the context of the COVID-19 pandemic, i.e., we compare and discuss already commercialized rapid test kits and describe emerging rapid tests with strong potential for commercialization. The application and development of diagnostic techniques in the context of a COVID-19 pandemic may provide a reliable reference for respiratory-transmitted viruses such as influenza virus, measles virus, and varicella-zoster virus. In fact, sensor platforms, which are considered to have great potential, have already been applied to the rapid detection of a variety of viruses other than SARS-CoV-2 [204, 205], and the novel sensing methods brought about by SARS-CoV-2 may provide new insights into the diagnosis of other diseases. We expect to accumulate experience in diagnostic techniques in the context of the COVID-19 pandemic in order to cope with future epidemics or outbreaks of novel viruses. Moreover, with the advancements in nanomaterial science and techniques, the novel coronavirus diagnosis will be updated in the coming time. The scope of development of a robust and rapid sensor for infectious diseases should be with successful commercialization and mass-scale production. In addition, the rapid assays of virus test results of patients could be recorded and researcher can monitor the health patients in real-time and take measures if necessary. It is conceivable that these biosensors will play a crucial role in controlling infectious diseases and public health. With this trend, the future will witness more breakthroughs and pioneering detection methods in attacking different viruses such as SARS-CoV-2.
Availability of data and materials
All data needed to evaluate the conclusions in this paper are either present in the paper or directly derived from the cited references.
Abbreviations
- RT-qPCR:
-
Reverse transcription-quantitative polymerase chain reaction
- Ct:
-
Cycle threshold
- NAATs:
-
Nucleic acid amplification technologies
- LFA:
-
Lateral flow assay
- Ag:
-
Antigen
- Ab:
-
Antibody
- S:
-
Spike protein
- E:
-
Envelope protein
- M:
-
Membrane protein
- N:
-
Nucleoside protein
- RT-LAMP:
-
Reverse transcriptase loop-mediated isothermal amplification
- RAT:
-
Rapid antigen test
- Cq:
-
Cycle of quantification
- VOCs:
-
Volatile organic compounds
- Mpro:
-
Main protease
- AuNPs:
-
Gold nanoparticles
- dPCR:
-
Digital PCR
- RT-HAD:
-
Reverse transcription helicase-dependent amplification
- PER:
-
Primer exchange reaction
- ELISA:
-
Enzyme-linked immunosorbent assay
- LFIA:
-
Lateral flow immunoassay
- RBD:
-
Receptor binding domain
- hACE2:
-
Human angiotensin-converting enzyme 2
- LOD:
-
Limits of detection
- SPCE:
-
Screen-printed carbon electrode
- GCE:
-
Glassy carbon electrode
- ECL:
-
Electrochemiluminescence
- BioFETs:
-
Field-effect transistor-based biosensors
- ST-NCF:
-
Slightly tapered no-core fiber
- SERS:
-
Surface-enhanced Raman scattering
- LSPR:
-
Localized surface plasmon resonance
References
WHO (2021) Global excess deaths associated with COVID-19 (modelled estimates). Available from. https://www.who.int/data/sets/global-excess-deaths-associated-with-covid-19-modelled-estimates (Accessed June 2023).
L. Camporota, J.N. Cronin, M. Busana, L. Gattinoni, F. Formenti, Pathophysiology of coronavirus-19 disease acute lung injury. Curr Opin Crit Care 28(1), 9–16 (2022). https://doi.org/10.1097/MCC.0000000000000911
K. Dhama, S. Khan, R. Tiwari, S. Sircar, S. Bhat, Y.S. Malik, K.P. Singh, W. Chaicumpa, D.K. Bonilla-Aldana, A.J. Rodriguez-Morales, Coronavirus disease 2019-COVID-19. Clin Microbiol Rev (2020). https://doi.org/10.1128/CMR.00028-20
D. Purohit, A.K. Ahirwar, A. Sakarde, P. Asia, N. Gopal, COVID-19 and lung pathologies. Horm Mol Biol Clin Investig 42(4), 435–443 (2021). https://doi.org/10.1515/hmbci-2020-0096
T.J. Louis, A. Qasem, L.S. Abdelli, S.A. Naser, Extra-pulmonary complications in SARS-CoV-2 infection: a comprehensive multi organ-system review. Microorganisms 10, 153 (2022). https://doi.org/10.3390/microorganisms10010153
I.O. Lawal, M.M. Kgatle, K. Mokoala, A. Farate, M.M. Sathekge, Cardiovascular disturbances in COVID-19: an updated review of the pathophysiology and clinical evidence of cardiovascular damage induced by SARS-CoV-2. BMC Cardiovasc Disord 22(1), 93 (2022). https://doi.org/10.1186/s12872-022-02534-8
J.M. Dubin, N.E. Bennett, J.A. Halpern, The adverse impact of COVID-19 on men’s health. Curr Opin Urol 32(2), 146–151 (2022). https://doi.org/10.1097/MOU.0000000000000966
N. Delli Muti, F. Finocchi, G. Tossetta, G. Salvio, M. Cutini, D. Marzioni, G. Balercia, Could SARS-CoV-2 infection affect male fertility and sexuality? APMIS 130(5), 243–252 (2022). https://doi.org/10.1111/apm.13210
X. Li, Z. Chen, J. Geng, Q. Mei, H. Li, C. Mao, M. Han, COVID-19 and male reproduction: a thorny problem. Am J Mens Health 16(1), 15579883221074816 (2022). https://doi.org/10.1177/15579883221074816
S. D’Ippolito, F. Turchiano, A. Vitagliano, G. Scutiero, A. Lanzone, G. Scambia, P. Greco, Is there a role for SARS-CoV-2/COVID-19 on the female reproductive system? Front Physiol 13, 845156 (2022). https://doi.org/10.3389/fphys.2022.845156
A. Carp-Veliscu, C. Mehedintu, F. Frincu, E. Bratila, S. Rasu, I. Iordache, A. Bordea, M. Braga, The effects of SARS-CoV-2 infection on female fertility: a review of the literature. Int J Environ Res Public Health 19(2), 984 (2022). https://doi.org/10.3390/ijerph19020984
D.J. Jamieson, S.A. Rasmussen, An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 226(2), 177–186 (2022). https://doi.org/10.1016/j.ajog.2021.08.054
B. Ebner, Y. Volz, J.N. Mumm, C.G. Stief, G. Magistro, The COVID-19 pandemic—what have urologists learned? Nat Rev Urol 19(6), 344–356 (2022). https://doi.org/10.1038/s41585-022-00586-1
S.A. Saeed, I.S. Pastis, M.G. Santos, COVID-19 and its impact on the brain and mind—a conceptual model and supporting evidence. Psychiatr Q 93(1), 271–284 (2022). https://doi.org/10.1007/s11126-022-09980-9
Ç.T.E. Ozkurt, COVID-19 Gastrointestinal manifestations, liver injury and recommendations. World J Clin Cases 10(4), 1140–1456 (2022). https://doi.org/10.1998/wjcc.v10.i4.1140
C. Villa, E. Rivellini, M. Lavitrano, R. Combi, Can SARS-CoV-2 infection exacerbate Alzheimer’s Disease? an overview of shared risk factors and pathogenetic mechanisms. J Pers Med 12(1), 29 (2022). https://doi.org/10.3390/jpm12010029
S. Soltani, M. Zandi, S.E. Ahmadi, B. Zarandi, Z. Hosseini, S. Akhavan Rezayat, M. Abyadeh, I. Pakzad, P. Malekifar, R. Pakzad, S.H. Mozhgani, Pooled prevalence estimate of ocular manifestations in COVID-19 patients a systematic review and meta-analysis. Iran J Med Sci 47(1), 2–14 (2022). https://doi.org/10.30476/ijms.2021.89475.2026
A.D. Desai, M. Lavelle, B.C. Boursiquot, E.Y. Wan, Long-term complications of COVID-19. Am J Physiol Cell Physiol 322(1), C1–C11 (2022). https://doi.org/10.1152/ajpcell.00375.2021
M. Rewerska-Jusko, K. Rejdak, Social stigma of patients suffering from COVID-19: challenges for health care system. Healthcare 10(2), 292 (2022). https://doi.org/10.3390/healthcare10020292
A.Y. Thye, J.W. Law, L.T. Tan, P. Pusparajah, H.L. Ser, S. Thurairajasingam, V. Letchumanan, L.H. Lee, Psychological symptoms in COVID-19 patients: insights into pathophysiology and risk factors of long COVID-19. Biology 11(1), 61 (2022). https://doi.org/10.3390/biology11010061
N. Al-Awwal, F. Dweik, S. Mahdi, M. El-Dweik, S.H. Anderson, A review of SARS-CoV-2 disease (COVID-19): pandemic in our time. Pathogens 11(3), 368 (2022). https://doi.org/10.3390/pathogens11030368
R. Khandia, S. Singhal, T. Alqahtani, M.A. Kamal, N.A. El-Shall, F. Nainu, P.A. Desingu, K. Dhama, Emergence of SARS-CoV-2 Omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ Res 209, 112816 (2022). https://doi.org/10.1016/j.envres.2022.112816
V.-M. Manoiu, K. Kubiak-Wójcicka, A.-I. Craciun, Ç. Akman, E. Akman, Water quality and water pollution in time of COVID-19: positive and negative repercussions. Water 14(7), 1124 (2022). https://doi.org/10.3390/w14071124
D. Zhang, S.S.F. Duran, W.Y.S. Lim, C.K.I. Tan, W.C.D. Cheong, A. Suwardi, X.J. Loh, SARS-CoV-2 in wastewater: from detection to evaluation. Mater Today Adv 13, 100211 (2022). https://doi.org/10.1016/j.mtadv.2022.100211
P. Foladori, F. Cutrupi, M. Cadonna, S. Manara, Coronaviruses and SARS-CoV-2 in sewerage and their removal: step by step in wastewater treatment plants. Environ Res 207, 112204 (2022). https://doi.org/10.1016/j.envres.2021.112204
F. Mollarasouli, N. Zare-Shehneh, M. Ghaedi, A review on corona virus disease 2019 (COVID-19): current progress, clinical features and bioanalytical diagnostic methods. Mikrochim Acta 189(3), 103 (2022). https://doi.org/10.1007/s00604-022-05167-y
H. Parmar, M. Montovano, P. Banada, S.R. Pentakota, S. Shiau, Z. Ma, K. Saibire, A. Chopoorian, M. O’Shaughnessy, M. Hirsch, P. Jain, G. Demirdjian, M. Karagueuzian, T. Robin, M. Salvati, B. Patel, D. Alland, Y.L. Xie, RT-PCR negative COVID-19. BMC Infect Dis 22(1), 149 (2022). https://doi.org/10.1186/s12879-022-07095-x
M.C. Saad Menezes, D.V. Santinelli Pestana, J.C. Ferreira, C.R. Ribeiro, M.C. de Carvalho, I.M. Felix, K.R. da Silva, V.C. Junior, J.F. Marchini, J.C. Alencar, L.M.G. Gomez, D.D. Maua, H.P. Souza, C.-G. Emergency Usp, G. Hcfmusp, Covid-study, distinct outcomes in COVID-19 patients with positive or negative RT-PCR Test. Viruses 14(2), 175 (2022). https://doi.org/10.3390/v14020175
W. Zhang, Y. He, Z. Feng, J. Zhang, Recent advances of functional nucleic acid-based sensors for point-of-care detection of SARS-CoV-2. Mikrochim. Acta 189(3), 128 (2022). https://doi.org/10.1007/s00604-022-05242-4
L. Benevides Lima, F.P. Mesquita, L.L. Brasil, F. de Oliveira, A. da Silva, M.E. Oliveira, P.F.N. Amaral de Moraes, R.C.M. Souza, True or false: what are the factors that influence COVID-19 diagnosis by RT-qPCR? Expert Rev Mol Diagn 22(2), 157–167 (2022). https://doi.org/10.1080/14737159.2022.2037425
A.R.N. Ko, K.K.K. Tan, S.Y.L. Chan, K.X.L. Goh, S.S. Sim, J.H.C. Lim, K.L. Tan, W.L. Chan, K.S. Oon, L.L.E. Nagarajan, N. Suphavilai, SARS-CoV-2 N Gene G29195T point mutation may affect diagnostic reverse transcription-PCR detection. Microbiol Spectr 23, 0222321 (2022). https://doi.org/10.1128/spectrum.02223-21
R.W. Peeling, D.L. Heymann, Y.-Y. Teo, P.J. Garcia, Diagnostics for COVID-19: moving from pandemic response to control. Lancet 399(10326), 757–768 (2022). https://doi.org/10.1016/s0140-6736(21)02346-1
N. Odiwuor, J. Xiong, F. Ogolla, W. Hong, X. Li, F.M. Khan, N. Wang, J. Yu, H. Wei, A point-of-care SARS-CoV-2 test based on reverse transcription loop-mediated isothermal amplification without RNA extraction with diagnostic performance same as RT-PCR. Anal Chim Acta 1200, 339590 (2022). https://doi.org/10.1016/j.aca.2022.339590
F.E. Marino, E. Proffitt, E. Joseph, A. Manoharan, A rapid, specific, extraction-less, and cost-effective RT-LAMP test for the detection of SARS-CoV-2 in clinical specimens. PLoS ONE 17(4), e0266703 (2022). https://doi.org/10.1371/journal.pone.0266703
S.A. El-Kafrawy, M.M. El-Daly, A.M. Hassan, S.M. Harakeh, T.A. Alandijany, E.I. Azhar, Rapid and reliable detection of SARS-CoV-2 using direct RT-LAMP. Diagnostics 12(4), 828 (2022). https://doi.org/10.3390/diagnostics12040828
Y. Artik, A.B. Cosgun, N.P. Cesur, N. Hizel, Y. Uyar, H. Sur, A. Ayan, Comparison of COVID-19 laboratory diagnosis by commercial kits: effectivity of RT-PCR to the RT-LAMP. J Med Virol 94(5), 1998–2007 (2022). https://doi.org/10.1002/jmv.27559
J.A. Batsis, G.G. Boateng, L.M. Seo, C.L. Petersen, K.L. Fortuna, E.V. Wechsler, R.J. Peterson, S.B. Cook, D. Pidgeon, R.S. Dokko, R.J. Halter, D.F. Kotz, Development and usability assessment of a connected resistance exercise band application for strength-monitoring. World Acad Sci Eng Technol 13(5), 340–348 (2019). https://doi.org/10.5281/zenodo
X. Lu, H. Lee, H.L. Yu, Y. Cao, I.M. Hsing, Rapid and highly specific detection of communicable pathogens using one-pot loop probe-mediated isothermal amplification (oLAMP). Sens Actuators B Chem (2022). https://doi.org/10.1016/j.snb.2022.131385
H. Jayakody, D. Rowland, C. Pereira, R. Blackwell, T. Lasota, M. Laverick, L. Tisi, H.S. Leese, A.D.S. Walsham, Development of a high sensitivity RT-PCR assay for detection of SARS-CoV-2 in individual and pooled nasopharyngeal samples. Sci Rep 12(1), 5369 (2022). https://doi.org/10.1038/s41598-022-09254-1
H. Tombuloglu, H. Sabit, H. Al-Khallaf, J.H. Kabanja, M. Alsaeed, N. Al-Saleh, E. Al-Suhaimi, Multiplex real-time RT-PCR method for the diagnosis of SARS-CoV-2 by targeting viral N, RdRP and human RP genes. Sci Rep 12(1), 2853 (2022). https://doi.org/10.1038/s41598-022-06977-z
O. Almendares, J.L. Prince-Guerra, L.D. Nolen, J.K.L. Gunn, A.P. Dale, S.A. Buono, M. Deutsch-Feldman, S. Suppiah, L. Hao, Y. Zeng, V.A. Stevens, K. Knipe, J. Pompey, C. Atherstone, D.P. Bui, T. Powell, A. Tamin, J.L. Harcourt, M. Petway, C. Bohannon, J.M. Folster, A. MacNeil, R. Salerno, W. Kuhnert-Tallman, J.E. Tate, N. Thornburg, H.L. Kirking, K. Sheiban, J. Kudrna, T. Cullen, K.K. Komatsu, J.M. Villanueva, D.A. Rose, J.C. Neatherlin, M. Anderson, P.A. Rota, M.A. Honein, W.A. Bower, CC-SDT laboratory, performance characteristics of the abbott BinaxNOW SARS-CoV-2 antigen test in comparison to real-time reverse transcriptase PCR and viral culture in community testing sites during november 2020. J Clin Microbiol 60(1), e0174221 (2022). https://doi.org/10.1128/JCM.01742-21
R.A. Mullinax, Antigen testing for COVID-19 asymptomatic screening: why it should be preferred over PCR insights from navy region Japan’s experience with a comprehensive antigen testing strategy. Mil Med 187(3–4), 68–72 (2022). https://doi.org/10.1093/milmed/usab331
C. Barrera-Avalos, R. Luraschi, E. Vallejos-Vidal, A. Mella-Torres, F. Hernandez, M. Figueroa, C. Rioseco, D. Valdes, M. Imarai, C. Acuna-Castillo, F.E. Reyes-Lopez, A.M. Sandino, The rapid antigen detection test for SARS-CoV-2 underestimates the identification of COVID-19 positive cases and compromises the diagnosis of the SARS-CoV-2 (K417N/T, E484K, and N501Y) variants. Front Public Health 9, 780801 (2021). https://doi.org/10.3389/fpubh.2021.780801
H.K. Sheikh, Colorimetric chromophoric rapid detection of SARS-CoV-2 through breath analysis Pakistan. J Pharm Sci 35(1), 157–160 (2022). https://doi.org/10.3721/PJPS.2022.35.1.REG.157-160.1
Z. Jin, Y. Mantri, M. Retout, Y. Cheng, J. Zhou, A. Jorns, P. Fajtova, W. Yim, C. Moore, M. Xu, M.N. Creyer, R.M. Borum, J. Zhou, Z. Wu, T. He, W.F. Penny, A.J. O’Donoghue, J.V. Jokerst, A charge-switchable zwitterionic peptide for rapid detection of SARS-CoV-2 main protease. Angew Chem Int Engl 61(9), e202112995 (2022). https://doi.org/10.1002/anie.202112995
Y. Farsi, A. Tahvildari, M. Arbabi, F. Vazife, L.A. Sechi, A.H. Shahidi Bonjar, P. Jamshidi, M.J. Nasiri, M. Mirsaeidi, Diagnostic, prognostic, and therapeutic roles of gut microbiota in COVID-19: a comprehensive systematic review. Front Cell Infect Microbiol 12, 80464 (2022). https://doi.org/10.3389/fcimb.2022.804644
B.M. Hryniewicz, J. Volpe, L. Bach-Toledo, K.C. Kurpel, A.E. Deller, A.L. Soares, J.M. Nardin, L.F. Marchesi, F.F. Simas, C.C. Oliveira, L. Huergo, D.E.P. Souto, M. Vidotti, Development of polypyrrole (nano)structures decorated with gold nanoparticles toward immunosensing for COVID-19 serological diagnosis. Mater Today Chem 24, 100817 (2022). https://doi.org/10.1016/j.mtchem.2022.100817
J. McClements, L. Bar, P. Singla, F. Canfarotta, A. Thomson, J. Czulak, R.E. Johnson, R.D. Crapnell, C.E. Banks, B. Payne, S. Seyedin, P. Losada-Perez, M. Peeters, Molecularly imprinted polymer nanoparticles enable rapid, reliable, and robust point-of-care thermal detection of SARS-CoV-2. ACS Sens. 7(4), 1122–1131 (2022). https://doi.org/10.1021/acssensors.2c00100
J. Cui, L. Kan, F. Cheng, J. Liu, L. He, Y. Xue, S. Fang, Z. Zhang, Construction of bifunctional electrochemical biosensors for the sensitive detection of the SARS-CoV-2 N-gene based on porphyrin porous organic polymers. Dalton Trans 51(5), 2094–2104 (2022). https://doi.org/10.1039/d1dt03869a
L. Liv, H. Kayabay, An electrochemical biosensing platform for the SARS-CoV-2 spike antibody detection based on the functionalised SARS-CoV-2 spike antigen modified electrode. ChemistrySelect 7(10), e202200256 (2022). https://doi.org/10.1002/slct.202200256
Z. Jin, J. Yeung, J. Zhou, Y. Cheng, Y. Li, Y. Mantri, T. He, W. Yim, M. Xu, Z. Wu, P. Fajtova, M.N. Creyer, C. Moore, L. Fu, W.F. Penny, A.J. O’Donoghue, J.V. Jokerst, Peptidic sulfhydryl for interfacing nanocrystals and subsequent sensing of SARS-CoV-2 protease. Chem Mater 34(3), 1259–1268 (2022). https://doi.org/10.1021/acs.chemmater.1c03871
Y. Zheng, S. Bian, J. Sun, L. Wen, G. Rong, M. Sawan, Label-free LSPR-vertical microcavity biosensor for on-site SARS-CoV-2 detection. Biosensors 12(3), 151 (2022). https://doi.org/10.3390/bios12030151
F. Haghayegh, R. Salahandish, M. Hassani, A. Sanati-Nezhad, Highly stable buffer-based zinc oxide/reduced graphene oxide nanosurface chemistry for rapid immunosensing of SARS-CoV-2 antigens. ACS Appl Mater Interf 14(8), 10844–10855 (2022). https://doi.org/10.1021/acsami.1c24475
Y. Zhao, J. Chen, Z. Hu, Y. Chen, Y. Tao, L. Wang, L. Li, P. Wang, H.Y. Li, J. Zhang, J. Tang, H. Liu, All-solid-state SARS-CoV-2 protein biosensor employing colloidal quantum dots-modified electrode. Biosens Bioelectron 202, 113974 (2022). https://doi.org/10.1016/j.bios.2022.113974
M. Mehmandoust, Z.P. Gumus, M. Soylak, N. Erk, Electrochemical immunosensor for rapid and highly sensitive detection of SARS-CoV-2 antigen in the nasal sample. Talanta 240, 123211 (2022). https://doi.org/10.1016/j.talanta.2022.123211
W.L. Ang, R.R.X. Lim, A. Ambrosi, A. Bonanni, Rapid electrochemical detection of COVID-19 genomic sequence with dual-function graphene nanocolloids based biosensor. FlatChem 32, 100336 (2022). https://doi.org/10.1016/j.flatc.2022.100336
Y. Peng, C. Lin, L. Long, T. Masaki, M. Tang, L. Yang, J. Liu, Z. Huang, Z. Li, X. Luo, J.R. Lombardi, Y. Yang, Charge-transfer resonance and electromagnetic enhancement synergistically enabling MXenes with excellent SERS sensitivity for SARS-CoV-2 S protein detection. Nanomicro Lett 13, 52 (2021). https://doi.org/10.1007/s40820-020-00565-4
M.A. Zamzami, G. Rabbani, A. Ahmad, A.A. Basalah, W.H. Al-Sabban, S. Nate Ahn, H. Choudhry, Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry 143, 107982 (2022). https://doi.org/10.1016/j.bioelechem.2021.107982
D. Naranbat, L. Schneider, R. Kantor, C.G. Beckwith, L. Bazerman, F. Gillani, S. Sahu, K. Rapoza, S. Sam, V. Novitsky, J. Shin, E. Hipolito, I. Diaz, D. Carnevale, A. Tripathi, DirectDetect SARS-CoV-2 direct real-time RT-PCR study using patient samples. ACS Omega 7(6), 4945–4955 (2022). https://doi.org/10.1021/acsomega.1c05595
Y. Lee, Y.S. Kim, D.I. Lee, S. Jeong, G.H. Kang, Y.S. Jang, W. Kim, H.Y. Choi, J.G. Kim, S.H. Choi, The application of a deep learning system developed to reduce the time for RT-PCR in COVID-19 detection. Sci Rep 12(1), 1234 (2022). https://doi.org/10.1038/s41598-022-05069-2
O. Delpuech, J.A. Douthwaite, T. Hill, D. Niranjan, N.T. Malintan, H. Duvoisin, J. Elliott, I. Goodfellow, M. Hosmillo, A.L. Orton, M.A. Taylor, C. Brankin, H. Pitt, D. Ross-Thriepland, M. Siek, A. Cuthbert, I. Richards, J.R. Ferdinand, C. Barker, R. Shaw, C. Ariani, I. Waddell, S. Rees, C. Green, R. Clark, A. Upadhyay, R. Howes, Heat inactivation of clinical COVID-19 samples on an industrial scale for low risk and efficient high-throughput qRT-PCR diagnostic testing. Sci Rep 12(1), 2883 (2022). https://doi.org/10.1038/s41598-022-06888-z
H. Chen, Y. Wang, H. Wei, Z. Rong, S. Wang, A rapid water bath PCR combined with lateral flow assay for the simultaneous detection of SARS-CoV-2 and influenza B virus. RSC Adv 12(6), 3437–3444 (2022). https://doi.org/10.1039/d1ra07756b
W Shin, CJ Lee, YM Lee, YB Choi, S Mun, K Han, Rapid identification of SARS-CoV-2 in the point-of-care using digital PCR-based Dr. PCR Di20K COVID-19 Detection Kit without viral RNA extraction. Genes Genomics. 44(5), 617–628 (2022). https://doi.org/10.1007/s13258-022-01242-z
R. Lu, J. Wang, M. Li, J. He, Y. Wang, J. Dong, W. Cai, Retrospective quantitative detection of SARS-CoV-2 by digital PCR showing high accuracy for low viral load specimens. J Infect Dev Ctries 16(1), 10–15 (2022). https://doi.org/10.3855/jidc.15315
T. Boogaerts, S. Van den Bogaert, L.A.E. Van Poelvoorde, D. El Masri, N. De Roeck, N.H.C. Roosens, M. Lesenfants, L. Lahousse, K. Van Hoorde, A.L.N. van Nuijs, P. Delputte, Optimization and application of a multiplex digital PCR assay for the detection of SARS-CoV-2 variants of concern in Belgian influent wastewater. Viruses 14(3), 610 (2022). https://doi.org/10.3390/v14030610
D. Yolda-Carr, D.A. Thammavongsa, N. Vega, S.J. Turner, P.J. Pickering, A.L. Wyllie, Evaluation of the liberty16 mobile real time PCR device for use with the salivadirect assay for SARS-CoV-2 Testing. Front Cell Infect Microbiol 11, 808773 (2021). https://doi.org/10.3389/fcimb.2021.808773
C Vogels, O M Allicock, D E. Brackney, C C Kalinich, I M Ott, N Grubaugh, A L Wyllie, SalivaDirect™: RNA extraction-free SARS-CoV-2 diagnostics V6. https://doi.org/10.17504/protocols.io.btdnni5e
H.Y. Chung, M.J. Jian, C.K. Chang, J.C. Lin, K.M. Yeh, C.W. Chen, S.S. Hsieh, K.S. Hung, S.H. Tang, C.L. Perng, F.Y. Chang, C.H. Wang, H.S. Shang, Emergency SARS-CoV-2 variants of concern: novel multiplex real-time RT-PCR assay for rapid detection and surveillance. Microbiol Spectr 10(1), e0251321 (2022). https://doi.org/10.1128/spectrum.02513-21
M. Fabiani, K. Margiotti, M. Sabatino, A. Viola, A. Mesoraca, C. Giorlandino, A rapid and consistent method to identify four SARS-CoV-2 variants during the first half of 2021 by RT-PCR. Vaccines 10(3), 483 (2022). https://doi.org/10.3390/vaccines10030483
D. Xiong, X. Zhang, M. Shi, N. Wang, P. He, Z. Dong, J. Zhong, J. Luo, Y. Wang, J. Yu, H. Wei, Developing an amplification refractory mutation system-quantitative reverse transcription-PCR assay for rapid and sensitive screening of SARS-CoV-2 variants of concern. Microbiol Spectr 10(1), e0143821 (2022). https://doi.org/10.1128/spectrum.01438-21
C. Dachert, M. Muenchhoff, A. Graf, H. Autenrieth, S. Bender, H. Mairhofer, P.R. Wratil, S. Thieme, S. Krebs, N. Grzimek-Koschewa, H. Blum, O.T. Keppler, Rapid and sensitive identification of omicron by variant-specific PCR and nanopore sequencing: paradigm for diagnostics of emerging SARS-CoV-2 variants. Med Microbiol Immunol 211(1), 71–77 (2022). https://doi.org/10.1007/s00430-022-00728-7
W. Liu, L.P. Lee, Toward rapid and accurate molecular diagnostics at home. Adv Mater 35(21), e2206525 (2023). https://doi.org/10.1002/adma.202206525
S.Y. Lee, J.S. Lee, J.J. Ahn, S.J. Kim, H. Sung, J.W. Huh, S.Y. Hwang, Simultaneous detection of SARS-CoV-2 and identification of spike D614G mutation using point-of-care real-time polymerase chain reaction. J Virol Methods 304, 114513 (2022). https://doi.org/10.1016/j.jviromet.2022.114513
A. Bhatt, Z. Fatima, M. Ruwali, C.S. Misra, S.S. Rangu, D. Rath, A. Rattan, S. Hameed, CLEVER assay: a visual and rapid RNA extraction-free detection of SARS-CoV-2 based on CRISPR-Cas integrated RT-LAMP technology. J Appl Microbiol 133(2), 410–421 (2022). https://doi.org/10.1111/jam.15571
Z. Yi, J. de Dieu Habimana, O. Mukama, Z. Li, N. Odiwuor, H. Jing, C. Nie, M. Hu, Z. Lin, H. Wei, L. Zeng, Rational programming of Cas12a for Early-Stage Detection of COVID-19 by lateral flow assay and portable real-time fluorescence readout facilities. Biosensors 12(1), 11 (2021). https://doi.org/10.3390/bios12010011
A.J. Colbert, D.H. Lee, K.N. Clayton, S.T. Wereley, J.C. Linnes, T.L. Kinzer-Ursem, PD-LAMP smartphone detection of SARS-CoV-2 on chip. Anal Chim Acta 1203, 339702 (2022). https://doi.org/10.1016/j.aca.2022.339702
T. Iijima, S. Ando, D. Kanamori, K. Kuroda, T. Nomura, L. Tisi, P.E. Kilgore, N. Percy, H. Kohase, S. Hayakawa, M. Seki, T. Hoshino, Detection of SARS-CoV-2 and the L452R spike mutation using reverse transcription loop-mediated isothermal amplification plus bioluminescent assay in real-time (RT-LAMP-BART). PLoS ONE 17(3), e0265748 (2022). https://doi.org/10.1371/journal.pone.0265748
R.K. Shanmugakani, M. Wu, An isothermal amplification-coupled dipstick for the rapid detection of COVID-19. J Med Microbiol (2022). https://doi.org/10.1099/jmm.0.001519
Y. Tang, Y. Wang, Y. Li, H. Zhao, S. Zhang, Y. Zhang, J. Li, Y. Chen, X. Wu, C. Qin, T. Jiang, X. Kang, An integrated rapid nucleic acid detection assay based on recombinant polymerase amplification for SARS-CoV-2. Virol Sin 37(1), 138–141 (2022). https://doi.org/10.1016/j.virs.2022.01.006
M. Zhou, C. Fan, L. Wang, T. Xu, X. Zhang, Enhanced isothermal amplification for ultrafast sensing of SARS-CoV-2 in microdroplets. Anal Chem 94(10), 4135–4140 (2022). https://doi.org/10.1021/acs.analchem.2c00008
Y. Kim, A.B. Yaseen, J.Y. Kishi, F. Hong, P. Yin, Single-strand RPA for rapid and sensitive detection of SARS-CoV-2 RNA. MedRxiv (2020). https://doi.org/10.1101/2020.08.17.20177006
D. Li, C. Duan, W. Cheng, Y. Gong, Y. Yao, X. Wang, Z. Wang, Y. Xiang, A simple and rapid method to assay SARS-CoV-2 RNA based on a primer exchange reaction. Chem Commun 58(28), 4484–4487 (2022). https://doi.org/10.1039/d2cc00488g
M. Ranjbar, M. Asadi, M. Nourigorji, B. Sarkari, Z. Mostafavi-Pour, K. Zomorodian, Z. Shabaninejad, M. Taheri-Anganeh, A. Maleksabet, M. Moghadami, A. Savardashtaki, Development of a recombinant nucleocapsid protein-based ELISA for the detection of IgM and IgG antibodies to SARS-CoV-2. Biotechnol Appl Biochem 69(6), 2592–2598 (2022). https://doi.org/10.1002/bab.2308
K.G. Beavis, S.M. Matushek, A.P.F. Abeleda, C. Bethel, C. Hunt, S. Gillen, A. Moran, V. Tesic, Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J Clin Virol 129, 104468 (2020). https://doi.org/10.1016/j.jcv.2020.104468
P.P. Liu, Y. Zong, S.P. Jiang, Y.J. Jiao, X.J. Yu, Development of a nucleocapsid protein-based ELISA for detection of human IgM and IgG antibodies to SARS-CoV-2. ACS Omega 6(14), 9667–9671 (2021). https://doi.org/10.1021/acsomega.1c00253
J. Luo, A. Brakel, A. Krizsan, T. Ludwig, M. Motzing, D. Volke, N. Lakowa, T. Grunewald, C. Lehmann, J. Wolf, S. Borte, S. Milkovska-Stamenova, J. Gabert, F. Fingas, M. Scholz, R. Hoffmann, Sensitive and specific serological ELISA for the detection of SARS-CoV-2 infections. Virol J. 19(1), 50 (2022). https://doi.org/10.1186/s12985-022-01768-4
E. Gonzalez-Gonzalez, R. Garcia-Ramirez, G.G. Diaz-Armas, M. Esparza, C. Aguilar-Avelar, E.A. Flores-Contreras, I.P. Rodriguez-Sanchez, J.R. Delgado-Balderas, B. Soto-Garcia, D. Araiz-Hernandez, M. Abarca-Blanco, J.R. Yee-de Leon, L.P. Velarde-Calvillo, A. Abarca-Blanco, J.F. Yee, Automated ELISA On-chip for the detection of anti-SARS-CoV-2 antibodies. Sensors 21(20), 6785 (2021). https://doi.org/10.3390/s21206785
S. Kasetsirikul, M. Umer, N. Soda, K.R. Sreejith, M.J.A. Shiddiky, N.T. Nguyen, Detection of the SARS-CoV-2 humanized antibody with paper-based ELISA. Analyst 145(23), 7680–7686 (2020). https://doi.org/10.1039/d0an01609h
M. Di Domenico, A. De Rosa, M. Boccellino, Detection of SARS-COV-2 proteins using an ELISA test. Diagnostics 11(4), 698 (2021). https://doi.org/10.3390/diagnostics11040698
M. Alafeef, D. Pan, Diagnostic approaches For COVID-19: lessons learned and the path forward. ACS Nano 16(8), 11545–11576 (2022). https://doi.org/10.1021/acsnano.2c01697
T. Peng, X. Jiao, Z. Liang, H. Zhao, Y. Zhao, J. Xie, Y. Jiang, X. Yu, X. Fang, X. Dai, Lateral flow immunoassay coupled with copper enhancement for rapid and sensitive SARS-CoV-2 nucleocapsid protein detection. Biosensors 12(1), 13 (2021). https://doi.org/10.3390/bios12010013
J. Szekely, J. Mongkolprasert, N. Jeayodae, C. Senorit, P. Chaimuti, P. Swangphon, N. Nanakorn, T. Nualnoi, P. Wongwitwichot, T. Pengsakul, Development, analytical, and clinical evaluation of rapid immunochromatographic antigen test for SARS-CoV-2 variants detection. Diagnostics 12(2), 381 (2022). https://doi.org/10.3390/diagnostics12020381
J.H. Lee, Y. Jung, S.K. Lee, J. Kim, C.S. Lee, S. Kim, J.S. Lee, N.H. Kim, H.G. Kim, Rapid biosensor of SARS-CoV-2 using specific monoclonal antibodies recognizing conserved nucleocapsid protein epitopes. Viruses 14(2), 255 (2022). https://doi.org/10.3390/v14020255
C. Chen, H. Hu, X. Li, Z. Zheng, Z. Wang, X. Wang, P. Zheng, F. Cui, G. Li, Y. Wang, Z. Liu, Y. Ma, Rapid detection of anti-SARS-CoV-2 antibody using a selenium nanoparticle-based lateral flow immunoassay. IEEE Trans Nanobiosci 21(1), 37–43 (2022). https://doi.org/10.1109/TNB.2021.3105662
G.Q. Zhang, Z. Gao, J. Zhang, H. Ou, H. Gao, R.T.K. Kwok, D. Ding, B.Z. Tang, A wearable AIEgen-based lateral flow test strip for rapid detection of SARS-CoV-2 RBD protein and N protein. Cell Rep Phys Sci 3(2), 100740 (2022). https://doi.org/10.1016/j.xcrp.2022.100740
X. Duan, Y. Shi, X. Zhang, X. Ge, R. Fan, J. Guo, Y. Li, G. Li, Y. Ding, R.A. Osman, W. Jiang, J. Sun, X. Luan, G. Zhang, Dual-detection fluorescent immunochromatographic assay for quantitative detection of SARS-CoV-2 spike RBD-ACE2 blocking neutralizing antibody. Biosens Bioelectron 199, 113883 (2022). https://doi.org/10.1016/j.bios.2021.113883
K. Dighe, P. Moitra, M. Alafeef, N. Gunaseelan, D. Pan, A rapid RNA extraction-free lateral flow assay for molecular point-of-care detection of SARS-CoV-2 augmented by chemical probes. Biosens Bioelectron 200, 113900 (2022). https://doi.org/10.1016/j.bios.2021.113900
A. Biby, X. Wang, X. Liu, O. Roberson, A. Henry, X. Xia, Rapid testing for coronavirus disease 2019 (COVID-19). MRS Commun 12(1), 12–23 (2022). https://doi.org/10.1557/s43579-021-00146-5
I. Polvere, S. Voccola, S. D’Andrea, L. Zerillo, R. Varricchio, J.R. Madera, R. Stilo, P. Vito, T. Zotti, Evaluation of FAST COVID-19 SARS-CoV-2 antigen rapid test kit for detection of SARS-CoV-2 in respiratory samples from mildly symptomatic or asymptomatic patients. Diagnostics 12(3), 650 (2022). https://doi.org/10.3390/diagnostics12030650
G.C. Mak, S.S. Lau, K.K. Wong, N.L. Chow, C.S. Lau, E.T. Lam, K.H. Ng, R.C. Chan, Evaluation of rapid antigen detection kits for detection of SARS-CoV-2 B16172 virus. Future Virol 129, 104500 (2022). https://doi.org/10.2217/fvl-2021-0229
T. Gracienta, R. Herardi, F. Santosa, T. Pasiak, Y. Tjang, Diagnostic accuracy of antibody-based rapid diagnostic test in detecting coronavirus disease 2019: systematic review. Arch Med Sci 18(4), 949–957 (2021). https://doi.org/10.5114/aoms/135910
J. Exinger, C. Hartard, F. Lafferriere, C. Fenninger, L.J. Charbonniere, H. Jeulin, Evaluation of a lateral flow immunoassay COVIDTECH((R)) SARS-CoV-2 IgM/IgG antibody rapid test. Jpn J Infect Dis 75(4), 334–340 (2022). https://doi.org/10.7883/yoken.JJID.2021.273
Y. Morinaga, H. Yamada, Y. Yoshida, H. Kawasuji, Y. Yamamoto, Analytical sensitivity of six lateral flow antigen test kits for variant strains of SARS-CoV-2. J Infect Chemother 29(2), 131–135 (2023). https://doi.org/10.1016/j.jiac.2022.10.004
G.C. Mak, S.S. Lau, K.K. Wong, N.L. Chow, C.S. Lau, E.T. Lam, R.C. Chan, D.N. Tsang, Analytical sensitivity and clinical sensitivity of the three rapid antigen detection kits for detection of SARS-CoV-2 virus. J Clin Virol 133, 104684 (2020). https://doi.org/10.1016/j.jcv.2020.104684
G.C. Mak, S.S. Lau, K.K. Wong, N.L. Chow, C.S. Lau, E.T. Lam, K.H. Ng, R.C. Chan, Evaluation of rapid antigen detection kits for detection of SARS-CoV-2 B16172 virus. Future Virol (2022). https://doi.org/10.2217/fvl-2021-0229
G.C.K. Mak, S.S.Y. Lau, K.K.Y. Wong, N.L.S. Chow, C.S. Lau, E.T.K. Lam, R.C.W. Chan, D.N.C. Tsang, Comparison of analytical sensitivity of the eight rapid antigen detection kits for detecting SARS-CoV-2 virus. J Clin Virol 144, 104994 (2021). https://doi.org/10.1016/j.jcv.2021.104994
U.V. Schneider, M.W. Forsberg, T.D. Leineweber, C.B. Jensen, K. Ghathian, C.N. Agergaard, K.K. Mortensen, A. Cohen, C.S. Jorgensen, H. Larsen, M.B. Hansen, U. Saleme, A. Koch, N.S. Kirkby, T. Kallemose, M.L. Schaadt, F.H. Jensen, R.L. Jorgensen, C.M.G. Ma, N. Steenhard, J.D. Knudsen, J.G. Lisby, RATtg National Danish, a nationwide analytical and clinical evaluation of 44 rapid antigen tests for SARS-CoV-2 compared to RT-qPCR. J Clin Virol 153, 105214 (2022). https://doi.org/10.1016/j.jcv.2022.105214
T. Peto, UC-LFO Team, COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: a national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine 36, 100924 (2021). https://doi.org/10.1016/j.eclinm.2021.100924
Q. Ye, W. Shao, H. Meng, Performance and application evaluation of SARS-CoV-2 antigen assay. J Med Virol 94(8), 3548–3553 (2022). https://doi.org/10.1002/jmv.27798
N. Samsunder, M. de Vos, S. Ngcapu, J. Giandhari, L. Lewis, A.B.M. Kharsany, C. Cawood, T. de Oliveira, Q.A. Karim, S.A. Karim, K. Naidoo, C. Escadafal, A. Sivro, Clinical evaluation of severe acute respiratory syndrome coronavirus 2 rapid antigen tests during the omicron wave in South Africa. J Infect Dis 226(8), 1412–1417 (2022). https://doi.org/10.1093/infdis/jiac333
K. Yu, J. Song, D. Kim, Y. Park, S.H. Jeong, Clinical evaluation of two rapid antigen tests for severe acute respiratory syndrome coronavirus 2 detection. Ann Lab Med 43(1), 120–123 (2023). https://doi.org/10.3343/alm.2023.43.1.120
Y. Takeuchi, Y. Akashi, Y. Kiyasu, N. Terada, Y. Kurihara, D. Kato, T. Miyazawa, S. Muramatsu, Y. Shinohara, A. Ueda, S. Notake, K. Nakamura, H. Suzuki, A prospective evaluation of diagnostic performance of a combo rapid antigen test QuickNavi-Flu+COVID19 Ag. J Infect Chemother 28(6), 840–843 (2022). https://doi.org/10.1016/j.jiac.2022.02.027
I. Wagenhauser, K. Knies, D. Hofmann, V. Rauschenberger, M. Eisenmann, J. Reusch, A. Gabel, S. Flemming, O. Andres, N. Petri, M.S. Topp, M. Papsdorf, M. McDonogh, R. Verma-Fuhring, A. Scherzad, D. Zeller, H. Bohm, A. Gesierich, A.K. Seitz, M. Kiderlen, M. Gawlik, R. Taurines, T. Wurmb, R.I. Ernestus, J. Forster, D. Weismann, B. Weissbrich, L. Dolken, J. Liese, L. Kaderali, O. Kurzai, U. Vogel, M. Krone, Virus variant-specific clinical performance of SARS coronavirus two rapid antigen tests in point-of-care use, from November 2020 to January 2022. Clin Microbiol Infect 29(2), 225–232 (2023). https://doi.org/10.1016/j.cmi.2022.08.006
S. Lee, K. Widyasari, H.R. Yang, J. Jang, T. Kang, S. Kim, Evaluation of the diagnostic accuracy of nasal cavity and nasopharyngeal swab specimens for SARS-CoV-2 detection via rapid antigen test according to specimen collection timing and viral load. Diagnostics 12(3), 710 (2022). https://doi.org/10.3390/diagnostics12030710
Z. Kawser, M. Hossain, S. Suliman, S. Lockman, J. Gitaka, G. Bandawe, R. Rahmat, I. Hasan, A.B. Siddik, M.H. Afrad, M.Z. Rahman, G. Miller, D.R. Walt, L.C. Ivers, R.C. LaRocque, J.B. Harris, F. Qadri, An assessment of a rapid SARS-CoV-2 antigen test in Bangladesh. Am J Trop Med Hyg 107(4), 845–849 (2022). https://doi.org/10.4269/ajtmh.22-0068
L. Porte, P. Legarraga, M. Iruretagoyena, V. Vollrath, G. Pizarro, J. Munita, R. Araos, T. Weitzel, Evaluation of two fluorescence immunoassays for the rapid detection of SARS-CoV-2 antigen-new tool to detect infective COVID-19 patients. PeerJ 9, e10801 (2021). https://doi.org/10.7717/peerj.10801
G. Pilarowski, P. Lebel, S. Sunshine, J. Liu, E. Crawford, C. Marquez, L. Rubio, G. Chamie, J. Martinez, J. Peng, D. Black, W. Wu, J. Pak, M.T. Laurie, D. Jones, S. Miller, J. Jacobo, S. Rojas, S. Rojas, R. Nakamura, V. Tulier-Laiwa, M. Petersen, D.V. Havlir, J. DeRisi, Performance characteristics of a rapid severe acute respiratory syndrome coronavirus 2 antigen detection assay at a public plaza testing site in San Francisco. J Infect Dis 223(7), 1139–1144 (2021). https://doi.org/10.1093/infdis/jiaa802
M. Alqahtani, A. Abdulrahman, F. Mustafa, A.I. Alawadhi, B. Alalawi, S.I. Mallah, Evaluation of rapid antigen tests using nasal samples to diagnose SARS-CoV-2 in symptomatic patients. Front Public Health 9, 728969 (2021). https://doi.org/10.3389/fpubh.2021.728969
Y. Seynaeve, J. Heylen, C. Fontaine, F. Maclot, C. Meex, A.N. Diep, A.F. Donneau, M.P. Hayette, J. Descy, Evaluation of two rapid antigenic tests for the detection of SARS-CoV-2 in nasopharyngeal Swabs. J Clin Med 10(13), 2774 (2021). https://doi.org/10.3390/jcm10132774
D.S. Faffe, R.L. Byrne, R. Body, T.M.P. Castiñeiras, A.P. Castiñeiras, L.S. Finch, K. Kontogianni, D. Bengey, R.M. Galliez, O.C. Ferreira Jr., D. Mariani, B.O. da Silva, S.S. Ribeiro, M. de Vos, C. Escadafal, E.R. Adams, A. Tanuri, AI cubas atienzar, multicenter diagnostic evaluation of a novel coronavirus antigen lateral flow test among symptomatic individuals in Brazil and the United Kingdom. Microbiol Spectr. 10(6), e0201222 (2022). https://doi.org/10.1128/spectrum.02012-22
F.M. Shurrab, N. Younes, D.W. Al-Sadeq, N. Liu, H. Qotba, L.J. Abu-Raddad, G.K. Nasrallah, Performance evaluation of novel fluorescent-based lateral flow immunoassay (LFIA) for rapid detection and quantification of total anti-SARS-CoV-2 S-RBD binding antibodies in infected individuals. Int J Infect Dis 118, 132–137 (2022). https://doi.org/10.1016/j.ijid.2022.02.052
D. Morales-Jadan, C. Viteri-Davila, B. Castro-Rodriguez, A.P. Vallejo-Janeta, I.A. Rivera-Olivero, F. Perez, M.A. Garcia-Bereguiain, Clinical performance of three commercial SARS-CoV-2 rapid antigen tests for community-dwelling individuals in a tropical setting. Front Cell Infect Microbiol 12, 832235 (2022). https://doi.org/10.3389/fcimb.2022.832235
L. Dong, W.F. Li, Y. Jiang, Performance evaluation of antigen detection rapid diagnostic test (Ag-RDT) for COVID-19 diagnosis in a primary healthcare center during the Shanghai COVID-19 quarantine period. Virol J. 19(1), 140 (2022). https://doi.org/10.1186/s12985-022-01871-6
A. Erman Daloglu, H. Er, N. Sepin Ozen, Y. Cekin, Evaluation of the rapid antigen detection kit with the polymerase chain reaction for detection of SARS-CoV-2 in respiratory samples. Mikrobiyol Bul 56(2), 263–273 (2022). https://doi.org/10.5578/mb.20229806
J. De Meyer, H. Goris, O. Mortele, A. Spiessens, G. Hans, H. Jansens, H. Goossens, V. Matheeussen, S. Vandamme, Evaluation of saliva as a matrix for RT-PCR analysis and two rapid antigen tests for the detection of SARS-CoV-2. Viruses 14(9), 1931 (2022). https://doi.org/10.3390/v14091931
C. Jiang, X. Mu, B. Du, Z. Tong, A review of electrochemical biosensor application in the detection of the SARS-COV-2. Micro Nano Lett 17(3), 49–58 (2022). https://doi.org/10.1049/mna2.12101
A.L. Lorenzen, A.M. Dos Santos, L.P. Dos Santos, L. da Silva Pinto, F.R. Conceicao, F. Wolfart, PEDOT-AuNPs-based impedimetric immunosensor for the detection of SARS-CoV-2 antibodies. Electrochim Acta 404, 13975 (2022). https://doi.org/10.1016/j.electacta.2021.139757
T. Li, S.D. Soelberg, Z. Taylor, V. Sakthivelpathi, C.E. Furlong, J.H. Kim, S.G. Ahn, P.D. Han, L.M. Starita, J. Zhu, J.H. Chung, Highly sensitive immunoresistive sensor for point-of-care screening for COVID-19. Biosensors 12(3), 149 (2022). https://doi.org/10.3390/bios12030149
H.A. Alhazmi, W. Ahsan, B. Mangla, S. Javed, M.Z. Hassan, M. Asmari, M. Al Bratty, A. Najmi, Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements. Nanotechnol Rev 11(1), 96–116 (2021). https://doi.org/10.1515/ntrev-2022-0009
Z. Rahmati, M. Roushani, SARS-CoV-2 virus label-free electrochemical nanohybrid MIP-aptasensor based on Ni(3)(BTC)(2) MOF as a high-performance surface substrate. Mikrochim. Acta 189(8), 287 (2022). https://doi.org/10.1007/s00604-022-05357-8
D. Soto, J. Orozco, Peptide-based simple detection of SARS-CoV-2 with electrochemical readout. Anal. Chim. Acta 1205, 339739 (2022). https://doi.org/10.1016/j.aca.2022.339739
J. Kim, S. Jeong, S. Sarawut, H. Kim, S.U. Son, S. Lee, G. Rabbani, H. Kwon, E.K. Lim, S.N. Ahn, S.K. Park, An immunosensor based on a high performance dual-gate oxide semiconductor thin-film transistor for rapid detection of SARS-CoV-2. Lab Chip 22(5), 899–907 (2022). https://doi.org/10.1039/d1lc01116b
W. Heo, K. Lee, S. Park, K.A. Hyun, H.I. Jung, Electrochemical biosensor for nucleic acid amplification-free and sensitive detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA via CRISPR/Cas13a trans-cleavage reaction. Biosens. Bioelectron. 201, 113960 (2022). https://doi.org/10.1016/j.bios.2021.113960
J.H. Park, G.Y. Lee, Z. Song, J.H. Bong, Y.W. Chang, S. Cho, M.J. Kang, J.C. Pyun, Capacitive biosensor based on vertically paired electrodes for the detection of SARS-CoV-2. Biosens Bioelectron 202, 113975 (2022). https://doi.org/10.1016/j.bios.2022.113975
C. Jiang, X. Mu, S. Liu, Z. Liu, B. Du, J. Wang, J. Xu, A study of the detection of SARS-CoV-2 ORF1ab gene by the use of electrochemiluminescent biosensor based on dual-probe hybridization. Sensors 22(6), 2402 (2022). https://doi.org/10.3390/s22062402
G. Seo, G. Lee, M.J. Kim, S.H. Baek, M. Choi, K.B. Ku, C.S. Lee, S. Jun, D. Park, H.G. Kim, S.J. Kim, J.O. Lee, B.T. Kim, E.C. Park, S.I. Kim, Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 14(4), 5135–5142 (2020). https://doi.org/10.1021/acsnano.0c02823
T.Y. Tai, A. Sinha, I. Sarangadharan, A.K. Pulikkathodi, S.L. Wang, G.Y. Lee, J.I. Chyi, S.C. Shiesh, G.B. Lee, Y.L. Wang, Design and demonstration of tunable amplified sensitivity of AlGaN/GaN high electron mobility transistor (HEMT)-based biosensors in human serum. Anal Chem 91(9), 5953–5960 (2019). https://doi.org/10.1021/acs.analchem.9b00353
D. Sung, J. Koo, A review of BioFET’s basic principles and materials for biomedical applications. Biomed Eng Lett 11(2), 85–96 (2021). https://doi.org/10.1007/s13534-021-00187-8
M. Chen, D. Cui, Z. Zhao, D. Kang, Z. Li, S. Albawardi, S. Alsageer, F. Alamri, A. Alhazmi, M.R. Amer, C. Zhou, Highly sensitive, scalable, and rapid SARS-CoV-2 biosensor based on In(2)O(3) nanoribbon transistors and phosphatase. Nano Res 15(6), 5510–5516 (2022). https://doi.org/10.1007/s12274-022-4190-0
P.H. Chen, C.C. Huang, C.C. Wu, P.H. Chen, A. Tripathi, Y.L. Wang, Saliva-based COVID-19 detection: a rapid antigen test of SARS-CoV-2 nucleocapsid protein using an electrical-double-layer gated field-effect transistor-based biosensing system. Sens Actuators B Chem 357, 131415 (2022). https://doi.org/10.1016/j.snb.2022.131415
M. Thanihaichelvan, S.N. Surendran, T. Kumanan, U. Sutharsini, P. Ravirajan, R. Valluvan, T. Tharsika, Selective and electronic detection of COVID-19 (Coronavirus) using carbon nanotube field effect transistor-based biosensor: a proof-of-concept study. Mater Today Proc 49, 2546–2549 (2022). https://doi.org/10.1016/j.matpr.2021.05.011
L. Wang, X. Wang, Y. Wu, M. Guo, C. Gu, C. Dai, D. Kong, Y. Wang, C. Zhang, D. Qu, C. Fan, Y. Xie, Z. Zhu, Y. Liu, D. Wei, Rapid and ultrasensitive electromechanical detection of ions, biomolecules and SARS-CoV-2 RNA in unamplified samples. Nat Biomed Eng. 6(3), 276–285 (2022). https://doi.org/10.1038/s41551-021-00833-7
M. Mahani, M. Faghihi-Fard, F. Divsar, M. Torkzadeh-Mahani, F. Khakbaz, Ultrasensitive FRET-based aptasensor for interleukin-6 as a biomarker for COVID-19 progression using nitrogen-doped carbon quantum dots and gold nanoparticles. Mikrochim Acta 189(12), 472 (2022). https://doi.org/10.1007/s00604-022-05570-5
H.A. Alhadrami, G. Suaifan, M.M. Zourob, A portable nanoprobe for rapid and sensitive detection of SARS-CoV-2 S1 protein. Biosensors 12(4), 232 (2022). https://doi.org/10.3390/bios12040232
J. Han, S.L. Lee, J. Kim, G. Seo, Y.W. Lee, SARS-CoV-2 spike protein detection using slightly tapered no-core fiber-based optical transducer. Mikrochim. Acta 189(9), 321 (2022). https://doi.org/10.1007/s00604-022-05413-3
B. Kang, Y. Lee, J. Lim, D. Yong, Y. Ki, S.W. Choi, S.S. Yoon, S. Jang, S. Uk Son, T. Kang, J. Jung, K.S. Lee, M.H. Kim, E.K. Lim, FRET-based hACE2 receptor mimic peptide conjugated nanoprobe for simple detection of SARS-CoV-2 Chem. Eng J 442, 136143 (2022). https://doi.org/10.1016/j.cej.2022.136143
W. Xu, J. Liu, D. Song, C. Li, A. Zhu, F. Long, Rapid, label-free, and sensitive point-of-care testing of anti-SARS-CoV-2 IgM/IgG using all-fiber Fresnel reflection microfluidic biosensor. Mikrochim. Acta 188(8), 261 (2021). https://doi.org/10.1007/s00604-021-04911-0
U.K. Sur, C. Santra, Spectroscopy: a versatile sensing tool for cost-effective and rapid detection of novel coronavirus (COVID-19). Emergent Mater 5(2), 249–260 (2022). https://doi.org/10.1007/s42247-022-00358-y
P.B.D. Silva, J.R.D. Silva, M.C. Rodrigues, J.A. Vieira, I.A. Andrade, T. Nagata, A.S. Santos, S.W.D. Silva, M. Rocha, S.N. Bao, P.M. Moraes-Vieira, J. Proenca-Modena, M.K.C. Angelim, G.F. Souza, S.P. Muraro, A.L.B. Barros, G.A. Souza Martins, F. Ribeiro-Dias, G. Machado, M.R. Fessel, A.M. Chudzinski-Tavassi, C.M. Ronconi, D. Goncalves, R. Curi, O.N. Oliveira, R.B. Azevedo, Detection of SARS-CoV-2 virus via dynamic light scattering using antibody-gold nanoparticle bioconjugates against viral spike protein. Talanta 243, 123355 (2022). https://doi.org/10.1016/j.talanta.2022.123355
D. Antoine, M. Mohammadi, M. Vitt, J.M. Dickie, S.S. Jyoti, M.A. Tilbury, P.A. Johnson, K.E. Wawrousek, J.G. Wall, Rapid, point-of-care scFv-SERS assay for femtogram level detection of SARS-CoV-2. ACS Sens 7(3), 866–873 (2022). https://doi.org/10.1021/acssensors.1c02664
D. Kawasaki, H. Yamada, K. Sueyoshi, H. Hisamoto, T. Endo, Imprinted photonic crystal-film-based smartphone-compatible label-free optical sensor for SARS-CoV-2 testing. Biosensors 12(4), 200 (2022). https://doi.org/10.3390/bios12040200
M.U. Hadi, M. Khurshid, SARS-CoV-2 detection using optical fiber based sensor method. Sensors 22(3), 751 (2022). https://doi.org/10.3390/s22030751
Z. Wang, J. Chen, S.A. Khan, F. Li, J. Shen, Q. Duan, X. Liu, J. Zhu, Plasmonic metasurfaces for medical diagnosis applications: a review. Sensors 22(1), 133 (2021). https://doi.org/10.3390/s22010133
P. Srithong, S. Chaiyo, E. Pasomsub, S. Rengpipat, O. Chailapakul, N. Praphairaksit, A novel delayed lateral flow immunoassay for enhanced detection of SARS-CoV-2 spike antigen. Mikrochim Acta 189(10), 386 (2022). https://doi.org/10.1007/s00604-022-05467-3
J. Liang, W. Zhang, Y. Qin, Y. Li, G.L. Liu, W. Hu, Applying machine learning with localized surface plasmon resonance sensors to detect SARS-CoV-2 particles. Biosensors 12(3), 173 (2022). https://doi.org/10.3390/bios12030173
P. Mohankumar, J. Ajayan, T. Mohanraj, R. Yasodharan, Recent developments in biosensors for healthcare and biomedical applications: a review. Measurement 167, 108293 (2021). https://doi.org/10.1016/j.measurement.2020.108293
M. Song, X. Lin, Z. Peng, S. Xu, L. Jin, X. Zheng, H. Luo, Materials and methods of biosensor interfaces with stability. Frontiers Mater 7, 583739 (2021). https://doi.org/10.3389/fmats.2020.583739
W.Y. Lim, B.L. Lan, N. Ramakrishnan, Emerging biosensors to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review. Biosensors 11(11), 434 (2021). https://doi.org/10.3390/bios11110434
S. Ahmadi, N. Rabiee, Y. Fatahi, S.E. Hooshmand, M. Bagherzadeh, M. Rabiee, V. Jajarmi, R. Dinarvand, S. Habibzadeh, M.R. Saeb, R.S. Varma, M. Shokouhimehr, M.R. Hamblin, Green chemistry and coronavirus. Sustain Chem Pharm 21, 100415 (2021). https://doi.org/10.1016/j.scp.2021.100415
H.K. Choi, M.J. Lee, S.N. Lee, T.H. Kim, B.K. Oh, Noble metal nanomaterial-based biosensors for electrochemical and optical detection of viruses causing respiratory illnesses. Front Chem 9, 672739 (2021). https://doi.org/10.3389/fchem.2021.672739
M. Que, C. Lin, J. Sun, L. Chen, X. Sun, Y. Sun, Progress in ZnO nanosensors. Sensors 21(16), 5502 (2021). https://doi.org/10.3390/s21165502
R. del Caño, T. García-Mendiola, D. García-Nieto, R. Álvaro, M. Luna, H.A. Iniesta, R. Coloma, C.R. Diaz, P. Milán-Rois, M. Castellanos, M. Abreu, R. Cantón, J.C. Galán, T. Pineda, F. Pariente, R. Miranda, Á. Somoza, E. Lorenzo, Amplification-free detection of SARS-CoV-2 using gold nanotriangles functionalized with oligonucleotides. Microchim. Acta 189(4), 171 (2022). https://doi.org/10.1007/s00604-022-05272-y
R. Stephanie, M.W. Kim, S.H. Kim, J.-K. Kim, C.Y. Park, T.J. Park, Recent advances of bimetallic nanomaterials and its nanocomposites for biosensing applications. TrAC Trends Anal Chem 135, 116159 (2021). https://doi.org/10.1016/j.trac.2020.116159
N. Alizadeh, A. Salimi, Multienzymes activity of metals and metal oxide nanomaterials: applications from biotechnology to medicine and environmental engineering. J Nanobiotechnology. 19(1), 26 (2021). https://doi.org/10.1186/s12951-021-00771-1
X. Liang, N. Li, R. Zhang, P. Yin, C. Zhang, N. Yang, K. Liang, B. Kong, Carbon-based SERS biosensor: from substrate design to sensing and bioapplication. NPG Asia Mater 13(1), 8 (2021). https://doi.org/10.1038/s41427-020-00278-5
J. Belza, A. Opletalova, K. Polakova, Carbon dots for virus detection and therapy. Mikrochim. Acta 188(12), 430 (2021). https://doi.org/10.1007/s00604-021-05076-6
V. Naresh, N. Lee, A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 21(4), 1109 (2021). https://doi.org/10.3390/s21041109
H.M. Fahmy, E.S. Abu Serea, R.E. Salah-Eldin, S.A. Al-Hafiry, M.K. Ali, A.E. Shalan, S. Lanceros-Mendez, Recent progress in graphene- and related carbon-nanomaterial-based electrochemical biosensors for early disease detection. Acs Biomater Sci Eng 8(3), 964–1000 (2022). https://doi.org/10.1021/acsbiomaterials.1c00710
A. Aykac, H. Gergeroglu, B. Besli, E.O. Akkas, A. Yavas, S. Guler, F. Gunes, M. Erol, An overview on recent progress of metal oxide/graphene/CNTs-based nanobiosensors. Nanoscale Res Lett 16(1), 65 (2021). https://doi.org/10.1186/s11671-021-03519-w
N. Chandrasekar, R. Balaji, R.S. Perala, N.Z. Nik Humaidi, K. Shanmugam, Y.C. Liao, M.T. Hwang, S. Govindaraju, A brief review of graphene-based biosensors developed for rapid detection of COVID-19 biomarkers. Biosensors 13, 3–307 (2023). https://doi.org/10.3390/bios13030307
M. Ebrahimi, M. Asadi, O. Akhavan, Graphene-based nanomaterials in fighting the most challenging viruses and immunogenic disorders. ACS Biomater Sci.Eng 8(1), 54–81 (2022). https://doi.org/10.1021/acsbiomaterials.1c01184
J. Sengupta, C.M. Hussain, The emergence of carbon nanomaterials as effective nano-avenues to fight against COVID-19. Materials 16(3), 1068 (2023). https://doi.org/10.3390/ma16031068
H. Riazi, G. Taghizadeh, M. Soroush, MXene-based nanocomposite sensors ACS. Omega 6(17), 11103–11112 (2021). https://doi.org/10.1021/acsomega.0c05828
X. Lin, Z. Li, J. Qiu, Q. Wang, J. Wang, H. Zhang, T. Chen, Fascinating MXene nanomaterials: emerging opportunities in the biomedical field. Biomater Sci 9(16), 5437–5471 (2021). https://doi.org/10.1039/d1bm00526j
V. Kumar, S. Mishra, R. Sharma, J. Agarwal, U. Ghoshal, T. Khanna, L.K. Sharma, S.K. Verma, P. Mishra, S. Tiwari, Development of RNA-based assay for rapid detection of SARS-CoV-2 in clinical samples. Intervirology 65(4), 181–187 (2022). https://doi.org/10.1159/000522337
T.A. Yano, T. Kajisa, M. Ono, Y. Miyasaka, Y. Hasegawa, A. Saito, K. Otsuka, A. Sakane, T. Sasaki, K. Yasutomo, R. Hamajima, Y. Kanai, T. Kobayashi, Y. Matsuura, M. Itonaga, T. Yasui, Ultrasensitive detection of SARS-CoV-2 nucleocapsid protein using large gold nanoparticle-enhanced surface plasmon resonance. Sci Rep 12(1), 1060 (2022). https://doi.org/10.1038/s41598-022-05036-x
G.A. Naikoo, F. Arshad, I.U. Hassan, T. Awan, H. Salim, M.Z. Pedram, W. Ahmed, V. Patel, A.S. Karakoti, A. Vinu, Nanomaterials-based sensors for the detection of COVID-19: A review. Bioeng Transl Med. 7(3), e10305 (2022). https://doi.org/10.1002/btm2.10305
M. Harun-Ur-Rashid, T. Foyez, I. Jahan, K. Pal, A.B. Imran, Rapid diagnosis of COVID-19 via nano-biosensor-implemented biomedical utilization: a systematic review. RSC Adv 12(15), 9445–9465 (2022). https://doi.org/10.1039/d2ra01293f
D. Lee, T. Lee, J.H. Hong, H.G. Jung, S.W. Lee, G. Lee, D.S. Yoon, State-of-the-art nanotechnologies used in the development of SARS-CoV-2 biosensors: a review. Meas Sci Technol 33(6), 1–21 (2022). https://doi.org/10.1088/1361-6501/ac51f1
M. Sher, A. Faheem, W. Asghar, S. Cinti, Nano-engineered screen-printed electrodes: a dynamic tool for detection of viruses. Trends Analyt Chem 143, 116374 (2021). https://doi.org/10.1016/j.trac.2021.116374
S.K. Kim, J.U. Lee, M.J. Jeon, S.-K. Kim, S.-H. Hwang, M.E. Hong, S.J. Sim, Bio-conjugated nanoarchitectonics with dual-labeled nanoparticles for a colorimetric and fluorescent dual-mode serological lateral flow immunoassay sensor in detection of SARS-CoV-2 in clinical samples. RSC Adv. 13(39), 27225–27232 (2023). https://doi.org/10.1039/d3ra04373h
S.M. Mousavi, M.Y. Kalashgrani, A. Gholami, N. Omidifar, M. Binazadeh, W.-H. Chiang, Recent advances in quantum dot-based lateral flow immunoassays for the rapid, point-of-care diagnosis of COVID-19. Biosensors 13(8), 786 (2023). https://doi.org/10.3390/bios13080786
W. Stokes, B.M. Berenger, A.A. Venner, V. Deslandes, J.L.V. Shaw, Point of care molecular and antigen detection tests for COVID-19: current status and future prospects. Expert Rev Mol Diagn. 22(8), 797–809 (2022). https://doi.org/10.1080/14737159.2022.2122712
R. Liu, Y. Hu, Y. He, T. Lan, J. Zhang, Translating daily COVID-19 screening into a simple glucose test: a proof of concept study. Chem. Sci. 12(26), 9022–9030 (2021). https://doi.org/10.1039/d1sc00512j
H. Chu, C. Liu, J. Liu, J. Yang, Y. Li, X. Zhang, Recent advances and challenges of biosensing in point-of-care molecular diagnosis. Sens Actuators B Chem 348, 130708 (2021). https://doi.org/10.1016/j.snb.2021.130708
Q. Li, X. Zhou, Q. Wang, W. Liu, C. Chen, Microfluidics for COVID-19: from current work to future perspective. Biosensors 13(2), 163 (2023). https://doi.org/10.3390/bios13020163
A. Kumar, A. Parihar, U. Panda, D.S. Parihar, Microfluidics-based point-of-care testing (POCT) devices in dealing with waves of COVID-19 pandemic: the emerging solution. ACS Appl Bio Mater 5(5), 2046–2068 (2022). https://doi.org/10.1021/acsabm.1c01320
J. Zhang, T. Lan, Y. Lu, Translating in vitro diagnostics from centralized laboratories to point-of-care locations using commercially-available handheld meters. Trends Analyt Chem. 124, 115782 (2020). https://doi.org/10.1016/j.trac.2019.115782
I.H. Cho, D.H. Kim, S. Park, Electrochemical biosensors: perspective on functional nanomaterials for on-site analysis. Biomater Res 24, 6 (2020). https://doi.org/10.1186/s40824-019-0181-y
A. Chakravarthy, A. Nandakumar, G. George, S. Ranganathan, S. Umashankar, N. Shettigar, D. Palakodeti, A. Gulyani, A. Ramesh, Engineered RNA biosensors enable ultrasensitive SARS-CoV-2 detection in a simple color and luminescence assay. Life Sci Alliance 4(12), e202101213 (2021). https://doi.org/10.6508/lsa.202101213
G. Nava, L. Casiraghi, T. Carzaniga, G. Zanchetta, M. Chiari, F. Damin, V. Bollati, L. Signorini, S. Delbue, T. Bellini, M. Buscaglia, Digital detection of single virus particles by multi-spot, label-free imaging biosensor on anti-reflective glass. Small 19(32), e2300947 (2023). https://doi.org/10.1002/smll.202300947
D. Lu, D. Liu, X. Wang, Y. Liu, Y. Liu, R. Ren, G. Pang, Kinetics of drug molecule interactions with a newly developed nano-gold-modified spike protein electrochemical receptor sensor. Biosensors 12(10), 888 (2022). https://doi.org/10.3390/bios12100888
M. Samir, D. Salah, S. Donia, A. Kasry, Specific chemical modification of nanohole edges in membrane graphene for protein binding. ACS Applied Nano Mater 5(3), 3733–3742 (2022). https://doi.org/10.1021/acsanm.1c04390
A. Pligovka, A. Lazavenka, U. Turavets, A. Hoha, M. Salerno, Two-level 3D column-like nanofilms with hexagonally-packed tantalum fabricated via anodizing of Al/Nb and Al/Ta layers-a potential nano-optical biosensor. Materials 16(3), 993 (2023). https://doi.org/10.3390/ma16030993
M. Vafabakhsh, M. Dadmehr, S Kazemi Noureini, Z Es’haghi, M Malekkiani, M Hosseini, Paper-based colorimetric detection of COVID-19 using aptasenor based on biomimetic peroxidase like activity of ChF/ZnO/CNT nano-hybrid. Spectrochim Acta A Mol Biomol Spectrosc 301, 122980 (2023). https://doi.org/10.1016/j.saa.2023.122980
W.I. Lee, A. Subramanian, S. Mueller, K. Levon, C.Y. Nam, M.H. Rafailovich, potentiometric biosensors based on molecular-imprinted self-assembled monolayer films for rapid detection of influenza a virus and SARS-CoV-2 spike protein. ACS Appl Nano Mater. 5(4), 5045–5055 (2022). https://doi.org/10.1021/acsanm.2c00068
Y. Sun, L. Zhou, Y. Ding, C. Liu, Z.S. Mao, Q.Y. Jiang, J. Chen, F. Chen, Y. Cao, Fabrication of flexible electrospinning nano-fiber membrane for detection of respiratory tract transmission virus based on SERS. Talanta 266(Pt 2), 125127 (2024). https://doi.org/10.1016/j.talanta.2023.125127
R. Liu, L. He, Y. Hu, Z. Luo, J. Zhang, A serological aptamer-assisted proximity ligation assay for COVID-19 diagnosis and seeking neutralizing aptamers. Chem. Sci. 11(44), 12157–12164 (2020). https://doi.org/10.1039/d0sc03920a
W. Zhang, N. Liu, J. Zhang, Functional nucleic acids as modular components against SARS-CoV-2: From diagnosis to therapeutics. Biosens. Bioelectron. 201, 113944 (2022). https://doi.org/10.1016/j.bios.2021.113944
A.F. Nahhas, T.J. Webster, The promising use of nano-molecular imprinted templates for improved SARS-CoV-2 detection, drug delivery and research. J Nanobiotechnology. 19(1), 305 (2021). https://doi.org/10.1186/s12951-021-01032-x
A. Bisht, A. Mishra, H. Bisht, R.M. Tripathi, Nanomaterial based biosensors for detection of viruses including sars-cov-2: a review. J Anal Test. 5(4), 327–340 (2021). https://doi.org/10.1007/s41664-021-00200-0
M. Drobysh, A. Ramanaviciene, R. Viter, C.F. Chen, U. Samukaite-Bubniene, V. Ratautaite, A. Ramanavicius, Biosensors for the determination of SARS-CoV-2 virus and diagnosis of COVID-19 infection. Int J Mol Sci 23(2), 666 (2022). https://doi.org/10.3390/ijms23020666
P.L. Truong, Y. Yin, D. Lee, S.H. Ko, Advancement in COVID-19 detection using nanomaterial-based biosensors. Exploration. 3(1), 20210232 (2023). https://doi.org/10.1002/exp.20210232
L.E. Breshears, B.T. Nguyen, S. Mata Robles, L. Wu, J.Y. Yoon, Biosensor detection of airborne respiratory viruses such as SARS-CoV-2. SLAS Technol 27(1), 4–17 (2022). https://doi.org/10.1016/j.slast.2021.12.004
H.O. Kaya, A.E. Cetin, M. Azimzadeh, S.N. Topkaya, Pathogen detection with electrochemical biosensors: advantages, challenges and future perspectives. J Electroanal Chem 882, 114989 (2021). https://doi.org/10.1016/j.jelechem.2021.114989
S. B. Somvanshi, P. B. Kharat, T. S. Saraf, S B. Somwanshi, S B. Shejul, K M. Jadhav, Multifunctional nano-magnetic particles assisted viral RNA-extraction protocol for potential detection of COVID-19. Mater. Res. Innov. 25(3), 169–174 (2020). https://doi.org/10.1080/14328917.2020.1769350
Acknowledgements
Authors are grateful to Seoul National University in Republic of Korea and Nantong University in China for the fnancial and infrastructural support. Authors extend their contributions to NantongEgens Biotechnology co., LTD in China for the substantial contributions to this paper.
Funding
This work was supported by Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P0012770) and grant from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2023–00208902)and SUN′s Materials Education/Research Division for Creative Global Leaders (4120200513611) and National Natural Science Foundation of China (81202249) and Nantong science and technology project (JC22022105).
Author information
Authors and Affiliations
Contributions
Writing, review and editing: YL, YL, YH, LW, JW, NB, YK and HWJ. YL and YL contributed equally to this work as the first author. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Li, Y., Hang, Y. et al. Rapid assays of SARS-CoV-2 virus and noble biosensors by nanomaterials. Nano Convergence 11, 2 (2024). https://doi.org/10.1186/s40580-023-00408-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40580-023-00408-z