Abstract
This work reports the rational design of MnOx nanorods on 3D crushed reduced graphene oxide (MnOx/C-rGO) by chemical reduction of Ni-incorporated graphene oxide (GO) followed by chemical etching to remove Ni. The resulting MnOx/C-rGO composite synergistically integrates the electronic properties and geometry structure of MnOx and 3D C-rGO. As a result, MnOx/C-rGO shows a significantly higher specific capacitance (Csp) of 863 F g−1 than MnOx/2D graphene sheets (MnOx/S-rGO) (373 F g−1) and MnOx (200 F g−1) at a current density of 0.2 A g−1. Furthermore, when assembled into symmetric supercapacitors, the MnOx/C-rGO-based device delivers a higher Csp (288 F g−1) than MnOx/S-rGO-based device (75 F g−1) at a current density of 0.3 A g−1. The superior capacitive performance of the MnOx/C-rGO-based symmetric device is attributed to the enlarged accessible surface, reduced lamellar stacking of graphene, and improved ionic transport provided by the 3D architecture of MnOx/C-rGO. In addition, the MnOx/C-rGO-based device exhibits an energy density of 23 Wh kg−1 at a power density of 113 Wkg−1, and long-term cycling stability, demonstrating its promising potential for practical application.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Supercapacitors are promising energy storage devices owing to their fast charge/discharge rate, excellent cyclic stability, high power density, and good environmental compatibility [1,2,3,4,5,6]. However, extensive practical applications of supercapacitors require improving the performance of the electrode materials [7,8,9,10,11]. Among various transition-metal-based electrode materials, manganese dioxide (MnO2) has been considered as a potential electrode material for supercapacitors due to its high theoretical capacitance of 1370 F g−1 [12,13,14]. However, the practical capacitance of MnO2 is usually much lower than the theoretical capacitance due to its inherent poor electrical conductivity and slow ion transport. Moreover, the electrochemical dissolution of MnO2 during charge–discharge cycles degrades the cycling stability, thus limiting its practical applications [14,15,16,17].
To date, different strategies, such as nanostructuring, defect engineering, hybridization, and surface modification have been used to improve the electrochemical performance of MnO2-based electrodes [1, 18, 19]. Among them, the hybridization of MnO2 with carbon-based nanomaterials, such as graphene, carbon nanotubes, fullerenes, etc., has been employed to overcome the existing limitations of MnO2 [20, 21]. It is worth mentioning that a two-dimensional (2D) graphene nanostructure with a single carbon atom layer of sp2 graphitic configuration offers superior electrical conductivity upon hybridization with metal oxides [22,23,24]. However, the reported MnO2/2D graphene composites usually show rapid capacitance decay due to the low accessibility of electrolyte ions into the electrode materials [25,26,27,28,29]. 2D graphene sheets are soft and can form conformal contact with a surface or each other with the high aspect ratio of 2D morphology, which increases the overlapping field and decreases the reciprocal distance (d), resulting in an intense attraction [30]. Moreover, 2D graphene sheets often result in irreversible agglomeration due to strong π–π interactions and van der Waals force. The van der Waals force-induced strong attraction between parallel sheets of 2D graphene correlates with the overlapping field and the fourth power of the reciprocal distance of separation (1/d4) [30, 31]. The consequent result is lamellar stacking in the horizontal alignment of graphene microstructures, which hinders charge transport. Despite several approaches to resolve such agglomeration in 2D sheets (e.g., graphene sheets), the inherent drawbacks of 2D-graphene sheets (S-rGO) associated with the irreversible agglomeration and restacking of S-rGO have not been solved completely.
Recently, compositing MnO2 with 3D graphene instead of 2D graphene has been proposed as a potential way to prevent aggregation and lamellar stacking of rGO [30, 32,33,34]. Additionally, the large surface area and the fast and reversible Faradic redox reactions of 3D-structured materials can greatly improve electrochemical performance. 3D graphene/MnO2 composite has been successfully synthesized by combining chemical vapor deposition (CVD) and hydrothermal methods. This composite had a Csp of 333 F g−1 at 0.2 A g−1 [33]. Biomass-derived N-doped 3D graphene@MnO2 (N-G@MnO2) composite was previously prepared by CVD method and it displayed a high Csp of 411.5 F g−1 at 0.5 A g−1 [34]. However, both composites were fabricated using the CVD process which typically requires special apparatus and produces highly toxic gaseous as by-products [35]. Lin et al. [36] reported the synthesis of well-organized ε-MnO2 hollow spheres/rGO composites which exhibited a Csp of 471 F g−1 at 0.8 A g−1. Although the use of 3D-structured composites of MnO2 and rGO may lead to a higher electrochemical performance, the fabrication of such composites is complicated and requires special attention to maintain the uniformity of the 3D structure.
Herein, we demonstrate a facile chemical route to fabricate MnOx on 3D crushed rGO (MnOx/C-rGO). The 3D-crushed graphene structure enhances the surface-to-volume ratio, while also enabling a high accessible surface area for fast ion transportation. The large number of voids and junction defects introduced by the highly disordered 3D-crushed structure provides sufficient active sites to more ions. Furthermore, the network of MnOx nanorods on the rGO matrix can help to reduce particle aggregation and prevent the restacking of rGO. As a result, the MnOx/C-rGO composite delivers a higher Csp than both MnOx/S-rGO and MnOx. Moreover, the capacitive performance of the MnOx/C-rGO composite is also superior compared to MnOx/S-rGO.
2 Experimental
2.1 Materials
Graphite powder (< 20 µm with purity > 99.9 wt.%, Sigma Aldrich, Germany), sodium nitrate (NaNO3; Lab-Scan, Ireland), potassium permanganate (KMnO4; Sigma Aldrich, Germany), sulfuric acid (18.4 M H2SO4; Lab-Scan, Ireland), hydrogen peroxide (H2O2; Sigma Aldrich, Germany), hydrochloric acid (12 M HCl; Lab-Scan, Ireland), aqueous ammonia (NH4OH; Lab-Scan, Ireland), hydrazine hydrate (Sigma Aldrich, Germany), benzyl alcohol (Merck, Germany), ethanol (Lab-Scan, Ireland), nickel chloride hexahydrate (NiCl2.6H2O; Merck, Germany), sodium hydroxide (NaOH; Lab-Scan, Ireland), sodium borohydride (NaBH4; Sigma Aldrich, Germany), ethylene glycol (Sigma Aldrich, Germany), polyvinylidene fluoride (PVDF; Sigma Aldrich, Germany), N-methyl-2-pyrrolidone (NMP; Sigma Aldrich, Germany) and sodium sulfate (Na2SO4; Sigma-Aldrich, Germany) were purchased and used without further purification. A water purification system (Barnstead nano pure, Thermo Scientific, USA) was used to obtain deionized (DI) water. A digital ultrasonic bath (Powersonic 505, Hwashin, South Korea) was used for sonication.
2.2 Preparation of MnOx/S-rGO and MnOx/C-rGO
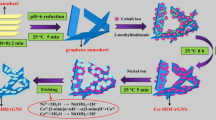
Graphene oxide (GO) was prepared from graphite powder following the modified Hummers method [37, 38]. A mixture of graphite powder (1.0 g), NaNO3 (0.5 g), KMnO4 (3.0 g), and DI water (46 mL) was prepared. A concentrated H2SO4 solution (23 mL) was slowly added into the mixture under strong stirring at about 35 °C. The temperature of the mixture was raised to about 98 °C and kept for 15 min. After that, the temperature of the mixture was lowered to 60 °C with the addition of 140 mL of warm DI water and 10 mL H2O2 (30% w/v). The mixture was further stirred for 2 h under continuous stirring to obtain the GO suspension. This suspension was washed several times with 1 M HCl solution. DI water was used to completely remove chloride ions. The suspension was dried in an oven to obtain GO powder. To synthesize rGO, a GO suspension was prepared by adding 360 mg of the GO powder into 400 mL of DI followed by ultrasonication. Aqueous ammonia (10 mL, 25% w/v) and hydrazine hydrate (10 mL, 80%) were then added under continuous stirring for 2 h at 98 °C. The dispersed 2D rGO sheets (S-rGO) were filtrated and dried in a drying oven at 60 °C. The synthesized S-rGO was used to prepare MnOx/S-rGO by mixing benzyl alcohol (100 µL) with the prepared S-rGO (200 mg) and DI water (30 mL) under slow stirring followed by the addition of KMnO4 (60 mg). The mixture was then stirred for 10 h at 60 °C. The obtained black MnOx/S-rGO powder was washed with DI water and ethanol. The solid powder was dried at 50 °C for 12 h in a drying oven and stored for further use.
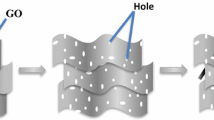
The C-rGO sample was obtained by first preparing Ni@rGO core–shell, where the Ni nanoparticles were synthesized according to a previous report [39]. Typically, 1000 mg of Ni NPs was dispersed in 100 mL ethylene glycol solution and sonicated for 60 min to obtain EG-wrapped Ni NPs. The previously prepared GO was then mixed with the EG-wrapped Ni NPs at a weight ratio of 1:5. The mixture was sonicated for 1 h followed by 24 h stirring, which resulted in a blackish-brown gel. The mixture of hydrazine hydrate (5 mL; 80% w/v) and aqueous ammonia (5 mL; 25% w/v) was added into the resulting blackish-brown gel under continuous stirring for 2 h at 98 °C. The solid black mass of Ni@rGO was separated by centrifugation and washed several times with DI water. 3D C-rGO was obtained from the prepared Ni@rGO by leaching the Ni core using HCl (25 mL, 37%) solution. DI water and ethanol were used to wash the prepared product, which was collected by centrifugation and dried overnight in an oven at 60 °C. The MnOx/C-rGO was prepared using the same procedures as MnOx/S-rGO, except that C-rGO was used instead of S-rGO.
2.3 Characterization
The surface morphology of the prepared composites was analyzed using field-emission scanning electron microscope (FESEM; JSM-7600F, JEOL, Japan) and transmission electron microscope (TEM, G2 F20, Tecnai, Japan). The phase composition and crystallinity of the samples were investigated using X-Ray diffractometer (XRD, Empyrean, PANalytical-Netherlands) equipped with Cu-Kα radiation (λ = 1.5418 Å). X-ray photoelectron spectrometer (XPS, Kratos AXIS Nova, Kyoto, Japan) equipped with a monochromator Al Kα (hv = 1486.6 eV) was used to analyze the surface compositions of the samples.
2.4 Electrochemical measurements
The electrochemical measurements were performed by cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) using an electrochemical workstation, (CHI 660E, CH Instruments, USA). In the three-electrode system, a single compartment electrochemical cell was produced with the sample-coated graphite rod (99.9% pure; OtoolWorld, USA; surface area ~ 0.28 cm2) as the working electrode, platinum (Pt) wire as the counter electrode and Ag/AgCl (sat. KCl) as the reference electrode. The improved solvent casting and drop drying processes were used to prepare the working electrode. A homogeneous slurry of the coating material was prepared by mixing the active material (95%) and PVDF binder (5%) with 150 μL of NMP followed by ultrasonication for 1 h. The mirror-polished graphite rod was then coated with 15–20 μL of the prepared slurry and dried at 60 °C in a vacuum oven for 5 h. The areal active mass density was ~ 3 mg cm−2. The CV measurements were conducted at various scan rates from 5 to 100 mV s−1. The GCD tests were performed at different current densities from 0.2 to 5.0 Ag−1 using 0.5 M Na2SO4 as the electrolyte within a stable potential window of − 0.1 to 0.8 V.
For the two-electrode system, a customized coin cell compartment with disk-shaped graphite electrodes (surface area ~ 0.45 cm2) was used to evaluate the electrochemical performance of the prepared composites. The disk-shaped graphite electrodes were drop-casted by following the same procedures as described for the three-electrode system. Whatman filter paper soaked with aqueous 1.0 M Na2SO4 electrolyte was used as a separator and placed between two modified disk-shaped electrodes. Here, the CV measurements were performed at different scan rates from 5 to 100 mV s−1. The GCD experiments were performed at different current densities from 0.3 to 5.0 A g−1 using an aqueous 1.0 M Na2SO4 electrolyte within a potential range of 0 to 1.5 V. EIS measurements were conducted using the two-electrode system within a frequency range of 0.01 to 100,000 Hz at the open-circuit potential (OCP) with an AC amplitude of 10 mV at room temperature. The long-term cyclability of the prepared electrode materials was performed by repeating GCD cycles up to 10,000 times at a current density of 5 A g−1 and potential range of 0 to 1.5 V in the two-electrode system. The voltage holding test was performed by subjecting a maximum potential of 1.5 V for 60 h after charging at a current density of 2 A g−1.
In the three-electrode system, the specific capacitance (Csp) was calculated from the GCD curves using Eq. (1) [29]
where I is the loaded current, Δt is the discharge time, ΔV is the potential range, and m is the mass loading of the active material. For the two-electrode system, Csp, energy density (E), and power density (P) were calculated using Eqs. (2)–(4) [40, 41].
The Coulombic efficiency (η) was calculated using Eq. (5) [40, 41].
where \(\Delta\) t is the time duration of charge or discharge part of GCD cycles. The capacitance retention (CR) values were calculated using Eq. (6).
3 Results and discussion
The morphological analysis of the as-prepared samples was conducted by FESEM. The FESEM images of S-rGO, C-rGO, MnOx/S-rGO, and MnOx/C-rGO are presented in Fig. 1. The S-rGO sample exhibits a flake-like architecture with isolated layers arranged intermittently edge-to-edge (Fig. 1a). The nanoscale interlocking of graphene sheets is responsible for this structure [42, 43]. The FESEM image of C-rGO is displayed in Fig. 1b. The C-rGO sample shows a nanoball-like structure with a crushed feature. The crushed graphene is formed due to the instability of graphene nanoballs as a result of the void formed at the core of Ni@rGO by etching with HCl solution. Figure 1c depicts the surface morphology of the MnOx/S-rGO composite. An interconnected network-like composite is formed with MnOx which covers the sheet-like S-rGO. However, these graphene nanosheets are slightly disrupted and not well-stacked. It is assumed that during the assembly of MnOx nanorods on the surface of the graphene nanosheets, a disruptive force separates these graphene sheets.
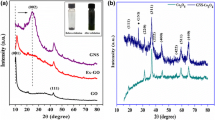
Figure 1d illustrates the FESEM image of the MnOx/C-rGO composite, in which many MnOx nanorods cover the C-rGO surface with high homogeneity. Interestingly, the ball-like shape of C-rGO is absent in the MnOx/C-rGO composite. The C-rGO is crushed and distributed in a well-dispersed network of MnOx nanorods. The TEM images (Additional file 1: Fig. S1a and b) show the formation of nanorod-shaped MnOx on S-rGO in MnOx/S-rGO and C-rGO in MnOx/C-rGO. These images also confirm the formation of layered birnessite MnOx. XRD was used to explore the crystal structures of the as-prepared samples. XRD patterns of MnOx/S-rGO and MnOx/C-rGO are shown in Fig. 2a. The diffraction peak at 2θ = 25° can be assigned to the (002) plane of rGO in MnOx/S-rGO and MnOx/C-rGO.[17] Here, the (002) peak in MnOx/S-rGO is broader than that of MnOx/C-rGO, indicating the more amorphous nature of S-rGO in MnOx/S-rGO. The diffraction peaks at 11.6°, 24.5°, 36.6° and 66° in the XRD patterns of MnOx/S-rGO and MnOx/C-rGO suggest the formation of a layered birnessite-type MnO2 phase (JCPDS No. 43-1456). The sharp peak at 36.6° corresponds to the ordered MnOx nanorods present in MnOx/C-rGO.
XPS analysis was conducted to further investigate the surface chemistry of the prepared samples. The comparative XPS survey spectra of the MnOx/S-rGO and MnOx/C-rGO are shown in Fig. 2b. The C 1 s peak at 284.5 eV and the O 1 s peak at 532.0 eV indicate the presence of carbon and oxygen from graphene sheets. The deconvoluted O 1 s peak at 530.0 eV can be assigned to the oxygen bound to manganese (Mn−O) in MnOx (Additional file 1: Fig. S2). The O 1 s peaks in both MnOx/S-rGO and MnOx/C-rGO composites at binding energies of 529.6, 530.0, and 532.4 eV are assigned to Mn–O–Mn, Mn–O–C, and C−O−C/C–OH bonds, respectively (Additional file 1: Fig. S2) [44, 45]. The above results confirm the formation of MnOx on the graphene oxide surface. The O 1 s peaks at 532.4 and 533.48 eV correspond to C–O–H and C–O–C of rGO in MnOx/S-rGO and MnOx/C-rGO composites, respectively (Additional file 1: Fig. S2).[46, 47] The deconvoluted C 1 s peaks of rGO reveal the presence of non-oxygenated carbon (C=C/C–C) at 284.6 eV, the epoxy group at 285.2 eV, and carbonyl carbon (C=O/–COOH) at 288.4 eV (Additional file 1: Fig. S3) [47,48,49]. The presence of N-doped graphene (NG) is also observed, as indicated by the small peak for N 1 s at ~ 400 eV in the XPS spectra [45]. The Mn 2p XPS spectra exhibit two characteristic peaks at 642.0 and 652.5 eV (Fig. 2b), corresponding to Mn 2p3/2 and Mn 2p1/2 spin–orbit peaks of MnOx, respectively, further confirming the presence of MnOx in the composite. The deconvoluted Mn 2p3/2 XPS spectra (Additional file 1: Fig. S4) of the MnOx/S-rGO and MnOx/C-rGO composites reveal the presence of two peaks centered at 642 and 644 eV corresponding to the spin–orbit doublet of Mn 2p3/2, confirming the presence of both Mn3+ and Mn4+ in these composites. The XPS analysis suggests the existence of Mn2O3 and MnO2 in the prepared composites, which is thus defined as MnOx in this study.[44, 49, 50] A small contribution of Mn(VII) from potassium manganate is confirmed by the presence of a trace amount of potassium on the surface in MnOx/S-rGO. It is noted that there is much more potassium in the MnOx/C-rGO composite without much increase of the Mn species. This K+ has a vital role in forming the layered birnessite phase. Some Mn4+ ions at the center of the MnO6 octahedral are replaced by Mn3+ ions, generating a net negative charge. The cations compensate for the net negative charges, leading to the formation of birnessite phase [25, 51,52,53].
Figure 3a shows the comparative CV curves of MnOx/S-rGO and MnOx/C-rGO obtained using a three-electrode system at a scan rate of 50 mV s−1 in the potential range of − 0.1 to 0.8 V in a 0.5 M Na2SO4 aqueous electrolyte. Both MnOx/S-rGO and MnOx/C-rGO composites exhibit symmetric rectangular CV curves, which are characteristic of EDLC. However, the integrated area of the CV curve of MnOx/C-rGO is significantly higher than that of MnOx/S-rGO. This indicates the higher charge storage capability of MnOx/C-rGO compared to MnOx/S-rGO [54,55,56]. The rectangular shape of the CV curves of MnOx, MnOx/S-rGO and MnOx/C-rGO is retained at all scan rates (5, 10, 20, 50, and 100 mV s−1), as shown in Additional file 1: Figs. S5a, S6a and S7a, respectively. The rectangular shape is well-retained with the increase of scan rate, indicating the good reversibility of the MnOx/C-rGO electrode.
Figure 3b displays the comparative GCD curves of the MnOx/S-rGO and MnOx/C-rGO composites at a current density of 0.2 A g−1. The MnOx/C-rGO composite exhibits a longer discharge time than MnOx/S-rGO. The GCD curves of MnOx, MnOx/S-rGO and MnOx/C-rGO at current densities; 0.2, 0.4, 0.8, 1, 2, 3, 4, and 5 A g−1 are shown in Additional file 1: Figs. S5b, S6b and S7b, respectively. The Csp values of MnOx/S-rGO and MnOx/C-rGO were calculated from the discharge curves of GCD using Eq. (1). MnOx/C-rGO exhibits a higher Csp (863 F g−1) than both MnOx/S-rGO (373 F g−1) and MnOx (200 F g−1) at 0.2 A g−1. The Csp values of MnOx/S-rGO and MnOx/C-rGO were also calculated individually at different current densities, as shown in Fig. 3c. The Csp values of MnOx/C-rGO, MnOx/S-rGO, and MnOx at a high current density of 5 A g−1 are 196, 43.0, and 20.0 F g−1, respectively. From Table 1, it can be observed that the prepared MnOx/C-rGO composite shows higher Csp than previously reported MnO2-based composites [33, 57,58,59,60,61,62,63].
Furthermore, symmetrical two-electrode supercapacitor devices with MnOx/S-rGO and MnOx/C-rGO were fabricated with a Whatman filter paper separator soaked in a 1 M Na2SO4 electrolyte. Figure 4a displays the comparative CV curves of the MnOx/S-rGO and MnOx/C-rGO-based symmetric devices at a scan rate of 20 mV s−1 in a potential range of 0 to 1.5 V. The symmetrical rectangular shape is present in the CV curves of both samples in the two-electrode system as well, indicating the EDLC behavior of both devices. With increasing scan rate, the MnOx/C-rGO-based device can maintain its CV curves, indicating its good rate performance (Additional file 1: Fig. S8a, b). The GCD curves of the MnOx/S-rGO and MnOx/C-rGO-based symmetric devices at a current density of 0.3 A g−1 are compared in Fig. 4b. The GCD curve of the MnOx/C-rGO-based device displays an almost symmetrical triangular shape, confirming its EDLC behavior. Moreover, the discharging time of the MnOx/C-rGO-based device is longer than that of the MnOx/S-rGO-based device. The GCD curves of the MnOx/S-rGO and MnOx/C-rGO-based symmetric devices at various current densities from 0.3 to 5 A g−1 are shown in Additional file 1: Fig. S8c, d, respectively. The Csp values of the MnOx/S-rGO and MnOx/C-rGO-based devices at different current densities were calculated using Eq. (2).
Two-electrode system measurements: a CV curves at a scan rate of 20 mV s−1; b GCD curves at a current density of 0.3 A g−1; c Specific capacitances of symmetric devices based on MnOx/S-rGO and MnOx/C-rGO at different current densities, and d Nyquist plots of symmetric devices based on MnOx/S-rGO and MnOx/C-rGO
The Csp as a function of current density is plotted in Fig. 4c. The Csp values of the MnOx/C-rGO-based symmetric device are 288, 234, 213, 196, 179, 160, and 133 F g−1 at current densities of 0.3, 0.5, 0.8, 1, 2, 3, and 5 A g−1, respectively. For comparison, the Csp values of the MnOx/S-rGO-based symmetric device are 76, 72, 66, 62, 54, 47, and 40 at current densities of 0.3, 0.5, 0.8, 1, 2, 3, and 5 A g−1, respectively. Obviously, the Csp values of the MnOx/C-rGO-based device are much higher than those of the MnOx/S-rGO-based device. The significantly enhanced capacitance of MnOx/C-rGO-based device may be attributed to the nanonet-like structure formed by the crushed rGO and MnOx nanorods, which may provide an effective network for ion transport. EIS measurements were performed to analyze the charge transfer resistance in the MnOx/S-rGO and MnOx/C-rGO-based symmetric devices (Fig. 4d). The corresponding fitted equivalent circuits and the obtained values are presented in Additional file 1: Fig. S9 and Table S1, respectively. The RS value of the MnOx/C-rGO-based device (3.742 Ω) is lower than that of the MnOx/S-rGO-based device (RS = 5.531 Ω), suggesting the enhanced electrical contact between MnOx/C-rGO and electrolyte. The charge transfer resistance (RCT) value of the MnOx/C-rGO-based device (8.734 Ω) is lower than that of the MnOx/S-rGO-based device (10.454 Ω). The superior ideal capacitive behavior of the MnOx/C-rGO-based device is indicated by the higher verticality of its Nyquist plot in the low-frequency region compared to the MnOx/S-rGO-based device. The sharp edges of C-rGO with the random distribution of multi-nanostructure provide pores and free volume inside and between rGO layers, thus facilitating a multi-channel structure for ion diffusion in MnOx/C-rGO.
The cycling performance of the fabricated symmetric devices with MnOx/S-rGO and MnOx/C-rGO was performed in the voltage range of 0 to 1.5 V at 5 A g−1. The Coulombic efficiency was calculated using Eq. (5) and the capacitance retention was determined using Eq. (6). Figure 5a compares the Coulombic efficiency and capacitance retention of the symmetric devices with MnOx/S-rGO and MnOx/C-rGO over 10,000 GCD cycles. Approximately 100% Coulombic efficiency is achieved for the MnOx/C-rGO-based device over the total cycle. However, the symmetric device fabricated using MnOx/S-rGO shows a decrease in Coulombic efficiency with consequent GCD cycles. The MnOx/C-rGO-based device retains 90% of its initial capacitance after 10,000 GCD cycles, while the MnOx/S-rGO-based device fades rapidly with only 70% of its initial capacitance being retained. This indicates the superior cycling stability of the MnOx/C-rGO-based device relative to the MnOx/S-rGO-based device. The slight decrease in the capacitance retention of the MnOx/C-rGO-based device may be due to the dissolution and detachment of electrolyte or oxygen evolution during charge–discharge cycles [15, 64, 65]. However, the dissolution of manganese oxide is not excluded here [14, 15, 64, 65]. The CV curves of the MnOx/C-rGO-based device before and after the stability test are almost similar (Fig. 5b), thus confirming the good stability of this composite.
The voltage holding (VH) test of MnOx/C-rGO was performed to investigate the reliability of the material upon exposure to the maximum working potential over a considerable time duration. A symmetric device with MnOx/C-rGO electrode was subjected to a maximum voltage of 1.5 V for 60 h. Figure 6a displays the variation of Csp with each holding time of 10 h. The Csp increases with increasing holding time. After 60 h, the Csp rises to 250% of its initial capacitance. This may be attributed to the activation of surface charges and pores of MnOx/C-rGO during the retention of voltage holding for a certain time duration. This is supported by comparing the GCD curves of the MnOx/C-rGO-based device before and after the VH test. An improvement of the discharge time is evident after 60 h holding time (Fig. 6b). We have compared the energy density (E) and power density (P) of the symmetric devices with MnOx/C-rGO and MnOx/S-rGO in the Ragone plot diagram, as shown in Fig. 7. The E and P values were calculated using Eqs. (3) and (4) from the GCD curves. The GCD curves are shown in Additional file 1: Fig. S8c, d. The E values of the MnOx/C-rGO-based device (23 Wh kg−1) are considerably higher than the MnOx/S-rGO-based device (5 Wh kg−1) at all current densities. The E of the supercapacitor depends on the cell voltage and specific capacitance. Understandably, the reason for the high E of the MnOx/C-rGO-based device lies in its high capacitance values. Due to the presence of crushed graphene, the energy density of a symmetric device with MnOx/C-rGO is enhanced without comprising the P values. Supercapacitors with high P and E values are highly desired for modern electronic devices to compete with lithium-ion batteries. Additionally, it is observed that the E value of the MnOx/C-rGO-based device is higher than those of the devices with various rGO composites reported in the literature (Fig. 7) [66,67,68,69,70,71,72,73].
To demonstrate the practical applicability of the MnOx/C-rGO composite, a mini-prototype device was fabricated. The photograph of the fabricated symmetric supercapacitor device is shown in Fig. 8a. The device was charged at 4.0 V using an electrometer for 120 s. During discharging, an LED light was connected to the device to track the response. The assembled supercapacitor can power a red LED light during discharging, as shown in Fig. 8b, c. After charging for 120 s, the device can power the LED light for about 540 s. These results indicate the promising practicality of the MnOx/C-rGO-based symmetric device.
4 Conclusions
In summary, we have demonstrated a facile route to prepare MnOx/C-rGO with high-level 3D structural disorders and exfoliated structures, which show high electrical conductivity and good structural integrity. The agglomeration and stacking problems of graphene sheets have been successfully minimized using a crushed 3D structure. The electrochemical measurements show that MnOx/C-rGO exhibits a considerably higher specific capacitance (863 F g−1) than MnOx/S-rGO (373 F g−1) and MnOx (200 F g−1) at a current density of 0.2 A g−1. When assembled into a symmetric supercapacitor, the MnOx/C-rGO-based device exhibits a higher Csp (288 F g−1) than the MnOx/S-rGO-based device (75 F g−1) at a current density of 0.3 A g−1. Moreover, the MnOx/C-rGO-based device exhibits a higher energy density of 23 Wh kg−1 than the MnOx/S-rGO-based device (5 Wh kg−1) at a power density of 113 W kg−1, further demonstrating the favorable assembly of chemically interface-tailored MnOx/C-rGO for use as a supercapacitor electrode. Such self-supporting 3D architecture avoids the occurrence of “dead mass” caused by the conductive agents and binders during cycling and exhibits excellent electrochemical performance, which will provide a promising candidate for next-generation electrochemical Na-storage systems.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
W. Raza, F. Ali, N. Raza, Y. Luo, K.-H. Kim, J. Yang, S. Kumar, A. Mehmood, E.E. Kwon, Nano Energy 52, 441–473 (2018)
R.R. Salunkhe, Y.V. Kaneti, Y. Yamauchi, ACS Nano 11, 5293–5308 (2017)
C. Li, Q. Li, Y.V. Kaneti, D. Hou, Y. Yamauchi, Y. Mai, Chem. Soc. Rev. 49, 4681–4736 (2020)
C. Young, T. Park, J.W. Yi, J. Kim, M.S.A. Hossain, Y.V. Kaneti, Y. Yamauchi, Chemsuschem 11, 3546–3558 (2018)
S. Liu, L. Kang, J. Zhang, S.C. Jun, Y. Yamauchi, ACS Energy Lett. 6, 4127–4154 (2021)
S. Liu, L. Kang, J. Hu, E. Jung, J. Zhang, S.C. Jun, Y. Yamauchi, ACS Energy Lett. 6, 3011–3019 (2021)
P.K. Panda, A. Grigoriev, Y.K. Mishra, R. Ahuja, Nanoscale Adv. 2, 70–108 (2020)
H. Fan, W. Shen, ACS Sustain. Chem. Eng. 4, 1328–1337 (2016)
F. Cheng, X. Yang, S. Zhang, W. Lu, J. Power Sour. 450, 227678 (2020)
R.R. Salunkhe, Y.V. Kaneti, J. Kim, J.H. Kim, Y. Yamauchi, Acc. Chem. Res. 49, 2796–2806 (2016)
S. Liu, L. Kang, J. Zhang, E. Jung, S. Lee, S.C. Jun, Energy Storage Mater. 32, 167–177 (2020)
S. Rusi, R. Majid, Sci. Rep. 5, 16195 (2015)
W. Huang, J. Li, Y. Xu, Materials 11, 716 (2018)
M.-M. Chen, X.-Y. Zhang, L.-Q. Wang, C.-Y. Wang, Ind. Eng. Chem. Res. 53, 10974–10981 (2014)
Y. Liu, D. Yan, R. Zhuo, S. Li, Z. Wu, J. Wang, P. Ren, P. Yan, Z. Geng, J. Power Sour. 242, 78–85 (2013)
J. Yan, Z. Fan, T. Wei, W. Qian, M. Zhang, F. Wei, Carbon 48, 3825–3833 (2010)
J. Deng, X. Wang, X. Duan, P. Liu, ACS Sustain. Chem. Eng. 3, 1330–1338 (2015)
C.V.V. Muralee Gopi, R. Vinodh, S. Sambasivam, I.M. Obaidat, H.-J. Kim, J. Energy Storage 27, 101035 (2020)
A.S. Lemine, M.M. Zagho, T.M. Altahtamouni, N. Bensalah, Int. J. Energy Res. 42, 4284–4300 (2018)
M. Wayu, Solids 2, 232–248 (2021)
D. Majumdar, ChemElectroChem 8, 291–336 (2021)
W. Yang, Z. Gao, J. Wang, B. Wang, Q. Liu, Z. Li, T. Mann, P. Yang, M. Zhang, L. Liu, Electrochim. Acta 69, 112–119 (2012)
Z. Li, Y. Mi, X. Liu, S. Liu, S. Yang, J. Wang, J. Mater. Chem. 21, 14706–14711 (2011)
L. Peng, X. Peng, B. Liu, C. Wu, Y. Xie, G. Yu, Nano Lett. 13, 2151–2157 (2013)
J. Zhang, J. Jiang, X.S. Zhao, J. Phys. Chem. C 115, 6448–6454 (2011)
J. Zhu, J. He, A.C.S. Appl, Mater. Interfaces 4, 1770–1776 (2012)
J. Yao, Q. Pan, S. Yao, L. Duan, J. Liu, Electrochim. Acta 238, 30–35 (2017)
S. Ghasemi, S.R. Hosseini, O. Boore-talari, Ultrason. Sonochem. 40, 675–685 (2018)
J. Dong, G. Lu, F. Wu, C. Xu, X. Kang, Z. Cheng, Appl. Surf. Sci. 427, 986–993 (2018)
J. Luo, H.D. Jang, J. Huang, ACS Nano 7, 1464–1471 (2013)
R.O. Brennan, J. Chem. Phys. 20, 40–48 (1952)
X.H. Xia, D.L. Chao, Y.Q. Zhang, Z.X. Shen, H.J. Fan, Nano Today 9, 785–807 (2014)
X.-L. Bai, Y.-L. Gao, Z.-Y. Gao, J.-Y. Ma, X.-L. Tong, H.-B. Sun, J.A. Wang, Appl. Phys. Lett. 117, 183901 (2020)
Q.J. Le, M. Huang, T. Wang, X.Y. Liu, L. Sun, X.L. Guo, D.B. Jiang, J. Wang, F. Dong, Y.X. Zhang, J. Colloid Interface Sci. 544, 155–163 (2019)
I. Ijaz, E. Gilani, A. Nazir, A. Bukhari, Green Chem. Lett. Rev. 13, 223–245 (2020)
M. Lin, B. Chen, X. Wu, J. Qian, L. Fei, W. Lu, L.W.H. Chan, J. Yuan, Nanoscale 8, 1854–1860 (2016)
W.S. Hummers, R.E. Offeman, J. Am. Chem. Soc. 80, 1339–1339 (1958)
J. Guerrero-Contreras, F. Caballero-Briones, Mater. Chem. Phys. 153, 209–220 (2015)
J.-Y. Choi, Y.-K. Lee, S.-M. Yoon, H.C. Lee, B.-K. Kim, J.M. Kim, K.-M. Kim, J.-H. Lee, J. Am. Ceram. Soc. 88, 3020–3023 (2005)
A.H. Reaz, H.R. Barai, S. Saha, K. Chowdhury, M.N. Mojumder, S.H. Firoz, A.-N. Chowdhury, S.W. Joo, C.K. Roy, J. Electrochem. Soc. 168, 080535 (2021)
O. Fasakin, J.K. Dangbegnon, D.Y. Momodu, M.J. Madito, K.O. Oyedotun, M.A. Eleruja, N. Manyala, Electrochim. Acta 262, 187–196 (2018)
S. Abdolhosseinzadeh, H. Asgharzadeh, H. Seop Kim, Sci. Rep. 5, 10160 (2015)
S. Deng, V. Berry, Mater. Today 19, 197–212 (2016)
B. Unnikrishnan, C.-W. Wu, I.W.P. Chen, H.-T. Chang, C.-H. Lin, C.-C. Huang, ACS Sustain. Chem. Eng. 4, 3008–3016 (2016)
H. Lei, J. Tu, D. Tian, S. Jiao, N. J. Chem. 42, 15684–15691 (2018)
K. Dai, L. Lu, C. Liang, J. Dai, Q. Liu, Y. Zhang, G. Zhu, Z. Liu, Electrochim. Acta 116, 111–117 (2014)
H. Liu, Z. Hu, L. Tian, Y. Su, H. Ruan, L. Zhang, R. Hu, Ceram. Int. 42, 13519–13524 (2016)
X. Li, X. Huang, D. Liu, X. Wang, S. Song, L. Zhou, H. Zhang, J. Phys. Chem. C 115, 21567–21573 (2011)
S. Yang, X. Song, P. Zhang, L. Gao, J. Mater. Chem. A 1, 14162–14169 (2013)
J. Jiang, A. Kucernak, Electrochim. Acta 47, 2381–2386 (2002)
D. Gueon, J.H. Moon, ACS Sustain. Chem. Eng. 5, 2445–2453 (2017)
L. Li, Z.A. Hu, N. An, Y.Y. Yang, Z.M. Li, H.Y. Wu, J. Phys. Chem. C 118, 22865–22872 (2014)
S.W. Lee, J. Kim, S. Chen, P.T. Hammond, Y. Shao-Horn, ACS Nano 4, 3889–3896 (2010)
B. Mu, W. Zhang, S. Shao, A. Wang, Phys. Chem. Chem. Phys. 16, 7872–7880 (2014)
R. Poonguzhali, N. Shanmugam, R. Gobi, A. Senthilkumar, R. Shanmugam, K. Sathishkumar, RSC Adv. 5, 45407–45415 (2015)
M. Hu, Y. Liu, M. Zhang, H. Wei, Y. Gao, J. Power Sources 335, 113–120 (2016)
X. Hong, X. Wang, Y. Li, J. Fu, B. Liang, J. Alloys Compd. 889, 161821 (2021)
Y. Guan, Z. Guo, H. Che, J. Mu, X. Zhang, Z. Zhang, G. Wang, Y. Bai, H. Xie, Chem. Eng. J. 331, 23–30 (2018)
G. Yang, S.-J. Park, J. Alloys Compd. 741, 360–367 (2018)
B. Liu, Y. Liu, H. Chen, M. Yang, H. Li, ACS Sustain Chem. Eng. 7, 3101–3110 (2019)
H. Wang, Q. Fu, C. Pan, Electrochim. Acta 312, 11–21 (2019)
Y. Chen, J. Zhang, M. Li, C. Yang, L. Zhang, C. Wang, H. Lu, Electrochim. Acta 292, 115–124 (2018)
T. Bi, H. Fang, J. Jiang, X. He, X. Zhen, H. Yang, Z. Wei, Z. Jia, J. Alloys Compd. 787, 759–766 (2019)
E. Umeshbabu, P. Justin, G.R. Rao, A.C.S. Appl, Energy Mater. 1, 3654–3664 (2018)
Z. Lei, F. Shi, L. Lu, A.C.S. Appl, Mater. Interfaces 4, 1058–1064 (2012)
K. Ghosh, C.Y. Yue, M.M. Sk, R.K. Jena, S. Bi, Sustain. Energy Fuels 2, 280–293 (2018)
B. Mendoza-Sánchez, J. Coelho, A. Pokle, V. Nicolosi, Electrochim. Acta 174, 696–705 (2015)
Z. Luan, Y. Tian, L. Gai, H. Jiang, X. Guo, Y. Yang, J. Alloys Compd. 729, 9–18 (2017)
A. Goljanian Tabrizi, N. Arsalani, A. Mohammadi, H. Namazi, L. Saleh Ghadimi, I. Ahadzadeh, N. J. Chem. 41, 4974–4984 (2017)
K. Makgopa, P.M. Ejikeme, C.J. Jafta, K. Raju, M. Zeiger, V. Presser, K.I. Ozoemena, J. Mater. Chem. A 3, 3480–3490 (2015)
Z.-S. Wu, W. Ren, D.-W. Wang, F. Li, B. Liu, H.-M. Cheng, ACS Nano 4, 5835–5842 (2010)
A. Bello, O.O. Fashedemi, J.N. Lekitima, M. Fabiane, D. Dodoo-Arhin, K.I. Ozoemena, Y. Gogotsi, A.T. Charlie Johnson, N. Manyala, AIP Adv. 3, 082118 (2013)
Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao, E. Xie, ACS Nano 7, 174–182 (2013)
Acknowledgements
The authors acknowledge the fellowship and instrumental support from the projects of Ministry of Science and Technology, Bangladesh and Bangladesh Energy and Power Research Council, Bangladesh (EPRC/58-2019-001-01). This work was performed in part at the Queensland node of the Australian National Fabrication Facility (ANFF), a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australia’s researchers.
Funding
This work is funded by the Committee for Advanced Studies and Research (CASR) of the Bangladesh University of Engineering and Technology (BUET) (Dhaka, Bangladesh), the JST-ERATO Yamauchi Materials Space-Tectonics Project (JPMJER2003), the Ministry of Science and Technology, Bangladesh and Bangladesh Energy and Power Research Council (EPRC/58-2019-001-01). Y. V. K. acknowledges the funding from Advance Queensland (AQIRF043-2020-CV). This work was performed in part at the Queensland node of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australia’s researchers. The authors are also grateful to the Taif University Researchers Supporting Project number (TURSP-2020/03), Taif University, Taif, KSA.
Author information
Authors and Affiliations
Contributions
A. H. R. performed the synthesis, characterization and electrochemical measurements, analyzed the data, wrote and prepared the original figures. S. S., C. K. R., and M. A. W. assisted with the materials characterization and/or electrochemical performance. M. A. W., G. W., M. A. A., S. L., Y. Y, Y. V. K., and M. S. H. provided technical feedback, revised the manuscript and figures and improved the quality of the language. S. H. F. designed the experiments, supervised the students while developing the manuscript and revising the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
TEM images of (a) MnOx/S-rGO and (b) MnOx/C-rGO and HRTEM images of (c) MnOx/S-rGO and (d) MnOx/C-rGO. Figure S2. High-resolution O1s XPS spectra of (a) MnOx/S-rGO and (b) MnOx/C-rGO. Figure S3. High resolution C 1 s XPS spectra of (a) MnOx/S-rGO and (b) MnOx/C-rGO. Figure S4. High resolution Mn 2p XPS spectra of (a) MnOx/S-rGO and (b) MnOx/C-rGO. Figure S5. (a) CV curves at different scan rates and (b) GCD curves at different current densities of MnOx in 0.5 M Na2SO4 electrolyte in the potential range of − 0.1 to 0.8 V. Figure S6. Electrochemical study in 0.5 M Na2SO4 electrolyte with a three-electrode system: (a) CV curves at different scan rates and (b) GCD curves at different current densities of MnOx/S-rGO in the potential range of − 0.1 to 0.8 V. Figure S7. (a) CV curves at different scan rates and (b) GCD at different current densities of MnOx/C-rGO in 0.5 M Na2SO4 electrolyte in the potential range of − 0.1 to 0.8 V. Figure S8. Electrochemical study in 1 M Na2SO4 electrolyte with a two-electrode system. CV curves of the symmetric devices prepared with (a) MnOx/S-rGO and (b) MnOx/C-rGO at different scan rates. GCD curves of the symmetric devices prepared with (c) MnOx/S-rGO and (d) MnOx/C-rGO at different current densities in the voltage range of 0 to 1.5 V. Figure S9. Equivalent fitting circuit. Table. S1 The obtained values of RS, Rct, Cdl, Zw, Cp, from EIS fitting.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reaz, A.H., Saha, S., Roy, C.K. et al. Boosting capacitive performance of manganese oxide nanorods by decorating with three-dimensional crushed graphene. Nano Convergence 9, 10 (2022). https://doi.org/10.1186/s40580-022-00300-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40580-022-00300-2