Abstract
Background
Canine idiopathic epilepsy (IE) is the most common chronic neurological brain disease in dogs, yet it can only be diagnosed by exclusion of all other potential causes. In people, epilepsy has been associated with a reduction in brain volume. The objective was to estimate the volume of the forebrain (FB), subarachnoid space (SAS) and lateral ventricles (LV) in dogs with IE compared to controls using Cavalieri’s principle. MRI scans of case and control dogs were identified from two neurology referral hospital databases. Eight breeds with increased odds of having IE were included: Golden Retriever, Labrador Retriever, Cocker Spaniel, Border terrier, German Shepherd dog, Parson Jack Russell terrier, Boxer, and Border Collie. Five dogs of each breed with IE and up to five controls were systematically and uniformly randomly sampled (SURS). The volume of the FB, SAS and LV were estimated from MRI scans by one blinded observer using Cavalieri’s principle.
Results
One hundred-two dogs were identified; 56 were diagnosed with IE and 46 were controls. There was no statistically significant difference in FB, SAS and LV volume between dogs with IE and controls. Dogs with a history of status epilepticus had significantly larger FB than those without (p = 0.05). There was a border-line trend for LV volume to increase with increasing length of seizure history in the IE group (p = 0.055).
Conclusion
The volumes of the FB, SAS and LV are not different between dogs with IE and controls, so IE remains a diagnosis of exclusion with no specific neuroanatomical biomarkers identified. This is the first time FB and SAS volume has been compared in dogs with IE. Unfortunately, we have shown that the results reporting significantly larger FBs in dogs with status epilepticus and LV volume increase with length of seizure history were likely confounded by breed and should be interpreted cautiously. Whilst these associations are interesting and clinically relevant, further investigation with breed-specific or larger, breed-diverse populations are required to permit strong conclusions. The Cavalieri principle provided an effective estimation of FB, SAS and LV volumes on MRI, but may be too time-intensive for use in clinical practice.
Similar content being viewed by others
Background
Idiopathic epilepsy (IE) is estimated to affect between 0.60–0.75% of the general canine population [1, 2]. Unlike structural epilepsy or reactive seizures, where a cause can be found with rigorous clinical investigations, the diagnosis of IE remains one of exclusion [3]. Magnetic Resonance Imaging (MRI) forms part of the Tier 2 confidence level of diagnostic certainty of IE recommendations made by the International Veterinary Epilepsy Task Force (IVETF), and is a common diagnostic tool in veterinary referral hospitals [3].
Research in people with epilepsy has uncovered a vast range of differences in brain volume of different anatomical structures compared to controls, such as the lateral ventricles (LV), hippocampus and thalamus, amongst others [4,5,6,7,8,9,10,11,12,13]. Increased volume or asymmetry of the LV have been associated with psychiatric and neurological conditions in people with epilepsy [12]. Interestingly, in people with temporal lobe epilepsy and idiopathic generalised epilepsy, significant brain atrophy has been reported compared to healthy controls when measured an average of 17.90 years after the onset of epilepsy, which could be the result of repeated seizures over time [5]. Unfortunately, the cross-sectional nature of this study may give rise to conclusions of causality resulting from primary abnormalities or secondary changes..
Veterinary volumetric studies have started to outline normal ranges for neuroanatomical structures of neurologically normal patients using MRI [14], however, target areas are still limited and additional studies are required to broaden the data available. This is particularly challenging given the conformational diversity of the dog [15], which is one of the most phenotypically diverse species on earth especially for the shape of the skull and cranial cavity [16]. In addition, automated voxel-based 2D morphometry techniques available in human medicine have only recently emerged in veterinary medicine [17, 18], meaning that in most instances copious time and practice is essential to take such measurements [19]. Commonly used methods in veterinary medicine include: estimations based on area width, length and depth [20, 21], volume rendering via slice-by-slice image segmentation [22] and manual pixel selection [22].
Similarly to human studies [12], many veterinary studies to date have focused on the volume or asymmetry of the lateral ventricles (LV) and have largely reported either no difference in total LV volume or left-right asymmetry in dogs with IE compared to controls [21, 23,24,25,26,27]. One veterinary study [28] found evidence of hippocampal atrophy in dogs with IE compared to controls whilst another found reduced grey matter volume in research beagles with and without spontaneous IE [17]. Most studies tentatively assume that these changes are incidental rather than evidence of pathology related to IE. Certainly, a hinderance in veterinary medicine is the expensive specialist software and time required for a clinician or researcher to individually map and estimate the volume of the anatomical structures of interest. Research in this area could be advanced by the discovery of a practical and time-saving alternative technique.
The objective of this study was to assess the practicality and ease of a design-based stereological method for volume estimation, the Cavalieri principle, and its use to estimate and compare the volume of the FB, SAS and LV in dogs with IE compared to controls. We hypothesise that the volume of the FB, SAS and LV will be different in dogs with IE compared to controls. We also hypothesise that these volumes will be associated with other clinical variables in the sub-population of dogs with IE, particularly with seizure history. Additionally, we will compare sub-groups of dogs with IE to compare volume of FB, SAS and LV in dogs with IE who experience cluster seizures (CS) and status epilepticus (SE) to dogs with IE who do not.
Results
One hundred-two dogs were identified; 56 were diagnosed with IE per the criteria above and 46 acted as controls. Forty-nine dogs were seen at Fitzpatrick Referrals Orthopaedics & Neurology, UK (48.04%; 26 cases and 23 controls), and 53 dogs were seen at the Queen Mother Hospital for Animals, UK (51.96%; 30 cases and 23 controls). This consisted of 37 Labrador Retrievers (36.27%), 15 Golden Retrievers (14.71%), 15 German Shepherds (14.71%), 15 Border Collies (14.71%), 13 Cocker Spaniels (12.75%), three Border terriers (2.94%), three Boxers (2.94%), and one Parsons Jack Russell terrier (0.98%). Table 1 reports the clinical diagnosis of control dogs. Neither age nor body weight were significantly different between dogs with IE and controls (Table 2). Mean MRI slice thickness was 3.79 mm (SD = 0.41) and mean slice interval was 22.58% (0.86 mm) (SD = 0.06) of the slice thickness.
All dogs with IE had a clinically normal inter-ictal neurological examination, haematology and biochemistry and an unremarkable brain on MRI. In addition to this, tests to give further confidence in diagnosis were done for 50 of the IE dogs (89.29%); 26 dogs also had both cerebrospinal fluid (CSF) and Bile Acid Stimulation Test (BAST) (46.43%), 20 dogs had CSF analysis (35.71%), and four dogs had BAST (7.14%). Two dogs had mildly elevated protein levels in their CSF, potentially associated with a recent seizure.
Dogs with IE had a mean inter-ictal period of 31.16 days (SD = 34.21) and, prior to the MRI scan, last had a seizure a mean of 16.46 days ago (SD = 16.61). At the time of MRI scan 27 dogs (51.79%) were drug-naïve, 20 (35.71%) were receiving one anti-epileptic drug (AED), eight (14.29%) were receiving two AEDs and one dog (1.79%) was receiving three AEDs.
Inter-observer agreement
Throughout training and the pilot study, measurements were taken in cm3 and retrospectively converted to mm3. The intra-class coefficient was 0.948, 0.936 and 0.977 for the FB, LV and SAS, respectively. Agreement was considered excellent for all three anatomic areas. A mean of 70.80, 70.20 and 85.80 points were counted within each region of interest for the FB, LV and SAS, respectively. This highlighted the need for a denser grid to ensure at least 200 points were counted per region of interest [29].
Effect of IE
Analyses included 37 randomly selected Labradors, Golden Retrievers, German Shepherd Dogs and Border Collies; 17 with IE and 20 controls. Mean FB volume for dogs with IE was 68,714.38mm3 (CV = 13.90; CE = 0.01) and 72,270.13mm3 (CV = 13.07; CE = 0.01) for controls. Mean SAS volume for dogs with IE was 10,491.44mm3 (CV = 27.19; CE = 0.04) and 10,756.49mm3 (CV = 25.39; CE = 0.02) for controls. Mean LV volume for dogs with IE was 3677.65mm3 (CV = 82.46; CE = 0.09) and 3319.53mm3 (CV = 71.70; CE = 0.05) for controls. There was no statistically significant difference in FB (p = 0.218), SAS (p = 0.828) and LV (p = 0.869) volume between dogs with IE and controls (Fig. 1). A mean of 3291, 473 and 162 points were counted for each brain region, FB, SAS and LV, respectively.
Effect of time since IE onset and LV volume
Time since onset of IE was available for 20 dogs. Mean time since onset of IE was 371.50 days (SD = 418.99 days). There was a trend towards LV volume increasing with the length of seizure history (r = 0.435, p = 0.055) (Fig. 2).
Cluster seizures
Twenty-one dogs were reported to have experienced CS, the remaining 37 were IE-controls (i.e. dogs diagnosed with IE with no history of CS) (Table 3). Five CS dogs and 5 IE-controls were randomly selected including Labradors, Golden Retrievers, German Shepherd Dogs and Border Collies. There were no significant differences between age or body weight for CS dogs and IE-controls. Mean FB volume was 68,747.88mm3 (CV = 14.44, CE = 0.10) for dogs with CS and 70,933.32mm3 (CV = 16.00,CE = 0.01) for IE-controls. Mean LV volume was 2465.69mm3 (CV = 33.39, CE = 0.05) for dogs with CS and 2546.86mm3 (CV = 19.16, CE = 0.05) for IE-controls. Mean SAS volume was 10,714.21mm3 (CV = 51.87, CE = 0.02) for dogs with CS and 9146.33mm3 (CV = 34.23, CE = 0.02) for IE-controls. There was no significant difference in FB (p = 0.754), SAS (p = 0.598) or LV (p = 0.854) volume between groups.
Status Epilepticus
Nine dogs were reported to have experienced SE, the remaining 49 were IE-controls (i.e. dogs diagnosed with IE with no history of SE). Descriptive data can be found in Table 4. Five SE dogs and 5 IE-controls were randomly selected including; Labradors, Golden Retrievers, German Shepherd Dogs and Border Collies. There were no significant differences between age or body weight for SE dogs and IE-controls. Mean FB volume was 72,345.62mm3 (CV = 7.91, CE = 0.01) for dogs with SE and 61,307.26mm3 (CV = 17.62, CE = 0.01) for IE-controls. Mean LV volume was 5062.18mm3 (CV = 85.40, CE = 0.04) for dogs with SE and 3508.40mm3 (CV = 92.87, CE = 0.04) for IE-controls. Mean SAS volume was 11,071.22mm3 (CV = 26.77, CE = 0.01) for dogs with SE and 10,197.66mm3 (CV = 55.83, CE = 0.02) for IE-controls. The FB volume of SE dogs was significantly larger than controls (p = 0.047) (Fig. 3). There was no significant difference in SAS (p = 0.527) or LV (p = 0.527) volume between groups.
Discussion
To the author’s knowledge this is the first study to use Cavalieri’s stereological principle to estimate brain volume in dogs with IE. In this study, no differences in FB, SAS and LV volume were found between dogs with IE and controls. Associations between clinical history and brain volume were found within the IE group but, as will be discussed further below, these results require further research to substantiate.
Effect of IE on FB, SAS and LV volume
This study found no statistically significant differences in FB, SAS and LV volume in dogs with IE compared to controls. Whilst many studies have focused on LV volume, we believe this is the first study to report FB and SAS volumes in dogs with IE compared to controls. Unfortunately, “negative” results such as these can be easily dismissed, but these results further support the need for a rigorous diagnosis of exclusion for dogs with IE, given that there are still no diagnostic biomarkers specific to IE. Furthermore, volumetric studies are important in veterinary medicine to establish a database of “normal” volumes for various breeds and disorders.
Most volumetric studies in dogs with IE compared to controls focus on total LV volume (as in this study) or on left/right LV asymmetry [13, 21, 24, 27, 28] reporting mixed findings. This study contributes to this existing body of research, perhaps strengthening the conclusion that there is no difference between total LV volume in dogs with IE compared to controls. Similarly, volumetric studies in people with epilepsy have reported various additional structural abnormalities [4,5,6,7,8,9, 11, 13], though a unanimous hypothesis on the cause of these changes has not been reached.
Historical veterinary studies [20, 25, 30] have suggested normalising the volume of the LV to the overall brain volume, especially when comparing different breeds. Using the data acquired in this study we expressed FB volume as a relative value and expressed LV volume as a percentage of FB volume, but results did not change the conclusion already made. Importantly, the body weights of dogs included in this study were not significantly different between IE and control groups, as Schmidt, et al. [31] have shown that brain volume is significantly affected by body weight in dogs. Though, it is worth mentioning that body condition score was not taken into account by either Schmidt, et al. [31] or the present study.
Pilegaard, et al. [32] found that breeds with a wide (laterolateral) but shallow (rostrocaudal) skull shape had a smaller cortical volume to ventricular volume ratio. Similarly, Bakici, et al. (2019) [33] used the Cavalieri method to compare brain fraction volumes, including lateral ventricles, of brachycephalic and mesocephalic breed types and found no significant difference, although the aforementioned authors’ findings should be interpreted with caution because, in stereology, volume fractions are normally limited and sometimes inconclusive and cannot be directly compared with more accurate global volumes as the ones we estimated in our study. Of the eight breeds included in this study, all are mesocephalic aside the Boxer, which was not measured due to low numbers. Therefore, the skull type of the dogs included is unlikely to have skewed the data as all dogs were a similar size and skull shape. Additional analysis to confirm this assumption by comparing FB, SAS and LV volume for Border Collies, Labradors, German Shepherd Dogs and Golden Retrievers with IE to controls separately by breed showed no significant differences in volumes for the FB, SAS or LV in any of the breeds, which further supports the negative findings reported.
Effect of time since the onset of IE
The time since the onset of IE was moderately-positively correlated to volume of the LV, but this finding was only a statistical trend. Figure 2 suggests this result is largely driven by two dogs, a Labrador Retriever and Golden Retriever, with large LVs where onset of IE was more than 1000 days prior to MRI. To assess this, a multivariate analysis of variance (MANOVA) was carried out, which showed no significant interactions between breed, LV volumes and time since seizure onset. Given the dataset was small, the dataset was copied once to double it. This second analysis with the doubled dataset showed a significant relationship between breed, LV volume and time since seizure onset (p = 0.005), of which the significant contributing interaction was breed and LV volume (p = 0.001). In this instance breed explained 34.8% of the LV volume difference. A follow-up one-way ANOVA showed Golden Retrievers to have significantly larger LV volumes than Border Collies and German Shepherd Dogs. Based on these rudimentary results, the authors would suggest careful selection and control of dog breed in the further longitudinal analysis of LV volume in dogs with IE recommended above. Though tempting to include many different dog breeds in a study to broaden the application of findings, the large conformational diversity of dogs may prohibit this by introducing avoidable variability.
A relationship between these variables in dogs with IE is biologically plausible; in a study on rat models of SE [34], it was found that LV volume increased over time. However, in dogs with IE, Kuwabara, et al. [28] reported no association between the hippocampi asymmetry ratio and the time since onset of seizures.
It has previously been reported that LV dilatation is positively correlated to increasing age in dogs [35,36,37] and people [38] and, therefore, age could be a confounding variable, in addition to the breed factors already discussed. Furthermore, Noh, et al. [39] have recently shown brain atrophy in a group of dogs over 9 years of age with cognitive dysfunction and, therefore, the effect of age on this population must be considered. The maximum age of dogs in this analysis was 79 months (6 years and 7 months), which would be considered middle-age. However, a previous study investigating canine cognitive dysfunction (CCD) in dogs with IE found onset of CCD-related clinical signs to be earlier than expected [40]. Without the support of MRI scans we cannot know if this is due to structural changes, but it would be an excellent route for further study. Dogs less than 6 months of age were not included, per the lower limit in the IVEFT guidelines for diagnosis of IE [3].
Though strong conclusions cannot be made, this study suggests that LV volume change over time could be a valuable future research hypothesis to test in a large veterinary longitudinal MRI study.
Cluster seizures and status Epilepticus
Whilst it is likely that dogs with CS or SE have been included in groups of dogs that have IE during volumetric studies, to the authors’ knowledge they have never been assessed as a separate entity. The FBs of dogs with SE were found to be significantly larger than controls, which was surprising and did not match clinical suspicions held prior to the study. Further investigation showed poor breed distribution in these two groups despite systematic and uniform random sampling (SURS); the SE group contained only Labradors and Golden Retrievers, whilst the control group only contained one of these dog breeds. Additionally, an outlier in the control group shows a Border Collie with a particularly small forebrain. It is likely that breed distribution is a factor in this result too and, therefore, we cannot confidently conclude that the FB is larger in dogs with IE who experience SE. Despite following standard methodology for stereological studies with regards to group sizes, we recommend breed-specific or larger breed-diverse analysis is carried out in future to identify significant trends should they exist..
Despite our results not aligning with expectations and likely breed-bias of our results, post-ictal changes in brain structure following a seizure or SE have been reported in people [41] and dogs [3]. In human medicine it is known that prolonged seizure activity causes neuronal death [42], and Hocker, et al. [43] demonstrated that cortical and subcortical atrophy occurs in people who experience super-refractory SE (SE that recurs or continues for more than 24 h despite the use of anaesthesia). Newey, et al. [44] reported one case where global cerebral atrophy occurred progressively in a patient with super-refractory SE.
Use of the Cavalieri principle
The Cavalieri principle was easy to use after a period of training and practice to familiarise the observer with the software, however, it was a time-consuming process with each brain taking 60–90 min to measure. The high density of cross-point markers was necessary to account for the relatively small volume of the LV so that a sufficient number of points could be counted to ensure an acceptable CE, meaning that thousands of cross-points were counted in larger areas such as the FB. If an observer was only interested in one anatomical structure then the density of the cross-points could be adjusted to account for this, thus speeding up the process.
Two studies have detailed the accuracy of the Cavalieri principle using MRI scans compared to the actual brain post-mortem; Furlong, et al. [45] found poor agreement between MRI and pathological volumetric analysis of the cortical and subcortical volume and thickness of the cerebral cortex in human brains post-mortem, and they concluded that this was likely due to the poor resolution provided by the 3 T MRI used. A similar study [46] used the Cavalieri principle to compare pig brain volumes on 3 T MRI under anaesthesia and on physical sections following immediate euthanasia. They found no statistically significant differences between estimation methods in all areas except the basal nuclei compartment. Whilst this is not considered an appropriate statistical measure for agreement, the authors reported mean differences of 3–5% for the total brain volume, which are acceptable, but up to 9–11% for the other compartments, indicating a less satisfactory level of agreement than the t-tests suggest. Given this evidence it could be that smaller and more subtly defined areas measured using the Cavalieri principle on MRI do not agree well with true physical sections due to poor resolution of the anatomical structure represented in the MRI or that a finer grid needs to be used. The use of contrast solutions (e.g. gadolinium) during MRI could be investigated as a potential way to improve the resolution required for the better visualisation of the anatomical area of interest.
One challenge of using the Cavalieri principle with digital images can be seen in Fig. 4. Due to the nature of MRI images representing the average of a slice thickness, potential for a partial volume effect, the use of a fine saturation gradient of white, grey and black, and the need to magnify an image for accuracy thus creating a very pixelated image, means measurements can have a certain degree of subjectivity.
Limitations
The presence of two dogs with mildly increased protein in their CSF presumed due to recent seizure activity could have biased the study. The IVEFT recommend repeating these tests 16 and six weeks later, respectively [3], however, in veterinary medicine this is unlikely to occur because care is privately funded. For further support of IE diagnosis follow-up clinical examination can be repeated 1–3 years later to confirm no development of interictal neurological deficits. Due to the referral population of these dogs the mean days since seizure was well under this recommendation.
The MRI scanners used in this study were 1.5 T, therefore, not comparable to 3 T and above scanners available in human medicine. It is possible that with a 3 T scanner, differences could be found or that longer scans on the 1.5 T scanners would be preferable for volumetric analysis. A study comparing image quality, resolution and contrast of MRI images of the canine brain taken on a 3 T and 7 T MRI scanner found that both had their preferred uses and that some structures were better visualised on 3 T and some on 7 T [47]. The authors suspected that the use of 7 T MRI could help in the explanation of certain pathology in brain disorders such as epilepsy, but access to these powerful magnets is very limited in a veterinary clinical or research environment, which inhibits further research in this area. T2 TRA sequences were chosen because they offered good tissue contrast and were commonly used across patients with epilepsy and controls, and across both hospital sites. This decision has potential to affect the measurement process and should be taken into consideration when comparing to the findings of existing or future research.
Conclusions
This research contributes to our current understanding of IE diagnosis in dogs, but also emphasises the need for further work in both healthy and pathological canine neuroanatomy, and the need for longitudinal investigation. No significant differences were found for FB, SAS or LV volume between dogs with IE and controls and, for now, IE remains a diagnosis of exclusion with no specific neuroanatomical biomarkers identified. Strong conclusions regarding association between LV volume and time since seizure onset or FB, SAS and LV volume in dogs with CS or SE compared to IE-controls cannot be made. The authors would advise strong controls for breed variation in future studies or active work on “normal ranges” for specific breeds so that these can be accounted for. Finally, the Cavalieri principle was an easy and effective estimation of FB, SAS and LV volumes on MRI images and shows promise for future research but may be too time-intensive for use in clinical practice.
Materials and methods
MRI scans were identified from the databases of two neurology referral hospitals with 1.5 T MRI scanners with at least T2-weighted transverse imaging of the entire brain, a common scan type in veterinary IE protocols. Eight breeds of dog previously identified as being at an increased odds of having IE compared to cross-breeds were included: the Golden Retriever, Labrador Retriever, Cocker Spaniel, Border terrier, German Shepherd dog, Parson Jack Russell terrier, Boxer, and Border Collie [2]. One database was searched manually and the other using VetCompass.Footnote 1 Dogs with IE were required to have at least a Tier I confidence level diagnosis [3] for inclusion. Controls were identified from dogs found to have an unremarkable brain on MRI (for reasons other than seizures) and following thorough examination of their clinical history. All dogs were assessed and diagnosed by a Diplomat of the European College of Veterinary Neurology (ECVN) or ECVN Resident. The term “unremarkable brain on MRI” means that it is perceived to be “normal” and that no anatomical brain abnormalities could be detected – it is similar to the human medical term, “MRI-negative”.
IE and control dogs of each breed were SURS from the resulting population of dogs for volumetric analysis. Breeds with three or less dogs in either IE or control groups were excluded from further analyses. The volume of the FB, SAS and LV (total left and right) were estimated by one blinded observer (FW) using the Cavalieri principle on the T2-weighted transverse brain MRI.
Cavalieri’s principle
The Cavalieri principle is an unbiased and efficient design-based stereological method used to accurately estimate the volume of three-dimensional structures using a series of SURS representative two-dimensional slices [48]. The Cavalieri principle uses a robust 3D stochastic sampling procedure, which means that a sample size of 5–10 subjects can be used effectively to detect inter-group differences [49, 50]. The estimated volume is calculated as follows,
Where Ap is the area associated with a point, m′ is the section evaluation interval, \( \overline{t} \) is the mean section cut thickness and Pi is the number of points counted on the grid. The associated coefficient of error (CE) is calculated as follows,
Where TotalVar is the total variance of the estimated volumes, n is the number of sections and Pi is the number of points counted on the grid. In this study all comparisons were made with a minimum group size of n = 5.
Estimation procedure
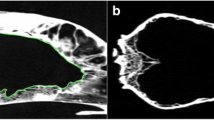
The stereological software StereoInvestigatorFootnote 2 was used to produce all final estimations. A minimum of 10 slices throughout a whole structure is recommended to decrease the CE and, therefore, MRI sequences with 20–29 and 30–39 slices were imported with a slice interval of 2 and 3, respectively. MRI slices were imported, and slice thickness was manually entered into the programme per the technical details of each scan. A 1.5mm2 point grid was chosen so that the smallest area of interest (LV) would contain a great enough number of points to achieve a low CE. The area associated with each point was informed by the software. The grid was superimposed over each slice at an angle randomly assigned by the software. Starting at the most rostral slice, points within an area of interest were highlighted using a different marker for each of the three structures, as shown in Fig. 5. Cross-points were counted when the top-right corner was within the structure of interest, as pictured in Fig. 6.
Measurement reliability
A training period to ensure accurate identification of desired anatomical structures and sufficient point grid density was carried out, followed by a pilot study of six dogs to check that the observer (FW, a Registered Veterinary Nurse) took measurements as accurately as a final-year ECVN Resident (AT). The training and pilot study were carried out manually, without the assistance of StereoInvestigator. An acetate page with a 1cm2 (for FB) and 2.5mm2 (for LV and SAS) cross-point grid was printed and attached to a computer screen displaying MRI slices at 1:1 magnification. Points were counted, manually recorded and volumes estimated. All other previously described methodology was upheld.
Statistical analyses
The data that support the findings of this study are openly available in Figshare at https://doi.org/10.5522/04/12578264.
Patient data was recorded on Microsoft ExcelFootnote 3 and exported to SPSS 23Footnote 4 to generate descriptive statistics. Raw stereological data was exported from StereoInvestigator to Microsoft Excel and exported to MiniTab 17Footnote 5 for inferential analyses. For the pilot study, mean volumes and the confidence intervals were reported, and for final measurements CE was used to express precision of the estimate [50]. Mean and standard deviation (SD) were reported for descriptive data regardless of data distribution and, for final volumetric data, the group mean was given alongside the coefficient of variance (CV) and the mean CE. CV represents the CE derived from the methodology and the genetic CV derived from the inherent biological variability of the animals. Inter-rater agreement was tested by calculating the intra-class coefficient (ICC) with a two-way mixed effects model for absolute agreement.
Data were tested for normality and homoscedasticity. Independent Samples t-tests, Kruskal-Wallis tests and Mood’s Median tests were used to compare groups, as appropriate. Where significant differences were found, Tukey’s Pairwise Comparisons were used for post-hoc analyses. The relationships between two continuous variables were tested with Pearson’s product-moment correlation. Statistical differences were considered significant at p < 0.05.
Ethics statement
Research was approved by the Royal Veterinary College Clinical Research Ethical Review Board (URN M2015 0053).
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the University College London Figshare repository, https://doi.org/10.5522/04/12578264.
Notes
Stereo Investigator, MBF BioScience
Microsoft Excel for Mac 2016
IBM SPSS Version 23
MiniTab®17
Abbreviations
- AED:
-
Anti-epileptic drug
- BAST:
-
Bile acid stimulation test
- CE:
-
Coefficient of error
- CS:
-
Cluster seizures
- CSF:
-
Cerebrospinal fluid
- CV:
-
Coefficient of variance
- ECVN:
-
European College of Veterinary Neurology
- FB:
-
Forebrain
- IE:
-
Idiopathic epilepsy
- IVETF:
-
International Veterinary Epilepsy Task Force
- LV:
-
Lateral ventricles
- MRI:
-
Magnetic resonance imaging
- SAS:
-
Subarachnoid space
- SE:
-
Status epilepticus
- SURS:
-
Systematic uniform random sampling
References
Heske L, Nødtvedt A, Jäderlund KH, Berendt M, Egenvall A. A cohort study of epilepsy among 665,000 insured dogs: incidence, mortality and survival after diagnosis. Vet J. 2014;202(3):471–6.
Kearsley-Fleet L, O’Neill D, Volk H, Church D, Brodbelt D. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet Rec. 2012;172(13):338.
De Risio L, Bhatti S, Muñana K, Penderis J, Stein V, Tipold A, et al. International veterinary epilepsy task force consensus proposal: Diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:148.
Zhou SY, Tong L, Song F, Hong XJ, Sun HF, Chang H, et al. Selective medial temporal volume reduction in the hippocampus of patients with idiopathic generalized tonic-clonic seizures. Epilepsy Res. 2015;110:39–48.
Goldberg H, Weinstock A, Bergsland N, Dwyer MG, Farooq O, Sazgar M, et al. MRI segmentation analysis in temporal lobe and idiopathic generalized epilepsy. BMC Neurol. 2014;14:131.
Ciumas C, Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67(4):683–6.
Kim JH, Lee JK, Koh SB, Lee SA, Lee JM, Kim SI, et al. Regional grey matter abnormalities in juvenile myoclonic epilepsy: a voxel-based morphometry study. Neuroimage. 2007;37(4):1132–7.
Du H, Zhang Y, Xie B, Wu N, Wu G, Wang J, et al. Regional atrophy of the basal ganglia and thalamus in idiopathic generalized epilepsy. J Magn Reson Imaging. 2011;33(4):817–21.
Woermann FG, Sisodiya SM, Free SL, Duncan JS. Quantitative MRI In patients with idiopathic generalized epilepsy. Evidence of widespread cerebral structural changes. Brain. 1998;121(9):1661–7.
Duncan JS. Neuroimaging methods to evaluate the etiology and consequences of epilepsy. Epilepsy Res. 2002;50(1–2):131–40.
Seeck M, Dreifuss S, Lantz G, Jallon P, Foletti G, Despland PA, et al. Subcortical nuclei volumetry in idiopathic generalized epilepsy. Epilepsia. 2005;46(10):1642–5.
Jackson DC, Irwin W, Dabbs K, Lin JJ, Jones JE, Hsu DA, et al. Ventricular enlargement in new-onset pediatric epilepsies. Epilepsia. 2011;52(12):2225–32.
Betting LE, Mory SB, Lopes-Cendes I, Li LM, Guerreiro MM, Guerreiro CAM, et al. MRI reveals structural abnormalities in patients with idiopathic generalized epilepsy. Neurology. 2006;67(5):848–52.
Milne ME, Anderson GA, Chow KE, O’Brien TJ, Moffat BA, Long SN. Description of technique and lower reference limit for magnetic resonance imaging of hippocampal volumetry in dogs. Am J Vet Res. 2013;74(2):224–31.
Boyko AR, Quignon P, Li L, Schoenebeck JJ, Degenhardt JD, Lohmueller KE, et al. A Simple Genetic Architecture Underlies Morphological Variation in Dogs. PLoS Biol. 2010:8.
Drake AG, Klingenberg CP. Large-scale diversification of skull shape in domestic dogs: disparity and modularity. Am Nat. 2010;175(3):289–301.
Frank L, Lüpke M, Kostic D, Löscher W, Tipold A. Grey matter volume in healthy and epileptic beagles using voxel-based morphometry – a pilot study. BMC Vet Res. 2018; 14(1):50 [cited 2019 4 Jan]
Nitzsche B, Boltze J, Ludewig E, Flegel T, Schmidt MJ, Seeger J, Barthel H, Brooks OW, Gounis MJ, Stoffel MH, Schulze S. A stereotaxic breed-averaged, symmetric T2w canine brain atlas including detailed morphological and volumetrical data sets. Neuroimage. 2019;15;187:93–103.
Rusbridge C, Long S, Jovanovik J, Milne M, Berendt M, Bhatti SFM, et al. International Veterinary Epilepsy Task Force recommendations for a veterinary epilepsy-specific MRI protocol. BMC Vet Res. 2015;11:194.
Kii S, Uzuka Y, Taura Y, Nakaichi M, Inokuma H, Onishi T. Developmental change of lateral ventricular volume and ratio in beagle-type dogs up to 7 months of age. Vet Radiol Ultrasound. 1998;39(3):185–9.
Schroder H, Meyer-Lindenberg A, Nolte I. Comparative examination of the lateral cerebral ventricles of different dog breeds using quantitative computed tomography. Berl Munch Tierarztl Wochenschr. 2006;119(11–12):506–11.
Schmidt MJ, Laubner S, Kolecka M, Failing K, Moritz A, Kramer M, et al. Comparison of the relationship between cerebral white matter and grey matter in normal dogs and dogs with lateral ventricular enlargement. PLoS One. 2015;
Estey CM, Dewey CW, Rishniw M, Lin DM, Bouma J, Sackman J, et al. A Subset of Dogs with Presumptive Idiopathic Epilepsy Show Hippocampal Asymmetry: A Volumetric Comparison with Non-Epileptic Dogs Using MRI. Front Vet Sci. 2017;4:183 [cited 2019 4 Jan]
De Haan CE, Kraft SL, Gavin PR, Wendling LR, Griebenow ML. Normal variation in size of the lateral ventricles of the Labrador retriever dog as assessed by Matgnetic resonance imaging. Vet Radiol Ultrasound. 1994;35(2):83–6.
Kii S, Uzuka Y, Taura Y, Nakaichi M, Takeuchi A, Inokuma H, et al. Magnetic resonance imaging of the lateral ventricles in beagle-type dogs. Vet Radiol Ultrasound. 1997;38(6):430–3.
Siniscalchi M, Franchini D, Pepe AM, Sasso R, Dimatteo S, Vallortigara G, et al. Volumetric assessment of cerebral asymmetries in dogs. Laterality. 2011;16(5):528–36.
Pivetta M, De Risio L, Newton R, Dennis R. Prevalence of lateral ventricle asymmetry in brain mri studies of neurologically normal dogs and dogs with idiopathic epilepsy. Vet Radiol Ultrasound. 2013;54(5):516–21.
Kuwabara T, Hasegawa D, Kobayashi M, Fujita M, Orima H. Clinical magnetic resonance volumetry of the hippocampus in 58 epileptic dogs. Vet Radiol Ultrasound. 2010;51(5):485–90.
Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63.
Vullo T, Korenman E, Manzo RP, Gomez DG, Deck MDF, Cahill PT. Diagnosis of cerebral ventriculomegaly in normal adult beagles using quantitative MRI. Vet Radiol Ultrasound. 1997;38(4):277–81.
Schmidt MJ, Amort KH, Failing K, Klingler M, Kramer M, Ondreka N. Comparison of the endocranial- and brain volumes in brachycephalic dogs, mesaticephalic dogs and Cavalier King Charles spaniels in relation to their body weight. Acta Vet Scand. 2014;56:30.
Pilegaard AM, Berendt M, Holst P, Møller A, McEvoy FJ. Effect of Skull Type on the Relative Size of Cerebral Cortex and Lateral Ventricles in Dogs. Front Vet Sci. 2017;4:30.
Bakici C, Akgun RO, Ozen D, Algin O, Oto C. The volume fraction values of the brain compartments using the Cavalieri principle and a 3T MRI in brachycephalic and mesocephalic dogs. Vet Med (Praha). 2019;64(11):482–9.
Nairismägi J, Gröhn OHJ, Kettunen MI, Nissinen J, Kauppinen RA, Pitkänen A. Progression of brain damage after status epilepticus and its association with epileptogenesis: a quantitative MRI study in a rat model of temporal lobe epilepsy. Epilepsia. 2004;45(9):1024–34.
Kimotsuki T, Nagaoka T, Yasuda M, Tamahara S, Matsuki N, Ono K. Changes of magnetic resonance imaging on the brain in beagle dogs with aging. J Vet Med Sci. 2005;67(10):961–7.
Su MY, Tapp PD, Vu L, Chen YF, Chu Y, Muggenburg B, et al. A longitudinal study of brain morphometrics using serial magnetic resonance imaging analysis in a canine model of aging. Prog Neuro-Psychopharmacol Biol Psychiatr. 2005;29(3):389–97.
Pugliese M, Carrasco JL, Gomez-Anson B, Andrade C, Zamora A, Rodríguez MJ, et al. Magnetic resonance imaging of cerebral involutional changes in dogs as markers of aging: an innovative tool adapted from a human visual rating scale. Vet J. 2010;186(2):166–71.
Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–70.
Noh D, Choi S, Choi H, Lee Y, Lee K. Evaluation of interthalamic adhesion size as an indicator of brain atrophy in dogs with and without cognitive dysfunction. Vet Radiol Ultrasound. 2017;58(5):581–7.
Packer RMA, McGreevy PD, Salvin HE, Valenzuela MJ, Chaplin CM, Volk HA. Cognitive dysfunction in naturally occurring canine idiopathic epilepsy. Ginsberg SD, editor. PLoS One. 2018;13(2):e0192182 [cited 2018 24 Aug]
Mendes A, Sampaio L. Brain magnetic resonance in status epilepticus: a focused review. Seizure. 2016;38:63–7.
Cole AJ. Status epilepticus and brain atrophy shrinkage is a growing problem. JAMA Neurol. 2016;73(10):1182–3.
Hocker S, Nagarajan E, Rabinstein AA, Hanson D, Britton JW. Progressive brain atrophy in super-refractory status epilepticus. JAMA Neurol. 2016;73(10):1201–7.
Newey CR, George P, Nattanmai P, Ahrens C, Hantus S, Sarwal A. Evolution of Cerebral Atrophy in a Patient with Super Refractory Status Epilepticus Treated with Barbiturate Coma. Case Rep Neurol Med. 2017;1–4 [cited 2019 4 Jan]
Furlong C, García-Fiñana M, Puddephat M, Anderson A, Fabricius K, Eriksen N, et al. Application of stereological methods to estimate post-mortem brain surface area using 3T MRI. Magn Reson Imaging. 2013;31(3):456–65.
Jelsing J, Rostrup E, Markenroth K, Paulson OB, Gundersen HJG, Hemmingsen R, et al. Assessment of in vivo MR imaging compared to physical sections in vitro - a quantitative study of brain volumes using stereology. Neuroimage. 2005;26(1):57–65.
Martín-Vaquero P, Da Costa RC, Echandi RL, Tosti CL, Knopp MV, Sammet S. Magnetic resonance imaging of the canine brain at 3 and 7T. Vet Radiol Ultrasound. 2011;52(1):25–32.
Howard CV, Reed MG. Unbiased stereology. Three-dimensional measurement in microscopy. 3rd ed. Liverpool: QTP Publications; 2010.
Baddeley A, Vedel Jensen EB. Stereology for statisticians. London: Chapman & Hall/CRC; 2004.
Gundersen HJG, Jensen EBV, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology - reconsidered. J Microsc. 1999;193(Pt3):199–211.
Acknowledgments
Many thanks to Eli Jovanovik for her help preparing and blinding the MRI images and to the reviewers for their time and constructive comments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. None of the associated institutions played a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript and only provided financial support in the form of authors’ salaries.
Author information
Authors and Affiliations
Contributions
FW is responsible for design, acquisition of data, data analysis, data interpretation, drafting of the manuscript. AC is responsible for design, acquisition of data, data analysis. HV is responsible for concept, design, data interpretation, critical revision of the manuscript, approval of the article. RMAP is responsible for data interpretation, critical revision of the manuscript, approval of the article. AT is responsible for data acquisition. CR is responsible for concept, design, data interpretation, critical revision of the manuscript, approval of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research was approved by the Royal Veterinary College Clinical Research Ethical Review Board (URN M2015 0053). Owners provided informed consent for all procedures undertaken based on the medical needs of their dog. Owners of dogs provided informed consent for their dog’s data to be used for research purposes.
Competing interests
HV: Served as contract researcher for: Nestle 2012–2014 and 2017–2019 - dietary modification of epilepsy in dogs; Desitin Pharma, 2012 - the role of levetiracetam in a referral hospital; industrial Funding 2014–2015 - investigating the effects of imepitoin behavioural, physiologic and owner reported indicators of anxiety in dogs treated for idiopathic epilepsy. Received competitive research grants for: RCVS pump primer grant 2010–2013 - pharmacometabonomic profiling of epileptic dogs; Waltham Foundation 2011–2014 - determination of plasma omega-3 fatty acid status in dogs with primary epilepsy and relationship to antiepileptic drug metabolism; CASE BBSRC PhD studentship 2012–2016 - metabolic profiling of epilepsy in dogs; American Kennel Club American Health Foundation, 2016–2018 - Investigating the Effect of a Ketogenic Medium Chain Triglycerides Supplement on the treatment of Canine Idiopathic Epilepsy and its behavioural comorbidities; BBSRC 2017–2020 - Investigating the relationship between epilepsy, drug-resistance and affective disorders in the domestic dog BB/P001874/1.
RMAP: Received industrial funding as a co-applicant from Boehringer Ingelheim (2014–15; Investigating the effects of imepitoin on behavioural, physiologic and owner-reported indicators of anxiety in dogs treated for idiopathic epilepsy) and Nestle (2017–19; Dietary modification of epilepsy in dogs). Received competitive research grants from the American Kennel Club (2016–18; Investigating the effect of a ketogenic medium chain triglycerides supplement on the treatment of canine idiopathic epilepsy and its behavioural comorbidities); BBSRC 2017–20; Investigating the relationship between epilepsy, drug-resistance and affective disorders in the domestic dog; BB/P001874/1 and 2017–2020; Comorbidity and characteristics of canine neurodevelopmental disorders and their impact on animal welfare; BB/P 010881/1.
CR: Served as a paid consultant for Boehringer Ingelheim in 2014.
FW, AC and AT declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Watson, F., Coppi, A.A., Volk, H.A. et al. Comparison of volume of the forebrain, subarachnoid space and lateral ventricles between dogs with idiopathic epilepsy and controls using a stereological approach: Cavalieri’s principle. Canine Genet Epidemiol 8, 3 (2021). https://doi.org/10.1186/s40575-021-00101-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40575-021-00101-6