Abstract
Background
Chest computed tomography findings are helpful for understanding the pathophysiology of severe acute respiratory distress syndrome (ARDS). However, there is no large, multicenter, chest computed tomography registry for patients requiring veno-venous extracorporeal membrane oxygenation (V-V ECMO). The aim of this study was to describe chest computed tomography findings at V-V ECMO initiation and to evaluate the association between the findings and outcomes in severe ARDS.
Methods
This multicenter, retrospective cohort study enrolled patients with severe ARDS on V-V ECMO, who were admitted to the intensive care units of 24 hospitals in Japan between January 1, 2012, and December 31, 2022.
Results
The primary outcome was 90-day in-hospital mortality. The secondary outcomes were the successful liberation from V-V ECMO and the values of static lung compliance. Among the 697 registry patients, of the 582 patients who underwent chest computed tomography at V-V ECMO initiation, 394 survived and 188 died. Multivariate Cox regression showed that traction bronchiectasis and subcutaneous emphysema increased the risk of 90-day in-hospital mortality (hazard ratio [95% confidence interval] 1.77 [1.19–2.63], p = 0.005 and 1.97 [1.02–3.79], p = 0.044, respectively). The presence of traction bronchiectasis was also associated with decreased successful liberation from V-V ECMO (odds ratio: 0.27 [0.14–0.52], p < 0.001). Lower static lung compliance was associated with some chest computed tomography findings related to changes outside of pulmonary opacity, but not with the findings related to pulmonary opacity.
Conclusions
Traction bronchiectasis and subcutaneous emphysema increased the risk of 90-day in-hospital mortality in patients with severe ARDS who required V-V ECMO.
Similar content being viewed by others
Background
Acute respiratory distress syndrome (ARDS) is a heterogeneous syndrome [1, 2], and the characteristics of “bilateral opacities”, according to the Berlin criteria for the definition of ARDS [3] differ from patient to patient [4,5,6]. ARDS can be classified under three severity categories [7]. The mortality rate of the most severe category—severe ARDS (PaO2/FiO2 ratio [P/F ratio] ≤ 100)—exceeds 50%, even if respiratory support with veno-venous extracorporeal membrane oxygenation (V-V ECMO) is attempted [8]. Considering this extremely high risk of mortality, studies focusing on severe ARDS requiring V-V ECMO support should be encouraged, although many previous studies, except for the Extracorporeal Life Support Organization registry analyses [8, 9], have been limited by small sample sizes [10, 11]. Analyses of data from a large multicenter database with novel findings may lead to the development of a new treatment strategy, including a more appropriate indication for V-V ECMO support, in this research field.
Therefore, we developed a retrospective database of patients with severe ARDS receiving V-V ECMO, named the Japan Chest CT for ARDS requiring V-V ECMO registry (J-CARVE registry), including data from 24 institutions across Japan. The J-CARVE registry is unique compared with other registries because it includes the chest computed tomography (CT) imaging data at V-V ECMO support initiation. Undoubtedly, chest CT findings are helpful in understanding the pathophysiology of ARDS [12, 13]; however, few studies have described the characteristics of chest CT findings in severe ARDS with V-V ECMO. This study aimed to describe the chest CT findings at the initiation of V-V ECMO support in patients with severe ARDS and evaluate the association between these findings and the risk of mortality.
Methods
Study design
Using data from the intensive care units (ICUs) at 24 institutions across Japan, we developed the J-CARVE registry—a retrospective database of patients with severe ARDS on V-V ECMO. Institutions that intended to participate in this registry had to submit a participation form available on the J-CARVE registry website (https://www.ace-registry.net) and needed to have treated at least 10 patients with severe ARDS who required V-V ECMO. The study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) before starting data collection (UMIN000048709). The registry was approved by the Institutional Review Board of Hiroshima University Hospital (E-2768), which waived the requirement for obtaining informed patient consent to ensure participant anonymity, as stipulated in the Japanese government guidelines. The details of the J-CARVE registry data collection and quality control are described in Additional file 1: SMethods.
Participants
The J-CARVE registry retrospectively enrolled adult (age ≥ 18 years) patients with severe ARDS for whom V-V ECMO support was initiated between January 2012 and December 2022. Severe ARDS was diagnosed based on the Berlin definition criteria (P/F ratio ≤ 100) [3]. In this study, patients were further excluded if they did not undergo chest CT examinations within a stipulated time window of 3 days from the start of V-V ECMO support or if the radiologists judged that the findings of the chest CT did not indicate ARDS.
Interpretation of chest CT scans
The chest CT findings were interpreted by five Japanese board-certified radiologists (WF, KN, HM, SK, and SM), all of whom had > 5 years of experience in interpreting chest CT images of patients with ARDS, based on a previous study [14]. The representative images for each finding are shown in Additional file 1: Fig. S1. For each CT data, two reviewers were randomly selected to interpret the images in a blinded manner. Prior to the interpretation of any CT findings, the diagnosis of ARDS was confirmed radiologically, and patients were only excluded from further assessments if both radiologists determined that there were no findings indicative of ARDS.
The concordance rates between the two evaluators for each chest CT finding are summarized in Additional file 1: Table S1. Any disagreements were resolved by a third reviewer in a blinded manner. Two patients had a history of right upper lobectomy and were regarded as having no signs in the right upper lobe.
Outcomes
The primary outcome was 90-day in-hospital mortality. The secondary outcomes were the successful liberation from V-V ECMO and values of static lung compliance.
Statistical analyses
The Chi-square and Mann–Whitney U tests were used to compare categorical and continuous variables, respectively. For the survival analysis, the survival time (number of days from V-V ECMO support initiation until death or the last follow-up) was considered uncensored if the patient died in the hospital on day 90 or earlier. Survival times were censored at the date of hospital discharge or day 90, whichever occurred first. Adjusted Cox proportional hazards regression analyses were performed using several adjusted variables. Besides information regarding basic demographics and comorbidities (age, sex, body mass index, and medical histories of hypertension, diabetes, chronic kidney disease, obstructive lung disease, interstitial lung disease, and chronic heart failure), we analyzed the following variables: time from the start of mechanical ventilation (MV) to V-V ECMO initiation, Sequential Organ Failure Assessment score at the start of V-V ECMO support as an indicator of the patients’ clinical severity, application of prone positioning before V-V ECMO support, and use of neuromuscular blockers before V-V ECMO support. These variables were reported to be associated with the mortality of patients with ARDS on V-V ECMO [10, 15]. We performed subgroup analyses according to the duration from the start of MV and V-V ECMO support initiation (early vs. late induction) or the primary cause of ARDS (bacterial, viral, or other pneumonia). We set 7 days as the cut-off value because many previous studies used 7 days [16,17,18,19]; in addition, patients receiving MV for > 7 days before V-V ECMO showed a higher mortality rate than those receiving MV for < 7 days [16].
The multivariate logistic regression analysis was performed to evaluate the association between each chest CT finding and successful liberation from V-V ECMO using the same adjustment factors as those for the survival analysis. The association between each chest CT finding and static lung compliance was also evaluated using the Mann–Whitney U test. All reported p-values were two-sided, and p < 0.05 indicated statistical significance. All analyses were performed using the R Package (R Foundation for Statistical Computing, Vienna, Austria).
Results
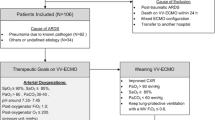
The patient flow diagram of this study is shown in Fig. 1. Among 697 patients who were admitted in 24 ICUs in Japan, 115 were excluded for the following reasons: 41 patients had unavailable chest CT images, 70 underwent chest CT examinations but not close to the start of V-V ECMO support (within 3 days), and 4 were judged as not having ARDS based on the radiologists’ interpretation of their chest CT findings. The remaining 582 patients were analyzed. The distribution of registered patients by years is shown in Figure E2. The time difference between chest CT examinations and the start of V-V ECMO support among the analyzed patients is shown in Additional file 1: Fig. S3. In approximately 80% of all analyzed patients, the CT examination was performed within 24 h, and 90% underwent the chest CT within 48 h.
Additional file 1: Table S2 shows the basic information of all participating institutes. Among the 24 participating hospitals, 14 (58.3%) were academhospitalsic , whereas the remaining 10 (41.7%) were nonacademic hospitals. Eleven (45.8%) of all institutes routinely employed an ultra-lung-protective strategy of a limited tidal volume of ≤ 3 mL/kg during V-V ECMO support. The usual setting values of positive end-expiratory pressure during V-V ECMO support varied from 5 to 15 cmH2O among hospitals.
Baseline characteristics from before and after V-V ECMO are summarized in Tables 1 and 2, respectively. With regard to the primary cause of ARDS, 93.5% (544/582) were intrapulmonary and 6.5% (38/582) were extrapulmonary cases. The mean duration (days) between the start of MV and V-V ECMO instauration was 2.0 (1.0–4.0) days, the mean value of the P/F ratio was 89.1 (± 37.0), and the mean score point of the Murray Lung Injury Score was 3.25 (2.75–3.50) (Table 1). The average setting values of positive end-expiratory pressure and dynamic driving pressure during days 1–3 after V-V ECMO support were 10.0 (± 3.4) and 10.0 (± 5.2) cmH2O, respectively, whereas the corresponding values during days 4–7 were 10.3 (± 5.2) and 10.0 (± 5.0) cmH2O, respectively (Table 2). The median length of hospital stay was 35.0 (19.0–61.0) days. The proportion of patients who were successfully liberated from V-V ECMO was 82.2% (475/582). The median duration of V-V ECMO support was 11.0 (7.0–19.0) days. Regarding the 90-day in-hospital mortality, 175 (30.1%) patients died within 90 days after the initiation of V-V ECMO support, whereas 407 (69.9%) survived. ICU mortality among all analyzed patients was 29.0% (169/582).
The interpretations of the chest CT findings of the analyzed patients are summarized in Additional file 1: Table S3. The interpretations of the chest CT findings analyzed according to the duration between the start of MV and V-V ECMO support initiation and primary reasons for ARDS are shown in Additional file 1: Fig. S4. We plotted Kaplan–Meier curves for the findings related to pulmonary opacity and subcutaneous emphysema (Fig. 2). The curves for the other findings are shown in Additional file 1: Fig. S5. The 90-day in-hospital mortality was significantly higher in patients with traction bronchiectasis than in those without (log-rank test: p < 0.001); however, no statistical significance was observed in the associations of other CT interpretations. The multivariate Cox regression analysis showed that the presence of traction bronchiectasis or subcutaneous emphysema increased the risk of 90-day in-hospital mortality (traction bronchiectasis; hazard ratio [HR] 1.77 [95% confidence interval {CI} 1.19–2.63], p = 0.005, subcutaneous emphysema; HR 1.97 [1.02–3.79], p = 0.044; see Additional file 1: Table S4).
For the subgroup analyses, we stratified all analyzed patients according to whether the duration between the start of MV and V-V ECMO support initiation was ≥ 7 days or not (early vs. late induction group). The 90-day in-hospital mortality was 39.8% (39/98) and 23.4% (92/393) in the early induction groups with and without traction bronchiectasis, respectively, and 67.7% (23/34) and 36.8% (21/57) in the late induction groups with and without traction bronchiectasis, respectively. The log-rank test showed that mortality was greater in patients with traction bronchiectasis than in those without, regardless of early or late induction (early, p = 0.020; late, p = 0.003). There was no significant difference between the patients with traction bronchiectasis in the early induction group and those without traction bronchiectasis in the late induction group (p = 0.32) (Fig. 3). Kaplan–Meier curves of traction bronchiectasis according to the primary reason for ARDS (excluding patients with extrapulmonary reasons as the primary cause of ARDS because there were only three with traction bronchiectasis on their chest CT scans) were also plotted. A trend was observed where the proportion of patients with traction bronchiectasis was greater than that of those without the sign, regardless of the primary reason for ARDS (see Additional file 1: Fig. S6). The presence of traction bronchiectasis also significantly decreased the odds ratio for successful liberation from V-V ECMO in the multivariate logistic regression analysis (odds ratio: 0.27 [95% CI 0.14–0.52], p < 0.001; see Additional file 1: Table S5).
In addition, for 453 patients for whom the values of static lung compliance were available, we evaluated the association between the values and each CT finding at the start of V-V ECMO support. Decreased values of static lung compliance were associated with the presence of pleural effusion, pneumothorax, and subcutaneous emphysema and a right atrium/left atrium ratio of > 1, although there were no findings related to the intensity of opacity, distribution of opacity, or fibroproliferative changes (Fig. 4).
Association between decreased value of static lung compliance and each chest computed tomography finding. Numbers close to the box plot represent the median values. The Mann–Whitney U test was performed. CTR: cardiothoracic ratio; IVC: inferior vena cava; LA: left atrium; PA: pulmonary artery; RA: right atrium
Discussion
In this study, we reported the profile of the J-CARVE registry, which is a large multicenter database of patients with severe ARDS on V-V ECMO support, including chest CT imaging data at the initiation of V-V ECMO support. This registry consists of data from 24 institutes across Japan, which has the highest number of CT scanners among the Organization for Economic Cooperation and Development countries and where CT examinations are much easier to access than those in other countries [20]. In fact, our database included the chest CT imaging data at the initiation of V-V ECMO support in most of the registered patients (> 80%).
Our registry is the first to report a large sample size from Japan to describe the outcomes of patients with severe ARDS on V-V ECMO. According to our data, > 80% of all patients were successfully liberated from V-V ECMO, and their ICU mortality was approximately 30%, which is comparable to that of 25–35% reported in previous studies, including an international report by Schmidt et al. [10, 15, 21]. The SOFA scores between our study and Schmidt et al.’s study were also comparable (9.8 ± 4.1 vs 10.4 ± 4.0), which means that the severity may be similar between the two studies. As for the treatment before and after V-V ECMO, the percentage of patients who underwent neuromuscular blocker therapy (56%) or prone positioning (26%) during V-V ECMO was higher than that reported in Schmidt et al.’s study (41% and 6%, respectively); in contrast, the percentage of patients who underwent these therapies before V-V ECMO was lower (our registry: 42% and 16%; Schmidt et al.’s study: 62% and 26%, respectively). This may be due to the difference in general treatment strategies between Japan, where a V-V ECMO is initiated without any attempt of other treatments, and other countries. We are interested in performing a future study to evaluate their outcomes after adjusting for the content of treatments before and after V-V ECMO between Japan and other countries.
Our findings highlight the importance of identifying traction bronchiectasis when evaluating the risk of mortality as well as predicting successful liberation in patients with severe ARDS on V-V ECMO, which aligns with previous findings targeting general populations of patients with ARDS without V-V ECMO [22,23,24]. Although our previous retrospective cohort study failed to show a statistically significant association between traction bronchiectasis and hospital mortality, this was possibly due to a small sample size [25]. Traction bronchiectasis is a reliable index of the degree of fibroproliferation in the pathophysiology of ARDS, especially ARDS with a diffuse alveolar damage pattern pathologically, and it was reported to be linked to the need for prolonged mechanical ventilatory support and worse outcomes [26]. Pathophysiologically, the presence of fibroproliferative changes may represent more severe lung injury like diffuse alveolar damage because ARDS is a complex syndrome with diverse pathological manifestations [27].
In our study, the mortality of patients in the late induction group was similar to that of those in the early induction group if traction bronchiectasis was absent. International guidelines suggest that the indication for V-V ECMO is limited to patients who receive MV within 7 days, based on previous studies showing the possibility that lung injuries in patients receiving MV for > 7 days are irreversible, leading to a decreased survival rate [21, 28]. However, our data suggest it is the existence of traction bronchiectasis, rather than the timing alone, that determines their survival, although the difference in survival during the short periods after V-V ECMO support has to be carefully evaluated in a future study because some survival curves in our results showed intersection during short periods.
In patients with severe ARDS, all of whom should have decreased static lung compliance, the values of static compliance were not associated with any findings related to pulmonary opacity, including intensity, distribution, and fibroproliferative changes. A previous study showed that static compliance was correlated with the amount of normally aerated lung tissues, and not with poorly aerated or nonaerated tissues [29], suggesting that the static compliance does not reflect the pathophysiology of severe ARDS, in which many parts of the lung are poorly aerated or nonaerated. Notably, the values of static compliance were similar between the patients who survived and those who died in our study (29.4 vs. 28.9 mL/cmH2O), which is consistent with the results of several studies reporting that static lung compliance is not associated with mortality [15]. We believe that our results support the importance of evaluation using other indices such as the presence of traction bronchiectasis, which cannot be assessed using respiratory mechanics.
In contrast, chest CT findings related to changes outside of pulmonary opacity, such as the presence of pneumothorax and subcutaneous emphysema, were significantly associated with a decreased value of static lung compliance. In particular, the presence of subcutaneous emphysema is the main macroscopic sign of barotrauma, which worsens the outcomes in patients with ARDS [30, 31]. Our data showed a significant association between the presence of subcutaneous emphysema and increased mortality, which is consistent with these previous findings.
Our study has some limitations. First, although all participating hospitals followed the guidelines for the indication for V-V ECMO, the final decision was made according to the preference of each participating hospital. Second, the values of positive end-expiratory pressure (PEEP) at the time of the chest CT examination were varied. PEEP-induced alveolar recruitment can transform poorly aerated lung areas into normally aerated lung areas [32]. Nevertheless, we believe that the interpretation of the presence of traction bronchiectasis and subcutaneous emphysema is unlikely to be significantly influenced by PEEP, because the values of PEEP during chest CT were similar between those with and without these findings (traction bronchiectasis (+) vs (−); 10.3 ± 4.4 cmH2O vs 10.7 ± 4.9 cmH2O, and subcutaneous emphysema (+) vs (−); 9.6 ± 3.3 cmH2O vs 10.7 ± 4.9 cmH2O). However, this does not mean that the continuous setting for MV until CT examination (not just timing at CT examination) does not influence the presence of any chest CT findings. Third, we cannot exclude a potential immortal time bias, although the 90-day survival rate was not markedly different between patients who underwent chest CT examination and those who did not (69.4% [455/656] vs 63.4% [26/41]). Fourth, several details regarding the measurements of some respiratory mechanics variables including static lung compliance at each participating hospital were unknown, which is a common limitation in large multicenter retrospective studies. Fifth, we cannot completely exclude the possibility that our database included the data of some patients with ARDS mimics, although we believe that our study is still worthwhile, as our registry contains real-world data. Finally, lung transplantation is rarely performed in Japan (none of the patients in this study underwent transplantation), which may have affected the indications and withdrawal of V-V ECMO support.
Conclusions
Traction bronchiectasis and subcutaneous emphysema increased the risk of 90-day in-hospital mortality in patients with severe ARDS who required V-V ECMO.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- HR:
-
Hazard ratio
- ICUs:
-
Intensive care units
- J-CARVE registry:
-
Japan Chest CT for ARDS requiring V-V ECMO registry
- MV:
-
Mechanical ventilation
- PEEP:
-
Positive end-expiratory pressure
- P/F ratio:
-
PaO2/FiO2 ratio
- UMIN-CTR:
-
University Hospital Medical Information Network Clinical Trials Registry
- V-V ECMO:
-
Veno-venous extracorporeal membrane oxygenation
References
Wilson JG, Calfee CS. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24:102.
Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. 1998;158:3–11.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.
Simon M, Braune S, Laqmani A, Metschke M, Berliner C, Kalsow M, Klose H, Kluge S. Value of computed tomography of the chest in subjects with ARDS: a retrospective observational study. Respir Care. 2016;61:316–23.
Coppola S, Pozzi T, Gurgitano M, Liguori A, Duka E, Bichi F, Ciabattoni A, Chiumello D. Radiological pattern in ARDS patients: partitioned respiratory mechanics, gas exchange and lung recruitability. Ann Intensive Care. 2021;11:78.
Desai SR, Wells AU, Suntharalingam G, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary injury: a comparative CT study. Radiology. 2001;218:689–93.
Villar J, Perez-Mendez L, Blanco J, Anon JM, Blanch L, Belda J, Santos-Bouza A, Fernandez RL, Kacmarek RM, Spanish Initiative for Epidemiology S, Therapies for AN. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting–a prospective, multicenter validation study. Intensive Care Med. 2013;39:583–92.
Friedrichson B, Mutlak H, Zacharowski K, Piekarski F. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care. 2021;25:38.
Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, Scheinkestel C, Cooper DJ, Brodie D, Pellegrino V, Combes A, Pilcher D. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–82.
Schmidt M, Stewart C, Bailey M, Nieszkowska A, Kelly J, Murphy L, Pilcher D, Cooper DJ, Scheinkestel C, Pellegrino V, Forrest P, Combes A, Hodgson C. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med. 2015;43:654–64.
Rilinger J, Zotzmann V, Bemtgen X, Schumacher C, Biever PM, Duerschmied D, Kaier K, Stachon P, von Zur MC, Zehender M, Bode C, Staudacher DL, Wengenmayer T. Prone positioning in severe ARDS requiring extracorporeal membrane oxygenation. Crit Care. 2020;24:397.
Sheard S, Rao P, Devaraj A. Imaging of acute respiratory distress syndrome. Respir Care. 2012;57:607–12.
Zompatori M, Ciccarese F, Fasano L. Overview of current lung imaging in acute respiratory distress syndrome. Eur Respir Rev. 2014;23:519–30.
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722.
Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, Vuylsteke A, Guervilly C, McGuinness S, Pierard S, Breeding J, Stewart C, Ching SSW, Camuso JM, Stephens RS, King B, Herr D, Schultz MJ, Neuville M, Zogheib E, Mira JP, Roze H, Pierrot M, Tobin A, Hodgson C, Chevret S, Brodie D, Combes A. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome. An international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200:1002–12.
Wu MY, Huang CC, Wu TI, Chang YS, Wang CL, Lin PJ. Is There a preinterventional mechanical ventilation time limit for candidates of adult respiratory extracorporeal membrane oxygenation. ASAIO J. 2017;63:650–8.
Olivier PY, Ottavy G, Hoff J, Auchabie J, Darreau C, Pierrot M. Prolonged time from intubation to cannulation in VV-ECMO for COVID-19: does it really matter? Crit Care. 2021;25:385.
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, Collaboration Ct. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–63.
Hermann M, Laxar D, Krall C, Hafner C, Herzog O, Kimberger O, Koenig S, Kraft F, Maleczek M, Markstaller K, Robak O, Rossler B, Schaden E, Schellongowski P, Schneeweiss-Gleixner M, Staudinger T, Ullrich R, Wiegele M, Willschke H, Zauner C, Hermann A. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann Intensive Care. 2022;12:6.
OECD database. August 22, 2023. https://data.oecd.org/healtheqt/computed-tomography-ct-scanners.htm.
Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, Haft JW, Swaniker F, Arbabi S, Hirschl RB, Bartlett RH. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240:595–605 (discussion 605-597).
Kamo T, Tasaka S, Suzuki T, Asakura T, Suzuki S, Yagi K, Namkoong H, Ishii M, Morisaki H, Betsuyaku T. Prognostic values of the Berlin definition criteria, blood lactate level, and fibroproliferative changes on high-resolution computed tomography in ARDS patients. BMC Pulm Med. 2019;19:37.
Hashimoto H, Yamamoto S, Nakagawa H, Suido Y, Sato S, Tabata E, Okamori S, Yoshida T, Ando K, Yoshitake S, Okada Y. Predictive value of computed tomography for short-term mortality in patients with acute respiratory distress syndrome: a systematic review. Sci Rep. 2022;12:9579.
Ichikado K, Muranaka H, Gushima Y, Kotani T, Nader HM, Fujimoto K, Johkoh T, Iwamoto N, Kawamura K, Nagano J, Fukuda K, Hirata N, Yoshinaga T, Ichiyasu H, Tsumura S, Kohrogi H, Kawaguchi A, Yoshioka M, Sakuma T, Suga M. Fibroproliferative changes on high-resolution CT in the acute respiratory distress syndrome predict mortality and ventilator dependency: a prospective observational cohort study. BMJ Open. 2012;2: e000545.
Nishikimi M, Ohshimo S, Fukumoto W, Anzai T, Awai K, Ogura T, Abe T, Masuda M, Fujizuka K, Nakamura M, Kyo M, Takahashi K, Shime N. Characteristics of the pulmonary opacities on chest CT associated with difficulty in short-term liberation from veno-venous ECMO in patients with severe ARDS. Respir Res. 2023;24:128.
Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–72.
Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet. 2022;400:1145–56.
Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226:544–64 (discussion 565-546).
Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis. 1987;136:730–6.
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36.
Lemmers DHL, Abu Hilal M, Bna C, Prezioso C, Cavallo E, Nencini N, Crisci S, Fusina F, Natalini G. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6:00385.
Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ, Group CTSAS. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1444–50.
Acknowledgements
We acknowledge and honor all of our team members who consistently put themselves in harm’s way during the COVID-19 pandemic. We dedicate this manuscript to them, as their vital contribution to knowledge about COVID-19 and sacrifices on the behalf of patients made it possible. We also want to thank Keigo Narita, Hidenori Mitani, Shota Kondo, and Shogo Maeda for interpreting chest CT scans. The collaborating authors in the J-CARVE Registry Group are the following. Junki Ishii1; Takayuki Ogura2; Mitsunobu Nakamura3; Keiki Shimizu4; Tatsutoshi Shimatani1; Mamoru Masuda3.
Affiliations of the J-CARVE Registry Group authors
1 Department of Emergency and Critical Care Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan; 2 Department of Emergency Medicine and Critical Care Medicine, SAISEIKAI Utsunomiya Hospital, Utsunomiya, Japan; 3 Advanced Medical Emergency Department and Critical Care Center, Japan Red Cross Maebashi Hospital, Maebashi, Japan; 4 Department of Critical Care and Emergency Medicine, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan.
Funding
This work was supported by JSPS KAKENHI (Grant Number JP22K09120) and the TSUCHIYA MEMORIAL MEDICAL FOUNDATION, a Grant-in-aid for multicenter clinical research from Japanese Association for Acute Medicine, and Japan Agency for Medical Research and Development (AMED, Grant Number JP23fk0108654).
Author information
Authors and Affiliations
Consortia
Contributions
Nishikimi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Nishikimi, Ohshimo, Fukumoto, Hamaguchi, Matsumura, Fujizuka, Hagiwara, Nakayama, Bunya, Maruyama, Abe, Anzai, Awai, Takahashi, Shime. Acquisition, analysis, or interpretation of data: Nishikimi, Fukumoto, Hamaguchi, Matsumura, Fujizuka, Hagiwara, Nakayama, Bunya, Maruyama, Ogata, Naito, Amemiya, Ikeda, Yagi, Furukawa, Taniguchi, Yagi, Katsuta, Konno, Suzuki, Kawasaki, Hattori, Nakamura, Kondo, Kikuchi, Kai. Drafting of the manuscript: Nishikimi, Ohshimo, Shime. Critical revision of the manuscript for important intellectual content: Ohshimo, Ogata, Naito, Amemiya, Ikeda, Yagi, Furukawa, Taniguchi, Yagi, Katsuta, Konno, Suzuki, Kawasaki, Hattori, Nakamura, Kondo, Kikuchi, Kai, Shime. Statistical analysis: Nishikimi, Anzai, Takahashi.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Boards of Hiroshima University Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
SMethods. Figure S1. Representative images of each of the characteristic pulmonary opacities on chest computed tomography scans. Figure S2. Distribution of registered patients by years. Figure S3. Cumulative proportion of the duration (h) between chest computed tomography examinations and initiation of veno-venous extracorporeal membrane oxygenation support. Figure S4. Characteristics of the chest computed tomography findings according to the mechanical ventilation–extracorporeal membrane oxygenation support duration and the underlying etiology of the acute respiratory distress syndrome. Figure S5. Survival curve of the chest computed tomography findings related to changes outside of the pulmonary opacity (excluding subcutaneous emphysema). Figure S6. Survival curve of participants with and without traction bronchiectasis separately according to the underlying etiology of acute respiratory distress syndrome. Table S1. Concordance rates between two evaluators. Table S2. Basic information of the participating hospitals. Table S3. Characteristics of chest computed tomography findings. Table S4. Results of multivariate Cox regression analysis of the relationship between V-V ECMO support initiation and 90-day in-hospital mortality. Table S5. Results of multivariate logistic regression analysis for successful ECMO liberation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nishikimi, M., Ohshimo, S., Fukumoto, W. et al. Chest CT findings in severe acute respiratory distress syndrome requiring V-V ECMO: J-CARVE registry. j intensive care 12, 5 (2024). https://doi.org/10.1186/s40560-023-00715-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-023-00715-x