Abstract
Background
Tracheostomy is commonly performed in critically ill patients because of its clinical advantages over prolonged translaryngeal endotracheal intubation. Early tracheostomy has been demonstrated to reduce the duration of mechanical ventilation and length of stay. However, its association with mortality remains ambiguous. This study aimed to evaluate the association between the timing of tracheostomy and mortality in patients receiving mechanical ventilation.
Methods
We performed a retrospective cohort analysis of adult patients who underwent tracheostomy during their intensive care unit (ICU) admission between April 2015 and March 2019. Patients who underwent tracheostomy before or after 29 days of ICU admission were excluded. Data were collected from the nationwide Japanese Intensive Care Patient Database. The primary outcome was hospital mortality. The timing of tracheostomy was stratified by quartile, and the association between patient outcomes was evaluated using regression analysis.
Results
Among the 85558 patients admitted to 46 ICUs during the study period, 1538 patients were included in the analysis. The quartiles for tracheostomy were as follows: quartile 1, ≤ 6 days; quartile 2, 7–10 days; quartile 3, 11–14 days; and quartile 4, > 14 days. Hospital mortality was significantly higher in quartile 2 (adjusted odds ratio [aOR]: 1.52, 95% confidence interval [CI]: 1.08–2.13), quartile 3 (aOR: 1.82, 95% CI: 1.28–2.59), and quartile 4 (aOR: 2.26, 95% CI: 1.61–3.16) (p for trend < 0.001) than in quartile 1. A similar trend was observed in the subgroup analyses of patients with impaired consciousness (Glasgow Coma Scale score < 8) and respiratory failure (PaO2:FiO2 ≤ 300) at ICU admission (p for trend = 0.081 and 0.001, respectively).
Conclusions
This multi-institutional observational study demonstrated that the timing of tracheostomy was significantly and independently associated with hospital mortality in a stepwise manner. Thus, early tracheostomy may be beneficial for patient outcomes, including mortality, and warrants further investigation.
Similar content being viewed by others
Background
Prolonged ventilation in critically ill patients is associated with long intensive care unit (ICU) and hospital stays and high mortality rates [1,2,3]. Compared to translaryngeal endotracheal intubation, tracheostomy has more potential advantages for patients with mechanical ventilation, such as lower airflow resistance and breathing effort, less administration of sedatives, better patient comfort and mobilization, weaning with less invasive attachment to and disconnection from the ventilator, and lower incidence of ventilator-associated pneumonia (VAP) [4,5,6,7,8]. Therefore, tracheostomy is commonly recommended for patients who are expected to require prolonged mechanical ventilation by international consensus [9, 10]. Over 20 years, the number of patients with mechanical ventilation in the ICU who underwent this procedure increased from 7 to 26% [11,12,13]. However, tracheostomy procedures pose a risk of complications, such as bleeding, stomal infection, and tube displacement, but these are mostly non-fatal [14, 15].
Numerous studies have been conducted concerning the optimal timing and benefits of early implementation to determine the appropriate indication for tracheostomy in critically ill patients with mechanical ventilation. In recent systematic reviews of randomized controlled trials [16,17,18], early tracheostomy demonstrated favorable outcomes, achieving a lower duration of mechanical ventilation and ICU stay than those of late tracheostomy. However, no association with mortality was found in these meta-analyses. Since the definition for the timing of tracheostomy varies among studies (early, within 2–10 days; late, 6–29 days or more) [19,20,21,22,23], direct two-arm comparisons remain challenging. Hence, evidence on the association between the timing of tracheostomy and mortality is still lacking.
Accordingly, we aimed to conduct a detailed statistical analysis of the impact of the timing of tracheostomy on the clinical outcomes of patients who received mechanical ventilation in a multicenter ICU setting.
Methods
Design and setting
This retrospective cohort study analyzed data from the Japanese Intensive Care PAtient Database (JIPAD), a multicenter observational data registry of critically ill patients established by the Japanese Society of Intensive Care Medicine. As of December 2019, the JIPAD study involves 70 ICUs and more than 100,000 patients in Japan. The Japanese Society of Intensive Care Medicine has a partnership agreement with the Australian and New Zealand Intensive Care Society Center for Outcome and Resource Evaluation. Consequently, the JIPAD collects data similar to those of the Australian and New Zealand Intensive Care Society Adult Patient Database (ANZICS APD), which is widely recognized as one of the largest ICU databases. A complete description of the JIPAD methodology has been previously provided [24].
Study population
We included adult patients aged ≥ 18 years who were admitted to the ICU between April 2015 and March 2019 and underwent a tracheostomy during their ICU admission. Patients with repeat ICU admissions from the same hospital episode, patients who underwent tracheostomy before the present ICU admission or after 29 days of ICU admission, and patients with missing tracheostomy dates were excluded.
Data collection
With the aim to improve the quality of care and develop intensive care practices, the JIPAD collects clinical data, including patient demographics, diagnosis at ICU admission, physiological data and treatment in the ICU, and patient outcomes. In this study, the following data were collected from the JIPAD: age, sex, body mass index, comorbidities (chronic heart failure, chronic respiratory failure, liver cirrhosis, liver failure, acute leukemia/multiple myeloma, lymphoma, metastatic cancer, immunosuppression, acquired immunodeficiency syndrome, and maintenance dialysis: [yes, no]), emergency admission (yes, no), surgical type of admission (yes, no), systematic diagnosis for ICU admission (cardiac, respiratory, gastrointestinal, neurological, sepsis, trauma, and other diagnoses), Acute Physiology And Chronic Health Evaluation (APACHE) II score, data within 24 h after ICU admission (incidence of acute kidney injury [yes, no], lowest PaO2:FiO2, Glasgow Coma Scale [GCS] score), length of hospital stay before ICU admission, ICU treatment (extracorporeal membrane oxygenation [yes, no], continuous renal replacement therapy [yes, no], duration of mechanical ventilation before tracheostomy, duration of mechanical ventilation in ICU, liberation of mechanical ventilation during ICU stay [yes, no]), length of ICU and hospital stay, and ICU and hospital mortality (yes, no). In the present study, immunosuppression and acquired immunodeficiency syndrome were collectively categorized under immunodeficiency. Comorbid acute leukemia/multiple myeloma, lymphoma, and metastatic cancer were categorized as malignancy. Chronic liver disease was defined as liver cirrhosis or liver failure. The data from the JIPAD used in this study, as well as from the ANZICS APD, were obtained exclusively during hospitalization at the registered institutions.
Outcomes
The primary outcome of this study was hospital mortality, and the secondary outcome was ICU mortality.
Statistical analyses
Continuous data were summarized as medians and interquartile ranges (IQRs), while categorical data were presented as numbers and percentages. The timing of tracheostomy starting from ICU admission was divided into quartiles: Q1, ≤ 6 days; Q2, 7–10 days; Q3, 11–14 days; and Q4, > 14 days. Differences in proportions were evaluated using the Chi-square test or Fisher’s exact test. Differences in distributed data were evaluated using the Kruskal–Wallis tests for the four groups.
To determine the relationships between the quartiles of the timing of tracheostomy and the primary and secondary outcomes, univariable and multivariable logistic regression models were developed, and the crude and adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. Age (1-year increment), sex (male, female), and APACHE II score (1-point increment) were added to the multivariable model for hospital and ICU mortality to adjust for potential confounders. We described the possible non-linear associations between the timing of tracheostomy and the estimated hospital and ICU mortality using restricted cubic splines in the univariable logistic regression model. Moreover, we performed a planned subgroup analysis stratified by the level of consciousness at ICU admission (GCS score < 8 or ≥ 8). Considering the effect of the timing of tracheostomy on lung injury, a subgroup analysis was performed in patients with respiratory failure. Respiratory failure was defined as the lowest PaO2:FiO2 ≤ 300 during the first 24 h of ICU admission; patients admitted to the ICU for cardiac diagnosis were excluded from the subgroup with respiratory failure. We also conducted a subgroup analysis of patients admitted to the ICU after cardiovascular surgery. The interaction effect between the timing of tracheostomy and subgroups of hospital mortality was assessed using the multivariable logistic regression model. All statistical analyses were conducted using R version 3.6.3 (2020, R Foundation for Statistical Computing, Vienna, Austria). A two-sided p value of 0.05 was considered significant.
Results
Study participants
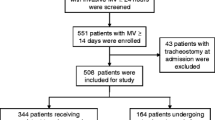
Among the 85558 admissions to 46 ICUs during the study period, 2073 patients who underwent tracheostomy and required mechanical ventilation were assessed for eligibility. Of them, we excluded 535 patients aged < 18 years (n = 93), with repeat ICU admissions (n = 266), with tracheostomy on ICU admission (n = 56), who underwent tracheostomy after day 29 (n = 49), and with missing data (n = 71). Finally, 1538 adult patients were included in the analysis (Fig. 1).
The characteristics of the study population divided by the quartiles of the timing of tracheostomy are presented in Table 1. The Q1 group was more likely to be admitted to the ICU for respiratory and neurological diagnoses than the other groups. In contrast, ICU admission for surgical, cardiac, and gastrointestinal diagnoses was more common for the Q4 group than for the other groups. Similar findings were observed in patients with chronic heart failure. In addition, the Q4 group had a lower PaO2:FiO2 and a higher GCS score within 24 h after ICU admission than those of the other groups. The frequency of comorbid maintenance dialysis, APACHE II score, and incidence of acute kidney injury within 24 h after ICU admission were the highest in the Q3 group. Patients who underwent tracheostomy were identified in 45 ICUs. The proportion of patients with the earliest tracheostomy classified as Q1 group ranged from 0 to 75% at each institution, and the timing of tracheostomy differed significantly between institutions (p < 0.001, Additional file 1).
Processes of care during ICU stay and length of hospital stay
The proportions of patients who received extracorporeal membrane oxygenation and continuous renal replacement therapy during ICU admission were higher in the Q4 group (18.7% and 44.2%, respectively) than in the other groups (p < 0.001 and p < 0.001, respectively; Table 2). In addition, the duration of mechanical ventilation in the ICU and the length of ICU stay were significantly longer in the Q4 group than in the other quartile groups (23.5 [IQR, 18.7–31.3] days and 27.5 [IQR, 21.7–35.0] days, respectively; p < 0.001 and p < 0.001, respectively). Moreover, the length of hospital stay was the longest in the Q4 group (78 [IQR, 51–119] days vs. 51 [IQR, 33–77] days for Q1; 56 [IQR, 36–90] days for Q2; and 64 [IQR, 44–103] days for Q3, p < 0.001).
Clinical outcomes
Hospital mortality, as the primary outcome, progressively increased with increasing quartiles of the timing of tracheostomy (Q1, 17.7%; Q2, 25.4%; Q3, 29.7%; Q4, 32.4%, p for trend < 0.001, Table 3). The adjusted multivariable analysis also showed a stepwise increase in hospital mortality rates across increasing quartiles (adjusted OR for quartile increment: 1.30, 95% CI: 1.17–1.44, p for trend < 0.001). The risk of hospital mortality was significantly higher in the Q4 group than in the Q1 group (adjusted OR: 2.26, 95% CI: 1.61–3.16). ICU mortality, as the secondary outcome, similarly showed a gradual increase as the quartile of the timing of tracheostomy increased (adjusted OR for quartile increment: 1.73, 95% CI: 1.45–2.07, p for trend < 0.001). The Q4 group also had a higher risk for ICU mortality than did the Q1 group (OR: 4.57, 95% CI: 2.59–8.04). Similarly, the restricted cubic spline showed a significant increase in ICU and hospital mortality with the timing of tracheostomy (Fig. 2). The overall hospital mortality rate at each institution varied from 0 to 42.9%, with significant differences between institutions (p = 0.027, Additional file 1).
Subgroup analyses
In total, 498 patients (32.4%) had a GCS score of < 8 on ICU admission (Table 4). Among them, hospital mortality tended to increase with increasing quartiles of the timing of tracheostomy (OR for quartile increment: 1.19, 95% CI: 0.98–1.44, p for trend = 0.081). In the 1040 patients with GCS score ≥ 8, there was a significant trend toward a stepwise increase in hospital mortality as the quartile of the timing of tracheostomy increased (OR for quartile increment: 1.33, 95% CI: 1.18–1.51, p for trend < 0.001). There was no significant interaction between the level of consciousness at ICU admission and hospital mortality (p = 0.310).
Meanwhile, 834 patients (54.2%) presented with respiratory failure on ICU admission. In the same group of patients, hospital mortality was significantly higher in the Q4 group than in the Q1 group (crude OR: 1.93, 95% CI: 1.24–3.01). Hospital mortality increased progressively with the increase in the quartile of the timing of tracheostomy in both patients with and without respiratory failure (p for trend = 0.001 and < 0.001, respectively; p for interaction = 0.218).
Furthermore, 202 patients (13.1%) were admitted to the ICUs after cardiovascular surgery. Hospital mortality tended to increase as the quartile of the timing of tracheostomy increased among these patients (OR for quartile increment: 1.41, 95% CI: 0.98–2.03, p for trend = 0.063). A similar trend toward a stepwise increase in hospital mortality rates across increasing quartiles was shown among those not admitted to the ICUs after cardiovascular surgery (OR for quartile increment: 1.3, 95% CI: 1.17–1.46, p for trend < 0.001; p for interaction = 0.681).
Discussion
Key findings
This multi-institutional observational study evaluated the clinical impact of the timing of tracheostomy on patient outcomes. The results showed that patients with delayed tracheostomy had prolonged mechanical ventilation and longer ICU and hospital stays. Extended mechanical ventilation prior to tracheostomy was associated with a high mortality rate in a time-dependent manner. This trend was the same for different diagnoses and levels of consciousness at the time of ICU admission.
Relationship with previous studies
Tracheostomy is a widely used alternative to translaryngeal endotracheal intubation in critical care settings. It can be performed both at the bedside and in the operating room; thus, its implementation is highly feasible [25].
The consensus established in 1989 recommends that mechanical ventilation by translaryngeal endotracheal intubation be performed in patients expected to be extubated within 10 days, while those expected to be mechanically ventilated for more than 21 days should be placed on tracheostomy [9]. Accordingly, tracheostomy is generally considered within 2 weeks after the commencement of mechanical ventilation in critically ill patients [10, 26, 27]. In the current study, 76.3% (1174 of 1538) of the patients were tracheostomized within the first 2 weeks. However, the international expert task force in 2017 has yet to specify the optimal timing of tracheostomy [28]. Furthermore, recent global guidelines on acute respiratory failure also do not address tracheostomy itself despite its integral role in the management of mechanical ventilation [29, 30].
Tracheostomy has been associated with patient outcomes, such as lengths of ICU and hospital stay, duration of mechanical ventilation, and cost, but large randomized controlled trials have not established an association with mortality [20, 31,32,33]. The largest randomized controlled trial, the TracMan trial conducted in the United Kingdom, compared early (≤ 4 days) and late tracheostomy (≥ 10 days) in 909 patients expected to require mechanical ventilation for at least 7 days [31]. Their results showed similar 30-day mortality between the early and late tracheostomy groups (30.8% vs. 31.5%). The mortality rates for other periods up to 2 years were also similar, substantially influencing the meta-analysis that included the study. Of the 454 patients in the early tracheostomy group in the TracMan trial, 385 (84.6%) underwent tracheostomy within 4 days of admission to the critical care unit. However, of the 454 patients in the late tracheostomy group, 244 (53.7%) did not undergo tracheostomy due to ICU discharge or weaning from mechanical ventilation. Meanwhile, 33 patients (7.3%) underwent tracheostomy before day 10, contrary to the protocol. Thus, the difficulty in predicting the required duration of mechanical ventilation has added an element of impracticality to the conduct of appropriate interventional studies [28].
Several large observational studies have described significant differences in patient outcomes, depending on the timing of tracheostomy. Patel et al. conducted a secondary analysis of a historical cohort of 1175 tracheostomy patients in the United States and found that mortality was 1.4-fold higher in late tracheostomy (≥ 14 days) than in early tracheostomy (< 14 days) [34]. Furthermore, a large multicenter study of 43,916 ICU patients reported that the timing of tracheostomy was significantly correlated with the duration of mechanical ventilation and length of ICU and hospital stay [35]. Our present study is one of the largest observational studies, and our investigation of a graded classification of the timing of tracheostomy showed a stepwise relationship between the timing of tracheostomy and various patient outcomes, including mortality.
Consistent with previous studies, the present study found that early tracheostomy was beneficial in the subgroups of patients requiring mechanical ventilation. Pinheiro et al. conducted a single-center cohort study of Brazilian patients with acute severe brain injury presenting with a GCS score < 8 and found that 28-day mortality was significantly lower in the early (≤ 8 days) tracheostomy group than in the late (> 8 days) tracheostomy group (9% vs. 47%) [36]. In subsequent studies of brain injury, early tracheostomy was found to shorten the duration of treatment (mechanical ventilation and lengths of ICU and hospital stay) and improve neurological outcomes, although the association with mortality remains potentially insignificant [37, 38].
Regarding the relationship between tracheostomy and patient outcomes in acute respiratory failure, a secondary analysis of the LUNG-SAFE study [39], an international cohort study of acute respiratory distress syndrome, was conducted [12]. Although the 60- and 90-day mortality rates were similar, the treatment duration (duration of mechanical ventilation, and lengths of ICU and hospital stay) and 28-day mortality rate (22.0% vs. 10.9%) were significantly lower in the early tracheostomy group (< 7 days) than in the late tracheostomy group (≥ 8 days). Other studies on patients with venovenous extracorporeal membrane oxygenation [40] or coronavirus disease 2019 [41] similarly found that early tracheostomy has a favorable impact on patient outcomes, mainly of shortening the duration of treatment. Our findings showed that early tracheostomy was associated with favorable patient outcomes in these subgroups as well as in the existing literature, and our study with a large population demonstrated that it also had a positive impact on mortality.
The other subgroup to consider is postoperative patients after cardiovascular surgery, who may require prolonged mechanical ventilation due to older age or more comorbidities [42]. However, concerns about surgical site infection and mediastinitis have led to the consideration of tracheostomy for patients who required mechanical ventilation for more than 2 weeks [43] and their optimal timing of tracheostomy is under investigation. Recently, Okada et al. reported an observational study of patients after cardiovascular surgery through a median sternotomy at a university hospital in Japan [44]. Compared with the late tracheostomy group (> 7 days after cardiovascular surgery), the early tracheostomy group (≤ 7 days) showed a shorter duration of ventilatory management and a higher 1-year survival rate (65% vs. 31%) without increased morbidities. This multicenter study using a nationwide database also demonstrated a stepwise association between the timing of tracheostomy and mortality.
Thus, the timing of tracheostomy was similarly associated with hospital mortality in all subgroups examined in this study. However, the main causes of death and detailed complications during mechanical ventilation were not obtained in our data, and the actual contribution of late tracheostomy to increased mortality and prolonged duration of treatment was not determined. Delayed indication of tracheostomy and prolonged translaryngeal endotracheal intubation may require prolonged administration of sedatives and opioids [45]. Therefore, late tracheostomy may lead to slower weaning from mechanical ventilation and a higher incidence of VAP, a critical complication of mechanical ventilation associated with morbidity and mortality [21, 46]. Indeed, a recent systematic review of randomized control studies which compare early (≤ 7 days) vs. late (> 7 days) tracheotomy demonstrated that late tracheostomy resulted in higher VAP incidence and longer duration of mechanical ventilation and ICU stay [17]. These factors may contribute to the increased mortality of late tracheostomy; however, the timing of tracheostomy and hospital mortality varied among the registered institutions, suggesting that differences in treatment strategies and facilities may have an impact on mortality. Further investigation, including detailed treatment and complications during mechanical ventilation in individual patients, is warranted.
Implications of study findings
Using data from the JIPAD, our findings indicate that the timing of tracheostomy is significantly associated with mortality. Compared with late tracheostomy, early tracheostomy after the initiation of mechanical ventilation is associated with a significantly high probability of favorable patient prognosis. Tracheostomy is a common and widely accepted procedure, and early tracheostomy can be used to improve patient prognosis. Furthermore, our findings provide the rationale for further investigation into the optimal timing of tracheostomy.
Strengths and limitations
The strength of this study is that it is a large multicenter cohort study that is inclusive and representative of numerous institutions and thus allows generalizability. However, our study also had some limitations. First, considering the nature of the observational study design, the results of this analysis are prone to misinterpretation and confounding that cannot be measured. Second, the indications and procedures for tracheostomy were based on institutional protocol, with no standardization. Third, the database used in this study contained treatment records during the stay at each registered institution. Therefore, our data did not provide information on treatment, including mechanical ventilation, prior to ICU admission and after the discharge. Fourth, the registry did not include information on the respiratory status or concomitant therapy at the time of tracheostomy. However, detailed clinical information on ICU admission enabled us to adjust our analysis for several major confounders using logistic regression models, and subgroup analyses showed robust associations with clinical outcomes.
Conclusion
Our study showed that the timing of tracheostomy is significantly associated with mortality in a stepwise manner. A similar trend was observed across all levels of consciousness and diagnoses at ICU admission. These findings support the need for early tracheostomy in critically ill patients requiring mechanical ventilation, with an emphasis on patient outcomes.
Availability of data and materials
The data that support the findings of this study are available from the JIPAD project, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Abbreviations
- ANZICS APD:
-
Australian and New Zealand Intensive Care Society Adult Patient Database
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- CI:
-
Confidence interval
- GCS:
-
Glasgow Coma Scale
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile ranges
- JIPAD:
-
Japanese Intensive Care PAtient Database
- OR:
-
Odds ratio
- VAP:
-
Ventilator-associated pneumonia
References
Beduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, et al. Epidemiology of weaning outcome according to a new definition. The WIND Study. Am J Respir Crit Care Med. 2017;195(6):772–83.
Penuelas O, Frutos-Vivar F, Fernandez C, Anzueto A, Epstein SK, Apezteguia C, et al. Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am J Respir Crit Care Med. 2011;184(4):430–7.
Thille AW, Richard JC, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–302.
Diehl JL, El Atrous S, Touchard D, Lemaire F, Brochard L. Changes in the work of breathing induced by tracheotomy in ventilator-dependent patients. Am J Respir Crit Care Med. 1999;159(2):383–8.
Freeman BD, Morris PE. Tracheostomy practice in adults with acute respiratory failure. Crit Care Med. 2012;40(10):2890–6.
Hosokawa K, Nishimura M, Egi M, Vincent JL. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. 2015;19:424.
Terragni P, Faggiano C, Brazzi L. Tracheostomy. In: Vincent J, Moore F, editors. Textbook of critical care. 7th ed. Philadelphia: Elsevier; 2016. p. 179–83.
Nieszkowska A, Combes A, Luyt CE, Ksibi H, Trouillet JL, Gibert C, et al. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005;33(11):2527–33.
Plummer AL, Gracey DR. Consensus conference on artificial airways in patients receiving mechanical ventilation. Chest. 1989;96(1):178–80.
McWhorter AJ. Tracheotomy: timing and techniques. Curr Opin Otolaryngol Head Neck Surg. 2003;11(6):473–9.
Mehta AB, Syeda SN, Bajpayee L, Cooke CR, Walkey AJ, Wiener RS. Trends in tracheostomy for mechanically ventilated patients in the United States, 1993–2012. Am J Respir Crit Care Med. 2015;192(4):446–54.
Abe T, Madotto F, Pham T, Nagata I, Uchida M, Tamiya N, et al. Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Crit Care. 2018;22(1):195.
Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–62.
Cheung NH, Napolitano LM. Tracheostomy: epidemiology, indications, timing, technique, and outcomes. Respir Care. 2014;59(6):895–915.
Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med. 1981;70(1):65–76.
Wang R, Pan C, Wang X, Xu F, Jiang S, Li M. The impact of tracheotomy timing in critically ill patients undergoing mechanical ventilation: a meta-analysis of randomized controlled clinical trials with trial sequential analysis. Heart Lung. 2019;48(1):46–54.
Chorath K, Hoang A, Rajasekaran K, Moreira A. Association of early vs. late tracheostomy placement with pneumonia and ventilator days in critically ill patients: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021.
Deng H, Fang Q, Chen K, Zhang X. Early versus late tracheotomy in ICU patients: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100(3):e24329.
Rumbak MJ, Newton M, Truncale T, Schwartz SW, Adams JW, Hazard PB. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004;32(8):1689–94.
Terragni PP, Antonelli M, Fumagalli R, Faggiano C, Berardino M, Pallavicini FB, et al. Early vs. late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303(15):1483–9.
Mohamed KAE, Mousa AY, ElSawy AS, Saleem AM. Early versus late percutaneous tracheostomy in critically ill adult mechanically ventilated patients. Egypt J Chest Dis Tuberc. 2014;63(2):443–8.
Koch T, Hecker B, Hecker A, Brenck F, Preuss M, Schmelzer T, et al. Early tracheostomy decreases ventilation time but has no impact on mortality of intensive care patients: a randomized study. Langenbecks Arch Surg. 2012;397(6):1001–8.
Barquist ES, Amortegui J, Hallal A, Giannotti G, Whinney R, Alzamel H, et al. Tracheostomy in ventilator dependent trauma patients: a prospective, randomized intention-to-treat study. J Trauma. 2006;60(1):91–7.
Irie H, Okamoto H, Uchino S, Endo H, Uchida M, Kawasaki T, et al. The Japanese Intensive care PAtient Database (JIPAD): a national intensive care unit registry in Japan. J Crit Care. 2020;55:86–94.
Shan L, Hao P, Xu F, Chen YG. Benefits of early tracheotomy: a meta-analysis based on 6 observational studies. Respir Care. 2013;58(11):1856–62.
Veenith T, Ganeshamoorthy S, Standley T, Carter J, Young P. Intensive care unit tracheostomy: a snapshot of UK practice. Int Arch Med. 2008;1(1):21.
Vargas M, Sutherasan Y, Antonelli M, Brunetti I, Corcione A, Laffey JG, et al. Tracheostomy procedures in the intensive care unit: an international survey. Crit Care. 2015;19:291.
Raimondi N, Vial MR, Calleja J, Quintero A, Cortes A, Celis E, et al. Evidence-based guidelines for the use of tracheostomy in critically ill patients. J Crit Care. 2017;38:304–18.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552.
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–63.
Young D, Harrison DA, Cuthbertson BH, Rowan K, TracMan C. Effect of early vs. late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–9.
Diaz-Prieto A, Mateu A, Gorriz M, Ortiga B, Truchero C, Sampietro N, et al. A randomized clinical trial for the timing of tracheotomy in critically ill patients: factors precluding inclusion in a single center study. Crit Care. 2014;18(5):585.
Hyde GA, Savage SA, Zarzaur BL, Hart-Hyde JE, Schaefer CB, Croce MA, et al. Early tracheostomy in trauma patients saves time and money. Injury. 2015;46(1):110–4.
Patel SA, Plowman EK, Halum S, Merati AL, Sardesai MG. Late tracheotomy is associated with higher morbidity and mortality in mechanically ventilated patients. Laryngoscope. 2015;125(9):2134–8.
Freeman BD, Borecki IB, Coopersmith CM, Buchman TG. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Crit Care Med. 2005;33(11):2513–20.
Pinheiro BDV, Tostes RDO, Brum CI, Carvalho EV, Pinto SP, Oliveira JC. Early versus late tracheostomy in patients with acute severe brain injury. J Bras Pneumol. 2010;36(1):84–91.
Jeon YT, Hwang JW, Lim YJ, Lee SY, Woo KI, Park HP. Effect of tracheostomy timing on clinical outcome in neurosurgical patients: early versus late tracheostomy. J Neurosurg Anesthesiol. 2014;26(1):22–6.
Khalili H, Paydar S, Safari R, Arasteh P, Niakan A, Abolhasani FA. Experience with traumatic brain injury: is early tracheostomy associated with better prognosis? World Neurosurg. 2017;103:88–93.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800.
DiChiacchio L, Boulos FM, Brigante F, Raithel M, Shah A, Pasrija C, et al. Early tracheostomy after initiation of venovenous extracorporeal membrane oxygenation is associated with decreased duration of extracorporeal membrane oxygenation support. Perfusion. 2020;35(6):509–14.
Queen Elizabeth Hospital Birmingham C-at. Safety and 30-day outcomes of tracheostomy for COVID-19: a prospective observational cohort study. Br J Anaesth. 2020;125(6):872–9.
Puentes W, Jerath A, Djaiani G, Cabrerizo Sanchez R, Wasowicz M. Early versus late tracheostomy in cardiovascular intensive care patients. Anaesthesiol Intensive Ther. 2016;48(2):89–94.
Walts PA, Murthy SC, Arroliga AC, Yared JP, Rajeswaran J, Rice TW, et al. Tracheostomy after cardiovascular surgery: an assessment of long-term outcome. J Thorac Cardiovasc Surg. 2006;131(4):830–7.
Okada M, Watanuki H, Masato T, Sugiyama K, Futamura Y, Matsuyama K. Impact of tracheostomy timing on outcomes after cardiovascular surgery. J Cardiothorac Vasc Anesth. 2021.
Dochi H, Nojima M, Matsumura M, Cammack I, Furuta Y. Effect of early tracheostomy in mechanically ventilated patients. Laryngoscope Investig Otolaryngol. 2019;4(3):292–9.
Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–21.
Acknowledgements
We would like to thank all the hospitals participating in the JIPAD for their contributions. We also thank our colleagues from Osaka University Center of Medical Data Science and Advanced Clinical Epidemiology Investigator’s Research Project for providing their insights and expert advice to help improve our research.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AT is the guarantor of the manuscript and takes responsibility for its content, including the data, analysis, review, and writing of the manuscript. AU contributed to the study design, data collection, data analysis, and review of the manuscript. TK contributed to the study design, data analysis, review, and writing of the manuscript. SK and TM contributed to the study design, data analysis, and review of the manuscript. RS, TY, NT, NI, and YF contributed substantially to the data collection and review of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Osaka University (approval number: 20026). The requirement for written informed consent was waived as all data were anonymized.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Patients who underwent tracheostomy and overall hospital mortality at each institution.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tanaka, A., Uchiyama, A., Kitamura, T. et al. Association between early tracheostomy and patient outcomes in critically ill patients on mechanical ventilation: a multicenter cohort study. j intensive care 10, 19 (2022). https://doi.org/10.1186/s40560-022-00610-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-022-00610-x