Abstract
Background
Most tropical regions have a climate characterized by marked seasonal rainfall patterns, and these seasonal patterns of rainfall directly affect anuran activity. However, in regions with temperate climates, the main aspect of climate related to anuran activity is the thermal regime. Thus, transitional climate regions represent good opportunities to study the effect of abiotic factors on anuran activity. In this study, we present new data on the activity pattern and calling season of Neotropical anurans in a subtemperate climate. Anuran activity was assessed based on the rate of capture of specimens in pitfall traps and calling surveys. The field study was conducted between May 2010 and April 2011 in wetlands in southernmost Brazil.
Results
An analysis based on directional (circular) statistics showed that general activity in the studied anurans was significantly seasonal. In addition, the general activity pattern of most species was regulated by temperature, not by rainfall, and approached that observed in regions with a temperate climate. However, we did not record a well-defined peak in the number of species displaying calling activity. This parameter did not exhibit any influence of temperature variations or rainfall.
Conclusions
The observed general activity pattern is different from that expected for the majority of anurans inhabiting eastern South America. The present study showed that the general activity patterns of most anurans in the wetland region in southernmost Brazil are regulated by temperature and not rainfall, similar to the pattern of anuran assemblies from temperate climates. However, the nonexistence of a significant relationship between calling activity and any of the environmental variables tested suggests the presence of a different environmental factor (e.g., photoperiod or the length of the hydroperiod of the relevant water bodies) as a trigger for the levels of general calling activity.
Similar content being viewed by others
Background
Many environmental factors act on such anuran activity patterns as reproduction, movements, and foraging. For example, reproductive activity depends on the acoustic quality of the habitat, availability of females, and presence and density of males at the breeding site (Both and Grant 2012). Nevertheless, reproduction is primarily associated with climate-specific abiotic factors (Bertoluci 1998; Oseen and Wassersug 2002; Xavier and Napoli 2011). Although some Neotropical anuran species breed throughout the year, general activity and reproduction typically exhibit remarkable seasonality (Jorgensen 1992; Bertoluci 1998). Other activities performed by anurans, such as dispersion, migration, or foraging, also appear to respond primarily to climatic variables. A wide range of methods are currently available for evaluating anuran activity, and the capture rate is the most frequently used indicator of general activity (Oliveira et al. 2013; Martins et al. 2014). Indeed, capture rate is an indirect measure of any activity performed by animals on the surface of a substrate (e.g., foraging, habitat exploration, dispersal, and migrations), as such activities increase their capture probability.
Associations between peaks of general activity and a particular behavior driven by physiological or metabolic events are poorly studied in the wild, especially in Neotropical anurans. An exception is reproductive activity, which can be evaluated through calling surveys. Environmental factors such as light intensity, relative humidity, and air temperature are relatively well-documented habitat cues that affect the calling activity of anurans (Fukuyama and Kusano 1992; Hatano et al. 2002; Almeida-Gomes et al. 2007). In contrast, photoperiod is a less well-studied climatic factor relative to seasonal changes in anuran behavior (Bradshaw and Holzapfel 2007). There is evidence that amphibians in subtemperate wetlands could be tracking photoperiod (as previously reported for temperature and rainfall) to determine their seasonal variations in activity (Canavero and Arim 2009). In addition, there has recently been significant improvement in the number of ecological studies of amphibians in the Neotropics, though most of these studies are concentrated in forested or tropical open habitats. Less attention has been dedicated to subtemperate open habitats, such as wetlands. Southern Brazilian wetlands possess unique climatic characteristics, such as colder winters, in comparison with other Brazilian regions (Huckembeck et al. 2012; Oliveira et al. 2013). Given the divergent opinions regarding the effects of climatic factors on the activity patterns of anurans (Oseen and Wassersug 2002; Saenz et al. 2006), transitional climatic regions represent an important natural laboratory for testing ecological hypotheses concerning the evolutionary process in ectotherms. In most Brazilian habitats (and other tropical areas), there is a small annual thermal amplitude (Maluf 2000), and precipitation appears to be the main determining factor of anuran activity patterns (Heyer 1973; Boquimpani-Freitas et al. 2007; Oliveira et al. 2013). However, in temperate climate regions, annual temperature variation appears to be the main factor that determines ectothermic activity (Gilbert et al. 1994; Navas and Bevier 2001; Oseen and Wassersug 2002). However, it is important to emphasize that studies performed in transitional regions between tropical and temperate climates have yielded conflicting results (Bernarde and Anjos 1999; Bernarde and Machado 2001; Conte and Rossa-Feres 2006).
The southernmost region of Brazil is in a transition zone between subtropical and temperate climates (Maluf 2000), thus constituting an important study area for understanding the activity patterns (reproductive or not) of Neotropical anurans. The relatively low temperatures, by tropical standards, and unpredictable rainfall can act as abiotic filters to limit activity and the establishment of species in this region. Studies on anuran general activity are important for species conservation purposes (Eterovick et al. 2005) and typically yield information about the species’ ecology and natural history (Santos et al. 2008). In Brazil, especially in Rio Grande do Sul State, the science of anuran general activity has not yet been pursued (Oliveira et al. 2013); thus, there is a lack of knowledge of this subject.
The present study aimed to evaluate the role of abiotic factors in the general activity pattern during the vocalization season in species associated with subtemperate wetlands in southernmost Brazil.
Methods
Study site
The study was conducted between May 2010 and April 2011 in an area formed by a mosaic of temporary wetlands (32° 08′ S and 52° 11′ E) located in Rio Grande, Rio Grande do Sul, southernmost Brazil. The samples were concentrated in an area of approximately 200 ha, which included a portion of the most extensive water bodies with the longest hydroperiods found at the study site. The predominant vegetation consisted of native field grass, which was relatively well preserved (Oliveira et al. 2013). The local climate was classified as humid subtemperate with an annual average temperature of 18.1 °C and an average temperature of 12.7 °C during the coldest month. The climatic seasonality in the study area was characterized by warm and cold seasons, which differed from much of the Brazilian territory climatic seasonality marked by dry and wet seasons (Maluf 2000; Tozetti et al. 2010).
During sampling, the mean minimum temperature of the air during the warm season ranged from 13.08 to 21.25 °C. During the cold season, the mean minimum temperature of the air ranged from 6.45 to 16.43 °C. The rainfall accumulation was 347.7 mm. Because of the small monthly variation in rainfall, the wettest period was defined by plotting the rainfall data (16.7 mm). June, July, October, and December of 2010 and January and April of 2011 were defined as dry months. The humid months were May, August, September, and November of 2010 and February and March of 2011. The climatological data were obtained from Meteorological Station 83,995 of Rio Grande, located 5 km from the study area.
Evaluation of anuran activity
Anuran activity was divided into two types: (1) general activity and (2) calling activity. These forms of activity were evaluated using the following two methods: general activity was evaluated in terms of the capture rate in pitfall traps with drift fences (Greenberg et al. 1994), and calling activity was evaluated through calling surveys. The capture rate in this study was considered an indicator of general activity. General activity corresponded to any activity by the animals on the surface of the substrate (e.g., foraging, habitat exploration, searching for partners, and migration) and increased the probability of capture. Because these methods are common in herpetology, they will be described only briefly in this paper.
We used two pitfall sets, each with two 40-m-long lines, distributed at selected locations around the water bodies (preferential sampling; Krebs 1999), with a distance of 3 km between them. These sites were defined in a manner so that flooding of the buckets was avoided. Each line had four 100-L buckets united by drift fences, for a total of 16 buckets (for more details, see Ximenez et al. 2014). The traps remained open for four consecutive days per month between May 2010 and April 2011. Each captured animal was identified and measured. Whenever possible, the sex of the specimen was determined. The animals were then marked by toe clipping and released 5 m away from one of the ends of the capture trap. The markings used aimed to differentiate between captures and recaptures. Recaptures represented less than 0.001 % of the captures.
A calling survey was performed twice per month between 1800 and 2300 h from May 2010 to April 2011 for a sample total of 120 h. To standardize the sampling effort, all samples were collected in a predefined area of approximately 1 ha located in the vicinity of the pitfall traps, for a total of two sampling sites. An estimate of the number of calling males was made during each sampling night for each site. The data were grouped into one of the following abundance classes (adapted from Bertoluci and Rodrigues 2002): a) 1–4 individuals in calling activity, b) 5–9 individuals, c) 10–20 individuals, or d) >20 individuals. In terms of the duration of the vocalization season, the species were classified as follows: 1) annuals—species that vocalized more than 70 % of the year or were recorded for at least 2 months in each season (warm and cold); 2) warm season—species that vocalized only during the warm months (November to April); and 3) cold season—species that vocalized only during the cold months (May to October). We calculated the frequency of calling occurrence, which corresponded to the number of samples (= months) in each vocalizing species divided by the total number of samples (12) of each species.
Data analysis
For all statistical analyses, we used data on anuran abundance to evaluate general activity and richness indices to evaluate calling activity. To evaluate general activity, only species with ten or more captured individuals were considered, thus making the analysis more robust. Additionally, arboreal species and/or species with “digital discs” were excluded from the analysis of general activity because they were able to escape from the traps (Enge 2001; Oliveira et al. 2013).
A circular statistical analysis (Zar 1999) was applied to evaluate the existence of a seasonal pattern in both general (estimated by the capture rate in traps) and calling activities (estimated by the number of calling species). The months were represented numerically by angles (intervals of 30 °C), and both the number of captures in the traps and number of calling species each month were used as frequencies for each month observed (Prado et al. 2005; Both et al. 2008). The following parameters were considered in the analysis: 1) the median angle (μ), which corresponded to the average period of the year (sampled days) in which most of the species were active and 2) the length of the mean vector (r), which indicated the clustering level of observations around μ. This value varied from 0 (least clustering) to 1 (greatest clustering), and 3) the circular standard deviation (SD). The seasonality tendencies for both activities were assessed using a nonparametric Rayleigh test (z). A significant Rayleigh test indicated the seasonal activity of the anuran assemblage studied. The niche breath of the calling season of each species was calculated using the inverse of the Simpson diversity index (Simpson 1949):
In the equation, p is the proportion of use (calling registers) of temporal category i (month survey), and n is the total number of categories. The values obtained for the diversity index as applied in our study range from 1 to 10, in which 1 corresponds to exclusive calling in only 1 month (specialists) and 10 corresponds to the use of all months in the same proportion (generalists; see Protázio et al. 2014). For a temporal niche analysis, we used the minimum number of calling males previously defined in each abundance class category in the sample (adapted from Bertoluci and Rodrigues 2002). Niche overlap was calculated using Pianka’s overlap index (Pianka 1973). The overlap index between species 1 and 2 (O 12) with temporal calling activities p 1i and p 2i , respectively, was calculated as follows:
In the equation, p represents the proportion of calling males in each month category i, n is the number of categories (months), and j and k represent compared species. The possible overlap scores range from 0 (no overlap) to 1 (complete overlap). We used a null model to investigate the presence of nonrandom patterns in the temporal distribution of calling. This null model was based on the niche overlap module of EcoSim (Gotelli and Entsminger 2003). For these null models, a data matrix with species, months, and the proportion of the number of calling males in each month was created. We performed 1000 randomizations of the data set to simulate random patterns that would be expected in the absence of seasonality in calling behavior (Gotelli and Graves 1996).
Comparisons of the capture rates between the months were analyzed using a Kruskal-Wallis analysis of variance followed by a Kruskal-Wallis post hoc test when necessary (Zar 1999). We applied a Mann–Whitney test to evaluate the variation in general activity between the warm/cold and dry/humid months (i.e., a Mann–Whitney U test: Zar 1999). A multiple regression was performed to detect possible associations between the climatic variables and the general and calling activities of the anurans (Zar 1999). The following climatic variables were considered for all regressions: maximum air temperature, minimum air temperature, relative air humidity, and accumulated rainfall. All statistical tests were performed with a significance level of 0.05 using the program Statistica 8.0.
Results
General activity
The circular statistical analysis revealed that anuran general activity was significantly seasonal (P < 0.001; Table 1). We captured 535 individuals belonging to nine species (Table 2). General activity was greater during warm months (N = 392) than during cold ones (N = 143, U = 160.50, P = 0.008). Multiple regression revealed the existence of a significant and positive relationship between the general activity, relative humidity, maximum and minimum temperatures, and accumulated rainfall (R 2 = 0.22, P = 0.05; Table 2). However, general activity did not vary significantly between dry and humid months (Table 2). When the species were evaluated separately, the relationship between general activity and abiotic variables was only significant for Physalaemus biligonigerus (R 2 = 0.40, P < 0.001), Pseudopaludicola falcipes (R 2 = 0.25, P = 0.02), Leptodactylus gracilis (R 2 = 0.23, P = 0.03), and Elachistocleis bicolor (R 2 = 0.51, P < 0.001; Table 1).
General activity was significantly higher during warm months for E. bicolor (N = 103), L. gracilis (N = 27), and P. biligonigerus (N = 53) than during cold months (N = 1, N = 4, N = 13; U = 77.50, P < 0.001; U = 130.00, P < 0.001; U = 130.00, P < 0.001, respectively; Table 2). Only P. falcipes presented greater general activity during cold months (N = 16) than during warm months (N = 1; U = 190.00, P < 0.01; Table 2). The general activity of Leptodactylus cf. latrans, Physalaemus gracilis, and Pseudis minuta did not vary significantly between warm and cold months (Table 2). There was no significant variation between dry and humid months for any of the species (Table 2). The multiple regression model showed a positive and significant relationship between the maximum air temperature, minimum air temperature, relative air humidity, accumulated rainfall, and activities of P. biligonigerus (R 2 = 0.40, P = 0.0004), P. falcipes (R 2 = 0.25, P = 0.02), L. gracilis (R 2 = 0.23, P = 0.03), and E. bicolor (R 2 = 0.51, P = 0.000007). The activity of the other species did not respond significantly to any of the climatic variables (Table 2).
Calling activity
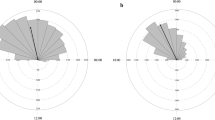
The circular statistical analysis revealed that anuran calling activity was not significantly seasonal (Table 1). Calling records were obtained for 13 species distributed in six families (Fig. 1). Two periods with a higher number of calling species were detected: between August and November and between January and March. A peak in the number of calling species occurred during October (N = 7 species) and September, February, and March (N = 6) (Fig. 1).
Anuran calling activity in southernmost Brazil. Calling activity sampling between May 2010 and April 2011 in subtemperate wetlands in southernmost Brazil. The abundance classes of individuals’ calling activities:  1–4 individuals;
1–4 individuals;  5–9;
5–9;  10–20; and
10–20; and  > 20. FO% occurrence frequency, CD cold and dry month, CH cold and humid month, WD warm and dry month, WH warm and humid month
> 20. FO% occurrence frequency, CD cold and dry month, CH cold and humid month, WD warm and dry month, WH warm and humid month
Regarding calling activity, five species were classified as “annual” (Dendropsophus sanborni, Hypsiboas pulchellus, Physalaemus sp., P. minuta, and Scinax squalirostris), six as “warm season” (Dendropsophus minutus, E. bicolor, L. gracilis, Odontophrynus maisuma, P. falcipes, and Rhinella dorbignyi) and two as “cold season” (L. cf. latrans and Scinax granulatus). The species with a higher frequency of calling were H. pulchellus (83.33 %) and S. squalirostris (75 %), whereas O. maisuma and L. cf. latrans only called during 1 month (Fig. 1). The number of species exhibiting calling activity did not show a significant relationship to any of the environmental variables (R 2 = 0.22; P = 0.53; sample number = 12). Because of the difficulty in differentiating the vocalizations of P. biligonigerus and P. gracilis, these species were not differentiated and were recorded as Physalaemus sp.
For all species, a higher calling activity occurred during warm months, which coincided with a peak in the capture rate in the traps. However, when examining the species separately, the calling and general activity periods only overlapped for E. bicolor, L. gracilis, and Physalaemus sp. Males of L. cf. latrans had a calling activity peak (October) that occurred before the general activity peak (November to January). An identical result occurred for P. minuta, which had calling peaks in September, October, March, and April, and general activity peaks in November, December, June, and July. However, P. falcipes males concentrated their vocalizations during warm months (November and January) and presented the greatest general activity during cold months (August and September). It should be emphasized that this comparison between the calling and general activity periods was not possible for R. dorbignyi and O. maisuma because of the low number of captures (<10), similar to the hylids because of their ability to climb and escape from the traps (with the exception of P. minuta).
Greater values of temporal niche breath were observed for H. pulchellus (B = 6.72), followed by D. sanborni (B = 4.81), and Physalaemus sp. (B = 4.13) (Table 3). Lower values were observed for E. bicolor, L. cf. latrans, and O. maisuma (B = 1). A niche overlap analysis revealed that the patterns varied from complete overlap to no overlap between species (Table 3). The mean overlap was 0.227 ± 0.267, and only 10.4 % of all species pairs showed a temporal overlap greater than 0.6. Greater overlap values were observed for R. dorbignyi and P. falcipes (0.9845988), Physalaemus sp. and S. fuscovarius (0.731399), and S. squalirostris and H. pulchellus (0.9035631). The null model showed that the observed temporal niche overlap index (0.227) did not vary significantly more than expected by chance (0.218) (P > 0.05). This suggests that the assemblage calling activity is randomly distributed.
Discussion
Our data suggest that the general activity of the studied anuran populations exhibits a seasonal pattern predominantly related to air temperature but not rainfall. This pattern was different from that expected for the majority of eastern South America (Bertoluci 1998; Vasconcelos et al. 2010). The relatively harsh winter in southernmost Brazil possibly imposes limitations on anuran activity due to a reduction in metabolic rate (Lillywhite 1974). During the coldest months, there would be a decreased in mobility and, consequently, in the probability of capture in traps (Toledo and Haddad 2005; Saenz et al. 2006).
The potential for cold as a factor limiting general activity was reinforced by the fact that captures during cold months were higher on days with higher minimum temperatures. In addition, species are expected to exhibit different ranges of optimal operative body temperature (Huey 1991). Although this hypothesis is speculative, it could explain the peak of activity during warm months in E. bicolor, L. gracilis, and P. biligonigerus, whereas P. falcipes presented a higher general activity during colder months. Another aspect of life history that affects general activity is the differences in the level of dependency on aquatic habitats. After an intense rain, the habitat could remain flooded for many weeks, promoting a long period of intense activity in some species and with no relationship to the precipitation rate. This effect appears to occur in L. cf. latrans, P. gracilis, and P. minuta, which exhibited no seasonal variation in activity pattern.
Although the peaks in calling activities correspond to warmer and moister months (Navas and Bevier 2001), many studies noted the importance of evaluating the effects of environmental variables independently for each species (Steelman et al. 2010). This pattern is reinforced by our data, which showed particular behaviors in certain species, such as P. falcipes, whose general activity was higher during colder months. However, this pattern may have been a secondary response because some of the coldest months had isolated rainfall peaks, which may have increased the species’ reproduction (Carvalho-e-Silva et al. 2000; Loebmann and Haddad 2010; Barreto et al. 2012).
Despite the occurrence of isolated peaks of rainfall during the coldest months, the region displayed a relatively homogeneous distribution pattern of rain throughout the year (Santos et al. 2008; Lingnau 2009; Oliveira et al. 2013). This pattern could further reinforce the effects of other factors, affording humidity only a secondary role as a moderator of anuran activity in southernmost Brazil. This scenario reduces the accuracy of predictions on activity patterns based on air temperature and humidity (Steelman et al. 2010). There was no significant relationship between calling activity and any of the environmental variables tested. We did not record a well-defined peak in the number of species showing calling activity. Such a peak has been demonstrated for other anuran communities in other locations in Brazil (Bertoluci 1998; Eterovick and Sazima 2000) and Uruguay (Canavero et al. 2008), including environments climatically similar to our study area (Bernarde and Anjos 1999; Conte and Machado 2005; Santos et al. 2008). Note that different species could respond differently to abiotic variables that influence reproductive activity (Hartel et al. 2007; Wells 2007). In addition to climatic variables, these triggers include other environmental parameters, such as photoperiod (Both et al. 2008; Canavero et al. 2009), which can make the detection of a general pattern of answers to the climatic variables difficult. In an attempt to predict mathematically the activity patterns of an anuran community in subtemperate wetlands in Uruguay, Canavero et al. (2008) observed that the annual pattern of calling activity is determined by a response to a set of seasonal variables or a variable that synthesizes the entire seasonal variation, such as photoperiod. This principle reinforces the fact that not all species respond to specific variables such as temperature or rainfall. Photoperiod appears to be a good predictor of activity in H. pulchellus, P. minuta, and P. gracilis. As also observed by Canavero et al. (2008), these species also presented a broad period of activity almost throughout the year in the present study.
The relatively low values of niche breath suggest that many species presents a short season of calling activity. Consequently, species would have a smaller overlap during reproductive periods, as occurred, e.g., between L. latrans, D. minutus, E. bicolor, and O. maiusma and between O. maiusma, E. bicolor, and L. gracilis. Finally, this behavior would reduce the overlap between species during the breeding seasons, thus reducing the possibility of competition for physical and acoustic space and diluting the effect of predators on nests (Kopp et al. 2010; Sinsch et al. 2012).
The majority of the species showed calling activity that was restricted to the warm season (46.15 %) or extended throughout the entire year (38.46 %). Even species that presented calling activity restricted to the cold season (L. cf. latrans and S. granulatus) were active during the transitional months between the cold and warm seasons. This result reinforces the finding that temperature was the predominant climatic factor for determining anuran calling activity. Note, however, that the calling season of S. granulatus may have been underestimated because of the low abundance of individuals in the studied area (personal observations).
The species with the longest calling periods were H. pulchellus and S. squalirostris. This pattern was most likely enhanced by their relatively high tolerance to low temperatures (Both et al. 2008). Species with higher tolerance to low temperatures may have greater reproductive success (Martins 2009), which could explain why those species were two of the most abundant in the study region. The prolonged calling pattern of H. pulchellus has also been recorded in other populations in southern South America (Achaval and Olmos 1997; Trindade et al. 2010) and in Uruguay (Canavero et al. 2008). However, the calling period of S. squalirostris in other subtemperate regions was concentrated during the warmer months between September and April (Achaval and Olmos 1997; Kwet and Di-Bernardo 1999). This result suggests a certain plasticity of the species regarding seasonality and reproductive activity. Such plasticity also appears to occur in P. minuta, whose reproductive activity was previously identified as brief (Santos et al. 2008) or long (Langone 1994; Melchiors et al. 2004). The low values of niche overlap reinforce the potential of the entire community to keep calling through all the year. Another important aspect of our work was that our results showed a temporal disjunction between the general and calling activities for some species (L. cf. latrans, P. minuta, and P. falcipes). The limitation of an ecological interpretation of activity data often occurs because of the low availability of basic information on the biology of the observed species, particularly regarding reproductive biology.
Conclusions
The present study is one of the first to describe anuran general activity in southern Brazil. The study showed that the general activity patterns of most anurans in the studied wetland habitats appear to be regulated by temperature and not rainfall, similar to the pattern of anuran assemblages in temperate climates (Oseen and Wassersug 2002; Saenz et al. 2006). The nonexistence of a significant relationship between calling activity and any of the environmental variables tested suggested the presence of a different environmental factor (e.g., photoperiod or the length of the hydroperiod of water bodies) or some untested biotic elements as a trigger for the levels of general calling activity. Nevertheless, the observed general activity pattern is different from that expected for most of eastern South America.
References
Achaval F, Olmos A (1997) Anfíbios y reptiles del Uruguay. Barreiro y Ramos S. A, Montevideo
Almeida-Gomes M, Van Sluys M, Rocha CFD (2007) Calling activity of Crossodactylus gaudichaudii (Anura: Hylodidae) in an Atlantic Rainforest area at Ilha Grande, Rio de Janeiro, Brasil. Belg J Zool 137:203–207
Barreto GS, Tinoco MS, Couto-Ferreira D, Browne-Ribeiro HC (2012) Distribuição de Pseudopaludicola aff. falcipes (Anura; Leiuperidae) na restinga do litoral norte da Bahia, Brasil. Revista Latino-Americana de Conservação 2(2)-3(1):27–36
Bernarde PA, Anjos L (1999) Distribuição espacial e temporal da anurofauna no Parque Estadual Mata dos Godoy, Londrina, Paraná, Brasil (Amphibia: Anura). Comunicações do Museu de Ciência e Tecnologia 12:127–140
Bernarde PS, Machado RA (2001) Riqueza de espécies, ambientes de reprodução e temporada de vocalização da anurofauna em Três Barras do Paraná, Brasil (Amphibia: Anura). Cuadernos de Herpetologia 14(2):93–104
Bertoluci J (1998) Annual patterns of breeding activity in Atlantic Rainforest anurans. J Herpetol 32(4):607–611
Bertoluci J, Rodrigues MT (2002) Seasonal patterns of breeding activity of Atlantic Rainforest anurans at Boracéia, Southeastern Brazil. Amphibia-Reptilia 23:161–167
Boquimpani-Freitas L, Marra RV, Van Sluys M, Rocha CFD (2007) Temporal niche of acoustic activity in anurans: interspecific and seasonal variation in a neotropical assemblage from south-eastern Brazil. Amphibia-Reptilia 28:269–276
Both C, Grant T (2012) Biological invasions and the acoustic niche: the effect of bullfrog calls on the acoustic signals of white-banded tree frogs. Biol Lett 8:714–716
Both C, Kaefer IL, Santos TG, Zechin STZ (2008) An austral anuran assemblage in the Neotropics: seasonal occurrence correlated with photoperiod. J Nat Hist 42(3):205–222
Canavero A, Arim M (2009) Clues supporting photoperiod as the main determinant of seasonal variation in amphibian activity. J Nat Hist 43(48):2975–2984
Canavero A, Arim M, Naya DE, Camargo A, Rosa I, Maneyro R (2008) Calling activity patterns in anuran assemblage: the role of seasonal trends and weather determinants. Northwest J Zool 4(1):29–41
Carvalho-e-Silva SP, Izecksohn E, Carvalho-e-Silva AMPT (2000) Diversidade e ecologia de anfíbios em restingas do sudeste brasileiro. In: Esteves FA, Lacerda LD (eds) Ecologia de Restingas e Lagoas Costeiras. NUPEM/UFRJ, Rio de Janeiro
Conte CE, Machado RA (2005) Riqueza de espécies e distribuição espacial e temporal em comunidade de anuros (Amphibia, Anura) em uma localidade de Tijucas do Sul, Paraná, Brasil. Rev Bras Zoo 22(4):940–948
Conte CE, Rossa-Feres DC (2006) Diversidade e ocorrência temporal da anurofauna (Amphibia, Anura) em São José dos Pinhais, Paraná, Brasil. Rev Bras Zoo 23(1):162–175
Enge KM (2001) The pitfalls of pitfall traps. J Herpetol 35:467–478
Eterovick PC, Sazima I (2000) Structure of an anuran community in a montane meadow in southeastern Brazil: effects of seasonality, habitat, and predation. Amphibia-Reptilia 21:439–461
Eterovick PC, Carnaval ACOQ, Borges-Nojosa DM, Silvano DL, Segalla MV, Sazima I (2005) Amphibian declines in Brazil: an overview. Biotropica 37(2):166–179
Fukuyama K, Kusano T (1992) Factors affecting breeding activity in a stream-breeding frog, Buergeria buergeri. J Herpetol 26:88–91
Gilbert M, Leclair R Jr, Fortin R (1994) Reproduction of the northern leopard frog (Rana pipiens) in floodplain habitat in the Richelieu River, P. Quebec, Canada. J Herpetol 28:465–470
Gotelli NJ, Graves GR (1996) Null models in ecology. Smithsonian Institution Press, Washington, DC
Greenberg CH, Neary DG, Harris LD (1994) A comparison of herpetofaunal sampling effectiveness of pitfall single-ended and double-ended funnel traps used with drift fences. J Herpetol 28:319–324
Hartel T, Sas I, Pernetta AP, Geltsh IC (2007) The reproductive dynamics of temperate amphibians: a review. North-West J Zool 3:127–145
Hatano FH, Rocha CFD, Van Sluys M (2002) Environmental factors affecting calling activity of a tropical diurnal frog (Hylodes phyllodes: Leptodactylidae). J Herpetol 36:314–318
Heyer WR (1973) Ecological interactions of frog larvae at a seasonal tropical location in Thailand. J Herpetol 7:337–361
Huckembeck S, Claudino M, Correa F, Bastos RF, Loebmann D, Tozetti AM, Garcia AM (2012) Pattern activity and microhabitat use of Pseudis minuta Günther, 1858 (Anura, Hylidae) in a subtropical Biosphere Reserve in Brazil. Braz J Biol 72:331–336
Huey RB (1991) Physiological consequences of habitat selection. Am Nat 137:91–115
Jorgensen CB (1992) Growth and reproduction. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibians. The University of Chicago Press, Chicago
Kopp K, Signorelli L, Bastos RP (2010) Distribuição temporal e diversidade de modos reprodutivos de anfíbios anuros no Parque Nacional das Emas e entorno, estado de Goiás, Brasil. Iheringia, Série Zoológica 100(3):192–200
Krebs CJ (1999) Ecological methodology. Addison Wesley Educational Publishers, Menlo Park, California
Kwet A, Di-Bernardo M (1999) Pró-Mata: Anfíbios-Amphibien-Amphibians. EDIPUCRS, Porto Alegre
Langone JA (1994) Ranas y Sapos del Uruguay (Reconocimiento y Aspectos Biológicos). Museo Dámaso Antonio Larranaga, Serie de Divulgación 5:1–123
Lillywhite HB (1974) How frogs regulate their body temperature. Environ Southwest 465:3–6
Lingnau R (2009) Distribuição temporal, atividade reprodutiva e vocalizações em uma assembleia de anfíbios anuros de uma floresta ombrófila mista em Santa Catarina, sul do Brasil. Pontifícia Universidade Católica do Rio Grande do Sul, Dissertation
Loebmann D, Haddad CFB (2010) Amphibians and reptiles from a highly diverse area of the Caatinga domain: composition and conservation implications. Biota Neotrop 10(3):227–256
Maluf JRT (2000) Nova classificação climática do Estado do Rio Grande do Sul. Revista Brasileira de Agrometeorologia 8(1):141–150
Martins LA (2009) Comportamento reprodutivo e social de Scinax squalirostris (Lutz, 1925) (Anura, Hylidae) sob influência de fatores ambientais. Pontifícia Universidade Católica do Rio Grande do Sul, Dissertation
Martins LS, Verrastro L, Tozetti AM (2014) The influences of habitat on body temperature control in a southern population of Liolaemus occipitalis (Boulenger, 1885) in Brazil. South American Journal of Herpetology 9(1):9–13
Melchiors J, Di-Bernardo M, Pontes GMF, Oliveira RB, Solé M, Kwet A (2004) Reprodução de Pseudis minuta (Anura, Hylidae) no sul do Brasil. Phyllomedusa 3(1):61–68
Navas CA, Bevier CR (2001) Thermal dependency of calling performance in the eurythermic frog Colostethus subpunctatus. Herpetologica 57:384–395
Oliveira MCLM, Santos MB, Loebmann D, Tozetti AM (2013) Diversity and associations between coastal habitats and anurans in southernmost Brazil. Anais da An Acad Bras Cienc 85:575–582
Oseen KL, Wassersug RJ (2002) Environmental factors influencing calling in sympatric anurans. Oecologia 133:616–625
Pianka ER (1973) The structure of lizard communities. Annu Rev Ecol Evol Syst 4:53–74
Prado CPA, Uetanabaro M, Haddad CFB (2005) Breeding activity patterns, reproductive modes, and habitat use by anurans (Amphibia) in a seasonal environment in the Pantanal, Brazil. Amphibia-Reptilia 26(2):211–221
Protázio AS, Albuquerquea RL, Falkenberga LM, Mesquita DO (2014) Acoustic ecology of an anuran assemblage in the arid Caatinga of northeastern Brazil. J Nat Hist 48:1–20
Saenz D, Fitzgerald LA, Kristen AB, Richard NC (2006) Abiotic correlates of anuran calling phenology: the importance of rain, temperature, and season. Herpetol Monogr 20(1):64–82
Santos TG, Kopp K, Spies MR, Trevisan R, Cechin SZ (2008) Distribuição temporal e espacial de anuros em área de Pampa, Santa Maria, RS. Iheringia, Série Zoologia 98(2):244–253
Sinsch U, Lümkemann K, Rosar K, Schwarz C, Dehling JK (2012) Acoustic niche partitioning in an anuran community inhabiting an Afromontane wetland (Butare, Rwanda). Afr Zool 47(1):60–73
Steelman CK, Dorcas ME (2010) Anuran calling survey optimization: developing and testing predictive models of anuran calling activity. J Herp 44(1):61–68
Toledo LF, Haddad CFB (2005) Acoustic repertoire and calling behavior of Scinax fuscomarginatus (Anura, Hylidae). J Herp 39(3):455–464
Tozetti AM, Pontes GMF, Borges-Martins M, Oliveira RB (2010) Temperature preferences of Xenodon dorbignyi: field and experimental observations. Herpetol J 20:277–280
Trindade AO, Oliveira SV, Cappellari LH (2010) Anfíbios anuros de uma área da serra do sudeste, Rio Grande do Sul (Caçapava do Sul). Biodiversidade Pampeana 8(1):19–24
Vasconcelos TS, Santos TG, Haddad CFB, Rossa-Feres DC (2010) Climatic variables and altitude as predictors of anuran species richnesss and number of reproductive modes in Brazil. J Trop Ecol 26(4):423–432
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Xavier AL, Napoli MF (2011) Contribution of environmental variables to anuran community structure in the Caatinga Domain of Brazil. Phyllomedusa 10(1):45–64
Ximenez SS, Oliveira MCLM, Santos MB, Tozetti AM (2014) The influence of habitat configuration on anuran species composition in subtemperate wetlands of southernmost Brazil. South American Journal of Herpetology 9(3):235–243
Zar JH (1999) Biostatistical analysis. Prentice-Hall, New Jersey
Acknowledgements
We thank the Rio Grande do Sul Research Foundation (FAPERGS) and the National Council for Scientific and Technological Development (CNPq) for the financial support and the Coordination for the Improvement of Higher Education Personnel (CAPES) for the Master’s Degree fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SSX was responsible for the field samples, performed most of the statistical analyses, and drafted the first version of the manuscript. AMT conceived the study, determined the sampling design, and reviewed the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ximenez, S.d.S., Tozetti, A.M. Seasonality in anuran activity and calling season in a Brazilian subtemperate wetland. Zool. Stud. 54, 47 (2015). https://doi.org/10.1186/s40555-015-0125-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40555-015-0125-8