Abstract
Seasonal fluctuations influence foodborne illness transmission and affect patterns of microbial contamination of food. Previous investigations on the seasonality of Salmonella enterica prevalence in dairy products in Ethiopia have been minimal. However, such data are needed to inform strategic development of effective interventions to improve food safety, as seasonal differences may affect intervention strategies. This study was conducted to identify differences in the prevalence of Salmonella in milk and cheese samples between wet and dry seasons. A longitudinal study design was utilized with a random sampling occurring during both dry and wet seasons. A total of 448 milk and cottage cheese samples were collected from Oromia, Sidama, and Amhara regions. Samples were tested for Salmonella using the ISO 6579-1: 2008 method, followed by PCR confirmation. A chi-square test was conducted to assess the significance of differences in the prevalence of Salmonella in the samples between the two seasons. Results from this study showed a higher prevalence of Salmonella in all sample types during the dry season (P < 0.05). Moreover, when comparing raw milk, pasteurized milk, and cottage cheese samples, a significant difference was observed in Salmonella prevalence from raw milk samples (27.08%) collected in the Oromia region. Additionally, data showed a significantly higher prevalence of Salmonella in samples collected from raw milk producers (29.17%) during the wet season (P < 0.05). This study indicates that in order to enhance the safety of dairy products in Ethiopia, comprehensive, long-term awareness building on hygienic milk production and handling that consider seasonal influence is warranted.
Similar content being viewed by others
Introduction
Foodborne illnesses continue to have significant adverse effects on global public health, the economy, and society (WHO, 2021). More than 600 million cases and 420, 000 deaths occur each year, with the proportion of this burden being predominately higher in low- and middle-income countries (Havelaar et al. 2015). In Ethiopia alone, around 60% of disease what type of diseases and in which population emerged from production and processing of perishable foods like dairy products (Wabto et al., 2017).
Milk and its products can be contaminated by pathogenic bacteria before it leaves the farm, mainly as a result of environmental contamination through air, water, barn, feed, and pasture during production and processing, (Lucey 2015; Tegegne and Tesfaye 2017). Thus, this will contribute to the acquisition of these bacteria by humans through consumption (Chen et al., 2018). In addition, direct passage from the blood (of cow) into milk (systemic infection), mastitis (udder infection), and fecal contamination (external contamination of milk from the environment during or after milking) are the main routes for the introduction of pathogenic microbes to milk (Lucey 2015; Belina et al., 2021). Most pathogenic bacteria are effectively controlled by milk pasteurization. However, inadequate pasteurization of milk results in human exposure to pathogenic bacteria in the pasteurized milk (Cancion-Padilla et al., 2017).
Salmonella eneterica is a foodborne pathogen with over 2500 serotypes, with more than 1540 belonging to the Salmonella enterica subspecies enterica; which accounts for the majority of Salmonella infections in humans (Eng et al. 2015). Salmonella is the leading cause of bacterial foodborne illness in the world (Keba et al. 2020; Pal et al. 2020). Foods contaminated with Salmonella, notably raw milk and its products, are responsible for an estimated 2,458,000 cases and 4,100 annual deaths in Africa (Majowicz et al., 2010; Pal et al. 2020). Furthermore, Salmonella has been linked to bacteremia in immunocompromised people, infants, and new babies in Africa, and dairy animals have been identified as primary sources of salmonellosis in humans (Feasey et al. 2012; Nyenje and Ndip., 2013).
Many infectious diseases exhibit seasonality, meaning that the prevalence of infections can vary due to seasonal weather patterns (Naumova et al. 2007). This is likely due to seasonal variation in temperature and precipitation, which result in seasonal prevalence peaks that are interspersed with low levels of infections (Green et al. 2006; Lal et al. 2012). Statistics show a correlation between short-term temperature variations and foodborne illnesses or infections (Ebi 2011; Semenza et al. 2012). Long-term climatic changes, such as elevated average air temperatures and increased precipitation frequency or intensity, have been shown to have an impact on short-term changes (Smith et al. 2019). Within certain bounds, the survival rates of most enteric pathogens in the environment are positively associated with ambient temperature (Semenza et al. 2012). Due to environmental influences such as seasonal weather and climate fluctuations, the patterns of pathogen prevalence consequently change (Semenza et al. 2012). Notably, Salmonella and Campylobacter infections have been shown to peak in the summer with demonstrated seasonal tendencies (Kovats et al. 2004; Rivero et al. 2012).
Environmental change, most notably season, weather, and climate, can also influence foodborne illness transmission indirectly by altering food consumption behaviors, livestock susceptibility to pathogens, and vector transmission due to range expansion, increased activity, and reproduction rates (Séguin et al., 2008; Smith et al. 2019). When livestock are stressed by temperature changes, pathogenic enteric bacteria, such as Salmonella, can colonize animals more readily (Keen et al. 2003; Pangloli et al. 2008). If processing hygiene standards are breached, this can raise the risk of fecal contamination of animal-based foods, such as raw milk and meat (Williams et al. 2015).
The relationship between temperature and precipitation and the frequency of salmonellosis cases is supported in multiple scientific publications (Rose et al. 2001; Zhang et al. 2010; Putturu et al. 2015; Stephen and Barnett 2016; Park et al. 2018; Judd et al. 2019; Ung et al. 2019). Recently, Bedassa et al. (2023) reported a 13.8% prevalence of the organism in milk and cheese in samples collected from the Oromia, Sidama, and Amhara regions of the country in the dry season. Studies on seasonal variations and regional distributions of pathogenic organisms are crucial for developing strategic interventions and anticipating outbreaks.
This research was planned with the intention of assessing whether seasonal variation affected the amount of Salmonella enterica found in milk and cottage cheese samples that were taken from study locations in the Amhara, Sidma, and Oromia regions of Ethiopia throughout the dry and wet seasons.
Materials and methods
Study area and study design
To determine the prevalence of Salmonella raw milk, pasteurized milk, and cottage cheese samples, a longitudinal study design was utilized in three important milk sheds in Ethiopia i.e. Deber-Zeit (Oromia), Hawassa (Sidama), and Bahir Dar (Amhara). The rainy (wet) and warm (dry) seasons of the nation’s climate profile were taken into consideration when designing a sampling plan to capture the seasonal differences. The selection criteria were based on the previously published data on prevalence of Salmonella in dry season (Bedassa et al.,2023), which showed that samples taken from these sites had a higher prevalence of Salmonella than those taken from the other study sites, most notably Hawassa City. Second, the selected study locations had a complete set of value chain actors, including producers, collectors, processors, and retailers. Due to the region’s recent reorganization, the SNNP region that was referenced in our previous report (Bedassa et al. 2023) was now the Sidama region.
Sample collection
In the wet season (June, July, and August), a total of 228 cow milk and cottage cheese samples (92 raw milk, 92 pasteurized milks, and 44 cottage cheese) were collected from Debre Zeit (n = 120), Hawassa (n = 60), and Bahir Dar (n = 48). Previously published data from the dry season was used for the seasonal comparison (Bedassa et al. 2023). The dry season samples were collected from the same producers, collectors, processors, and retailers as during wet season (January, February, March, and April). Dairy food samples were collected aseptically using sterile containers and transported by a portable refrigerator, which was maintained at 4 ℃, to the Holeta Microbial Biotechnology Laboratory of the National Agricultural Biotechnology Research Center (NABRC) for microbial analysis. Microbiological analyses were conducted within 12 h from sample collection.
Detection and conformation of S. Enterica
Figure 1 illustrates Salmonella enrichment and isolation according to ISO 6579-1: (ISO 2017). The full detailed procedure of Salmonella enrichment and isolation were outlined in our prior report (Bedassa et al. 2023). Buffered Peptone Water (BPW) (Oxoid, CM 0509) was used for the pre-enrichment of Salmonella, and it was incubated for 18 h at 35 °C. Salmonella was selectively enriched using sterile Muller Kaufmann Tetrathionate (MKTTn) broth (HiMedia) and Rappaport Vassiliadis (RV) broth (HiMedia), respectively. An inoculated RV broth was incubated at 41 °C for 24 h, while an inoculated MKTTn broth was incubated at 37 °C. Selective enriched presumed Salmonella were plated on Xylose Lysine Deoxycholate (XLD) agar and Hektoen Enteric (HE) agar (HiMedia). For 24 h, inoculated plates were incubated aerobically at 37 °C. Presumptive isolates were further subcultured onto Brain Heat Infusion (BHI) agar and incubated at 35 °C for 24 h for molecular conformation. Salmonella isolates were confirmed utilizing PCR targeting the invA gene (Galán and Curtiss III, 1991). The full and detailed procedure of Salmonella PCR conformation was outlined in our prior report (Bedassa et al. 2023). Each electrophoresis run included a 100 bp DNA ladder, as well as positive (Salmonella enterica ATTCC 35,664) and negative controls (nuclease free water).
Statistical analysis
Descriptive statistics of the prevalences were performed using Microsoft Excel, and a chi-square test was performed using SPSS version 20.0 software to evaluate the statistical significance of variations in Salmonella enterica prevalence among sample types (raw and pasteurized milk, cottage cheese), seasons, and across the diary value chain. P < 0.05 was used to determine statistical significance for differences.
Results
Comparison of overall prevalence of S. Enterica between dry and wet seasons across the sample type
The overall prevalence of S.entericafor all sample types (raw milk, pasteurized milk, and cottage cheese), collected from all three study sites, during the wet season was 13.2%. Raw milk samples collected during the wet season had a significantly higher prevalence of S.entericawhen compared to pasteurized milk and cottage cheese samples (χ2 = 11.41, P = 0.003). Likewise, during dry season, S.enterica was significantly recovered more from raw milk (χ2 = 34.53, P = 0.000)… Among the study regions, the samples collected in Oromia during the wet season had a significantly higher S.enterica prevalence as compared to Sidama and Amhara regions (Table 1). The variation in S.entericaprevalence is attributed to a higher detection rate of S.enterica from raw milk (χ2 = 18.72, P = 0.000) when compared to pasteurized milk and cottage cheese in Oromia region. This was the case also in the dry season (χ2 = 45.1, P = 0.000), 92.85% (n = 26) of tested raw milk samples tested positive for S.enterica. In Sidama, during wet season the prevalence of S.entericawas higher in pasteurized milk 3% (n = 6), but not significantly higher compared to raw milk and cottage cheese as shown in (Table 1). Even though the pattern detection of S.enterica was higher in raw milk 29.2% (n = 7) and pasteurized milk 25% (n = 6) in the dry season compared to cottage cheese 8.3% (n = 1), there was no significant variation (χ2 = 2.00, P = 0.367. The pattern of S.enterica prevalence in the Amhara region revealed that it was highly detected in raw milk 20% (n = 4) than in pasteurized milk 15% (n = 3) during the wet season but no significant variation has observed (Table 1). Similarly, during the dry season, it was recovered primarily from raw milk 25% (n = 5) and pasteurized milk 20% (n = 4), and no significant variation has been observed (χ2 = 1.77 P = 0.41).In terms of seasonal variation in the prevalence of S.enterica, we found significant differences in prevalence between wet and dry seasons only for raw milk samples collected from Oromia (χ2 = 7.3; P = 0.007; Fig. (2). In contrast, the differences were insignificant in Amhara and Sidama. Furthermore, no significant difference was found in cumulative S.enterica prevalence in pasteurized milk in three study sites (Fig. 3).
Comparison of overall prevalence of S.enterica between dry and wet seasons across the value chain
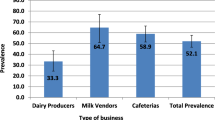
Significant differences in S.enterica prevalence in the wet season at different levels of the dairy value chains were found only in Oromia (χ2 = 19.31, P = 0.02) as shown in Table (2). The prevalence of S.enterica was similar at the producer 29% (n = 7) and collector 25% (n = 6) levels of the dairy value chain of raw milk. This finding is consistent with what was observed in the dry season, where the prevalence of S.enterica was found to be significantly varied (χ2 = 47.3, P = 0.00) among dairy value chains due to higher detection of S.enterica in producer 66,7% (n = 16) and collector 41.7% (n = 10) value chains of raw milk.
In the Sidama region, S.enterica predominantly recovered from the processer 25% (n = 3) and retailer 25% (n = 3) of the value chain of pasteurized milk during the wet season but doesn’t significantly higher as mentioned in Table (2). Likewise, no significant variation has been observed in the dry season (χ2 = 8.3, P = 0.14) in S.enterica prevalence at different levels of the dairy value chain. Even though there was no significant variation in S.enterica prevalence in the dry season it was typically recovered from the collector 41.67% (n = 5) and processer 41.67% (n = 5) value chain.
The prevalence of S.enterica in the dairy value chain that was located in the Amhara region had no significant variation as Table (2) demonstrated. Nevertheless, producer 20% (n = 2) and collector 20% (n = 2) value chains of the raw milk and processer 20% (n = 2) value chain of pasteurized milk had relatively higher S.enterica detection during wet season. The detection of S.entericaduring dry season in Amhara region was highly detected in the producer 40% ( n = 4) value chain of raw milk and retailer 30% (n = 3) value chain of pasteurized even though no significant variation has been observed (χ2 = 2.7, P = 0.74).
Seasonality of S.enterica prevalence among dairy value chain only observed in the Oromia region, in which the detection of S.enterica was significantly higher during the dry season (χ2 = 5.7, P = 0.017) (Fig. 4). This variation comes only from producer value chain (χ2 = 5.4, P = 0.02) than collector (χ2 = 2.3, P = 0.13), processor (χ2 = 2.09, P = 0.15) and farm market (χ2 = 1.0, P = 0.31); whereas in Sidama (χ2 = 1.345, P = 0.294) and Amhara (χ2 = 0.643, P = 0.425) no significant difference has been observed in S.enterica prevalence during dry and wet seasons. Fig. 5)
Discussion
The ability of S.enterica to resist environmental stress as well as proliferate is enhanced when temperature increases (Montville et al., 2012). Almashhadany et al. (2019) reported that the detection of the S.entericain cow milk samples gradually increased with r² =0.854 value in the spring season and early summer; the prevalence of the S.enterica was 19.51% (n = 41) in June and May and 2.4% (n = 41) in January and February. Pangloli et al. (2008) reported that contamination of S.entericain raw milk found to be lower in winter (June-March) (n = 24, 6%) and fall (October-December) (n = 24, 7%) than in spring (April-June) (n = 24, 17%) and summer (July-September) (n = 24, 29%). However, Gebeyehu et al. (2022) found the rate of detection of bacteria in raw milk samples was higher in the wet season 11.98% (n = 192) than in the dry season 8.85% (n = 192). They also reported that even though the detection rate of bacteria was higher in the wet season, it wasn’t significantly different from that of the dry season, which supports the current findings in Sidama and Amhara regions for the raw milk S.enterica seasonal prevalence.
The previous S.enterica prevalence reports from the Oromia region by Abunna et al. (2017) (Mojo) revealed a lower prevalence, but Mossie and Dires (2016) (Debre Zeit) reported a (23.5%) higher prevalence compared to the wet season prevalence of S.enterica. The higher prevalence could be attributed to the study’s large sample size and the fact that the samples were collected during the dry season. In the case of Sidama region, Madalcho et al. (2019) (Wolayta), Fesseha et al. (2020) (Hawassa), and Belay et al. (2022) (Gamo Zone) have reported 1.8%, 3.3%, and 8.3% prevalence, respectively, which was lower than the current study finding of wet season S.enterica in the SIDAMA region. While Senbetu (2014) and Habtamu et al. (2018) from Hawassa reported a higher prevalence rate of S. enterica, 25%, and 17.8%, respectively. Among the S.enterica prevalence reports from the Amhara region, Abebe et al. (2023) (Dessie and Kombolcha), Mulaw (2017), and Hailu et al. (2015) (Gondor) reported 5.92%, 9.35%, and 3.24%, respectively, which is lower than the S.enterica prevalence report during the wet season.
Several studies have revealed the importance of milk and dairy products in the transmission of S.enteric aand the development of salmonellosis in humans due to seasonal fluctuation (Mazurek et al. 2004; Dominguez et al. 2009; van Duynhoven et al. 2009; Giacometti et al. 2015; Putturu et al. 2015; Ung et al. 2019). Stephen and Barnett (2016) reported that the standard regression model estimated salmonellosis (59.4%) cases were increased when there was a 5 °C increase in mean temperature. Zhang et al. (2010) reported a 62% increase with a 5 °C mean temperature change. Similarly, Judd et al. (2019) (USA) found that the overall case count of salmonellosis became higher in the summer (38.6%) and lower in the winter (14.5%).
The impact of seasonal fluctuation on S.enterica detection in food items other than dairy products revealed the prevalence predominantly recovered from the samples that were collected in the warmer season (Lal et al. 2012). Calle et al. (2021) reported the contamination of S.enterica on beef carcasses was found to be higher in samples collected during the dry season (n = 103, 6%) than those collected during the rainy season (n = 102, 1.96%) with (OR 5.90, 95% CI 1.18–29.57) value. Barkocy-Gallagher et al. (2003) revealed that the detection of S.enterica from evisceration of beef carcasses was higher in summer and fall than in the winter and spring.
The super-shedding phenomena and changes in animal feeding habits brought on by climate change can both affect the prevalence and shedding rates of infection agents (Williams et al. 2015; Smith et al.,2019). When temperature is higher, cattle may graze outside more frequently. This can increase pathogen survival and shedding rates, and lactating cows infected with S.enterica may contaminate milk if proper hygiene practices aren’t followed (Jacob et al. 2009). There is evidence for the higher detection rate of the S.enterica in the dry (Sunny) season than wet (rainy) season. Fosseler et al. (2005) found that seasonal impacts of season on the detection of the S.enterica in the fecal materials of the cows revealed that the bacterium was detected at higher rates in summer (OD = 2.7) and spring (OD = 2.3) and slightly higher in fall (OD = 2.1) than winter (OD = 1). In addition, Pangloli et al. (2008) reported the S.enterica predominantly detected in those lactating cow facial samples that were gathered in the summer (72%) rather than in the fall (27%) and winter (13%). The recovery of the S.enterica was also 3.49-fold (Mental-Haenszel’s weighted odd ratio) higher during the months of May-July compared to February-April according to Wells et al. (2001).
Conclusion
In this study, we showed that S.enterica prevalence varied with the seasons, geographic locations, and dairy product type. In comparison to the wet seasons, the prevalence of Salmonella was notably higher during the dry season. In dry and wet seasons, samples of raw milk taken at the production stage were substantially more contaminated by S.enterica than pasteurized milk and cottage cheese, which justifies thorough heat treatment before consuming raw milk. Additionally, it was alarming to find that the pasteurized samples obtained from processor gates and retailers were tainted and required stringent intervention by means of enforcement of the implementation of Hazard Analysis and Critical Control Points (HACCP) and thorough follow-up by regulatory bodies for consumer health. The best way to ensure sustainable interventions is through the application of behaviour change communication training on good hygienic practices (GHP) and good agricultural practices (GAP) by identifying the natural ecosystems that contribute to contamination in order to ensure the microbiological safety of milk and cottage cheese.
Data availability
Not applicable.
References
Abebe E, Gugsa G, Ahmed M, Awol N, Tefera Y, Abegaz S (2023) Prevalence and Antimicrobial Resistance Pattern of Salmonella species from Foods. of Bovine Origin in Dessie and Kombolcha Towns, Ethiopia
Abunna F, Ashenafi D, Beyene T, Ayana D, Mamo B, Duguma R (2017) Isolation, identification and antimicrobial susceptibility profiles of Salmonella isolates from dairy farms in and around Modjo town, Ethiopia. Ethiop Veterinary J 21(2):92
Almashhadany DA, Osman AA (2019) Isolation, serotyping, and antibiogram of Salmonella isolates from raw milk sold at retail vending in Erbil city, Iraq. Bull UASVM Anim Sci Biotechnologies 76:2
Barkocy-Gallagher GA, Arthur TM, Rivera-Betancourt M, Nou X, Shackelford SD, Wheeler TL, Koohmaraie M (2003) Seasonal prevalence of Shiga toxin–producing Escherichia coli, including O157: H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. J Food Prot 66(11):1978–1986
Bedassa A, Nahusenay H, Asefa Z, Sisay T, Girmay G, Kovac J, Zewdu A (2023) Prevalence and associated risk factors for Salmonella enterica contamination of cow milk and cottage cheese in Ethiopia. Int J Food Contam 10(1):1–11
Belay N, Mohammed N, Seyoum W (2022) Bovine mastitis: prevalence, risk factors, and bacterial pathogens isolated in Lactating cows in Gamo Zone, Southern Ethiopia. Veterinary Medicine: Res Rep 13:9
Belina D, Hailu Y, Gobena T, Hald T, Njage PMK (2021) Prevalence and epidemiological distribution of selected foodborne pathogens in human and different environmental samples in Ethiopia: a systematic review and meta-analysis. One Health Outlook 3(1):1–30
Calle A, Carrascal AK, Patiño C, Carpio C, Echeverry A, Brashears M (2021) Seasonal effect on Salmonella, Shiga toxin-producing E. Coli O157: H7 and non-O157 in the beef industry in Colombia, South America. Heliyon, 7(7), e07547
Cancino-Padilla N, Fellenberg MA, Franco W, Ibáñez RA, Pérez EVB (2017) Foodborne bacteria in dairy products: detection by molecular techniques. Ciencia E investigación Agraria: Revista Latinoam De ciencias de la agricultura 44(3):215–229
Chen L, Alali W (2018) Recent discoveries in human serious foodborne pathogenic Bacteria: resurgence, pathogenesis, and control strategies. Front Microbiol 9:2412
Dominguez M, Jourdan-Da Silva N, Vaillant V, Pihier N, Kermin C, Weill FX, de Valk H (2009) Outbreak of Salmonella enterica serotype Montevideo infections in France linked to consumption of cheese made from raw milk. Foodborne Pathog Dis 6(1):121–128
Ebi K (2011) Climate change and health risks: assessing and responding to them through ‘adaptive management’. Health Aff 30(5):924–930
Eng SK, Pusparajah P, Ab Mutalib NS, Ser HL, Chan KG, Lee LH (2015) Salmonella: a review on pathogenesis, epidemiology, and antibiotic resistance. Front Life Sci 8(3):284–293. https://doi.org/10.1080/21553769.2015.1051243
Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA (2012) Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379(9835):2489–2499
Fesseha H, Aliye S, Kifle T, Mathewos M (2020) Isolation and multiple drug resistance patterns of Salmonella isolates from selected dairy farms in Hawassa town, Ethiopia. J Veterinary Sci Med, 8(7)
Fossler CP, Wells SJ, Kaneene JB, Ruegg PL, Warnick LD, Bender JB, Halbert LW (2005) Herd-level factors associated with isolation of Salmonella in a multi-state study of conventional and organic dairy farms: I. Salmonella shedding in cows. Prev Vet Med 70(3–4):257–277
Galán JE, Curtiss R 3rd (1991) Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are defcient for entry into mammalian cells. Infect Immun 59(9):2901–2908. https://doi.org/10.1128/iai.59.9.2901-2908.1991 PMID: 1879916; PMCID: PMC258111
Gebeyehu A, Taye M, Abebe R (2022) Isolation, molecular detection and antimicrobial susceptibility profile of Salmonella from raw cow milk collected from dairy farms and households in southern Ethiopia. BMC Microbiol 22(1):1–10
Giacometti F, Bonilauri P, Albonetti S, Amatiste S, Arrigoni N, Bianchi M, Serraino A (2015) Quantitative risk assessment of human salmonellosis and listeriosis related to the consumption of raw milk in Italy. J Food Prot 78(1):13–21
Green CG, Krause DO, Wylie JL (2006) Spatial analysis of Campylobacter infection in the Canadian province of Manitoba. Int J Health Geogr 5(1):1–14
Habtamu K, Ajebu N, Edessa N (2018) Microbiological quality and safety of milk production and marketing in Hawassa district, Ethiopia. Afr J Microbiol Res 12(25):587–594
Hailu D, Gelaw A, Molla W, Garedew L, Cole L, Johnson R (2015) Prevalence and antibiotic resistance patterns of Salmonella isolates from lactating cows and in-contact humans in dairy farms, Northwest Ethiopia. J Env Occup Sci 4(4):171–178. https://doi.org/10.5455/jeos.20151102014711
Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, World Health Organization Foodborne Disease Burden Epidemiology Reference Group (2015) … World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med, 12(12), e1001923
ISO (2017) International Standard Organization. Horizontal method for the detection, enumeration, and serotyping of Salmonella 2017
Jacob ME, Callaway TR, Nagaraja TG (2009) Dietary interactions and interventions affecting Escherichia coli O157 colonization and shedding in cattle. Foodborne Pathog Dis 6(7):785–792
Judd MC, Hoekstra RM, Mahon BE, Fields PI, Wong KK (2019) Epidemiologic patterns of human Salmonella serotype diversity in the USA, 1996–2016. Epidemiology & Infection, 147
Keba A, Rolon ML, Tamene A, Dessie K, Vipham J, Kovac J, Zewdu A (2020) Review of the prevalence of foodborne pathogens in milk and dairy products in Ethiopia. Int Dairy J 109:104762
Keen JE, Laegreid WW, Mckown C, Bono CG, Fox JL, Clawson JM, M., Heaton M (2003), November Effect of exogenous glucocorticoids and dietary change on winter and summer STEC O157 fecal shedding in naturally-infected beef cattle. In Research Workers in Animal Diseases Conference Proceedings
Kovats RS, Edwards SJ, Hajat S, Armstrong BG, Ebi KL, Menne B (2004) The effect of temperature on food poisoning: a time-series analysis of salmonellosis in ten European countries. Epidemiol Infect 132(3):443–453
Lal A, Hales S, French N, Baker MG (2012) Seasonality in human zoonotic enteric diseases: a systematic review. PLoS ONE, 7(4), e31883
Lubote R, Shahada F, Matemu A (2014) Prevalence of Salmonella spp. and Escherichia coli in raw milk value chain in Arusha, Tanzania. American J Res Com 2(9):1–13
Lucey JA (2015) Raw milk consumption: risks and benefits. Nutr Today 50(4):189
Madalcho EB (2019) A study on the prevalence of bovine mastitis and isolation of Major Pathogens Associated with it in and around Wolaita Sodo, Southern Ethiopia. Int J Res Stud Biosci 7(2):40–48
Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ … International collaboration on enteric Disease Burden of Illness studies. (2010). The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis, 50(6), 882–889
Mazurek J, Salehi E, Propes D, Holt JO, Bannerman T, Nicholson LM, Moolenaar RL (2004) A multistate outbreak of Salmonella enterica serotype typhimurium infection linked to raw milk consumption—Ohio, 2003. J Food Prot 67(10):2165–2170
Montville TJ, Matthews KR (2012) Physiology, growth, and inhibition of microbes in foods. Food microbiology: fundamentals and frontiers, 1–18
Mossie T, Dires A (2016) Prevalence of antimicrobial resistant Salmonella isolated from bulk milk of dairy cows in and around Debre-Zeit, Ethiopia. World’s Veterinary J 6(3):110–116
Mulaw G (2017) Prevalence and antimicrobial susceptibility of Salmonella species from lactating cows in dairy farm of bahir dar town, Ethiopia. Afr J Microbiol Res 11(43):1578–1585
Naumova EN, Jagai JS, Matyas B, DeMaria A, MacNeill IB, Griffiths JK (2007) Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol Infect 135(2):281–292
Nyenje ME, Ndip RN (2013) The challenges of foodborne pathogens and antimicrobial chemotherapy: a global perspective. Afr J Microbiol Res 7(14):1158–1172
Pal M, Teashal BM, Gizaw F, Alemayehu G, Kandi V (2020) Animals and food of animal origin as a potential source of salmonellosis: a review of the epidemiology, laboratory diagnosis, economic impact and public health significance. Am J Microbiol Res 8(2):48–56
Pangloli P, Dje Y, Ahmed O, Doane CA, Oliver SP, Draughon FA (2008) Seasonal incidence and molecular characterization of Salmonella from dairy cows, calves, and farm environment. Foodborne Pathog Dis 5(1):87–96
Park MS, Park KH, Bahk GJ (2018) Combined influence of multiple climatic factors on the incidence of bacterial foodborne diseases. Sci Total Environ 610:10–16
Putturu R, Eevuri T, Ch B, Nelapati K (2015) Salmonella enteritidis-foodborne pathogen-a review. Int J Pharm Biol Sci 5(1):86–95
Rivero MA, Passucci JA, Rodríguez EM, Parma AE (2012) Seasonal variation of HUS occurrence and VTEC infection in children with acute diarrhoea from Argentina. Eur J Clin Microbiol Infect Dis 31(6):1131–1135
Rose JB, Epstein PR, Lipp EK, Sherman BH, Bernard SM, Patz JA (2001) Climate variability and change in the United States: potential impacts on water-and foodborne diseases caused by microbiologic agents. Environ Health Perspect 109(suppl 2):211–221
Séguin J, Berry P, Bouchet V, Clarke KL, Furgal C, Environmental I, MacIver D (2008) Human health in a changing climate: a Canadian assessment of vulnerabilities and adaptive capacity. Human health in a changing climate, p 1
Semenza JC, Herbst S, Rechenburg A, Suk JE, Höser C, Schreiber C, Kistemann T (2012) Climate change impact assessment of food-and waterborne diseases. Crit Rev Environ Sci Technol 42(8):857–890
Senbetu DT (2014) Hazard analysis of cheese provided for consumers in Hawassa/Ethiopia. J Food Process Technol 5(2):1
Smith BA, Meadows S, Meyers R, Parmley EJ, Fazil A (2019) Seasonality and zoonotic foodborne pathogens in Canada: relationships between climate and Campylobacter, E. Coli and Salmonella in meat products. Epidemiology & Infection, p 147
Stephen DM, Barnett AG (2016) Effect of temperature and precipitation on salmonellosis cases in South-East Queensland, Australia: an observational study. BMJ open, 6(2), e010204
Tegegne B, Tesfaye S (2017) Bacteriological milk quality: possible hygienic factors and the role of Staphylococcus aureus in raw bovine milk in and around Gondar, Ethiopia. Int J Food Contam 4(1):1–9
Ung A, Baidjoe AY, Van Cauteren D, Fawal N, Fabre L, Guerrisi C, Le Hello S (2019) Disentangling a complex nationwide Salmonella Dublin outbreak associated with raw-milk cheese consumption, France, 2015 to 2016. Eurosurveillance 24(3):1700703
Van Duynhoven YTHP, Isken LD, Borgen K, Besselse M, Soethoudt K, Haitsma O, Van Steenbergen J (2009) A prolonged outbreak of Salmonella Typhimurium infection related to an uncommon vehicle: hard cheese made from raw milk. Epidemiol Infect 137(11):1548–1557
Wabeto W, Abraham Y, Anjulo AA (2017) Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. J Health Popul Nutr 36(1):1–7
Wells SJ, Fedorka-Cray PJ, Dargatz DA, Ferris K, Green A (2001) Fecal shedding of Salmonella spp. by dairy cows on farm and at cull cow markets. J Food Prot 64(1):3–11
Williams KJ, Ward MP, Dhungyel OP, Hall EJS (2015) Risk factors for Escherichia coli O157 shedding and super-shedding by dairy heifers at pasture. Epidemiol Infect 143(5):1004–1015
World Health Organization (2021) Estimating the burden of foodborne diseases: a practical handbook for countries: a guide for planning, implementing and reporting country-level burden of foodborne disease. World Health Organization. http://www.jstor.org/stable/resrep39061
Zhang Y, Bi P, Hiller JE (2010) Climate variations and Salmonella infection in Australian subtropical and tropical regions. Sci Total Environ 408(3):524–530
Acknowledgements
This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation and The Foreign, Commonwealth and Development Office of UK [Grant Number INV-008459]. We thank the farmers and agricultural development agents (DEAs) of all sites in the three regions of Ethiopia for allowing us to take samples. Lastly, we would like to thank all the staff and students of the ENSURE Dairy Ethiopia Project and National Agricultural Biotechnology Research Center for their assistance with the laboratory work of the study.
Funding
This work was supported by the Bill & Melinda Gates Foundation and The Foreign, Commonwealth, and Development Office of the UK [Grant Number INV-00845] awarded to AZ of Addis Ababa University. The work of JK was supported by the USDA National Institute of Food and Agriculture Hatch Appropriations under Project PEN04646 and Accession 1015787.
Author information
Authors and Affiliations
Contributions
H.N: Conceptualization; Investigation; Formal analysis; Data Collection; Data Analysis; Writing-original draft, Writing-review & Editing A.B: Conceptualization, Investigation; Formal analysis; Data Collection; Writing-review & Editing T.S: Conceptualization; Methodology; Supervision; Writing-review & EditingJ.V: Conceptualization; Methodology; Supervision, Fund Acquisition; Writing-review & EditingJ.K: Conceptualization; Methodology; Supervision; Fund Acquisition; Writing-review & Editing A.Z: Conceptualization; Data Curation; Fund Acquisition; Supervision; Writing-review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved, and ethical clearance was obtained from the Institutional Review Board of the College of Natural and Computational Sciences, Addis Ababa University (CNS-IRB 42/2019).
Consent for publication
All authors consent to publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Admasu, H.N., Bedassa, A., Tessema, T.S. et al. Seasonal variation of Salmonella enterica prevalence in milk and cottage cheese along the dairy value chain in three regions of Ethiopia. Food saf. and Risk 11, 2 (2024). https://doi.org/10.1186/s40550-024-00108-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40550-024-00108-4