Abstract
Background and objective
Little is known about how much public payers spend on orphan medicines. This study aimed at identifying information on orphan medicine expenditure incurred by public payers that was published in literature globally and at possibly synthesising their shares as portion of the total pharmaceutical expenditure.
Methods
A literature review was undertaken using Medline, the Orphanet Journal of Rare Diseases and Google Scholar. Titles and abstracts were screened, and full texts of potentially qualifying studies were reviewed for inclusion. Included articles were analysed, and bibliometric parameters as well as public expenditure data on orphan medicines were retrieved.
Results
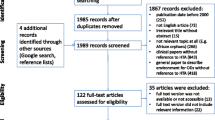
Six hundred forty three articles excluding duplicates were identified. After screening of the abstracts and a review of the full texts, 13 articles qualified for in-depth analysis.
The 13 selected articles on orphan pharmaceutical expenditure were published between 2010 and 2018. Survey periods varied between 1 year and 12 years. One publication included 22 countries but the majority of the studies were related to a single country. Expenditure data was available in five of the 13 articles, and eight articles used ‘expenditure proxies’ such as sales data. Spending data had been sourced from public institutions (4 studies), private providers (5 studies) and a combination of both (3 studies, no information on data source in 1 study). In all included studies, secondary data were analysed. Reported expenditure shares for orphan medicines in relation to total pharmaceutical spend was frequently below 3%. Countries with higher shares included the USA, Canada, the Netherlands and Bulgaria—the latter reporting spending on orphan medicines as high as 9%.
Conclusions
A low number of studies that informed about pharmaceutical spending on orphan medicines was published, thereof only a few explicitly analysed expenditure data of public payers. A conclusive synthesis of public spending on orphan medicines is a challenge given to the diversity in methodologies to measure expenditure. There is a need for further research to survey primary data of public spending for orphan medicines, based on a sound methodology to measure these data and to compare them internationally.
Similar content being viewed by others
Introduction
Rare diseases are conditions and illnesses that, per definition, affect a comparably low number of patients. The prevalence thresholds differ between countries and world regions. In the European Union (EU), for example, a rare disease is defined as a disease that affects fewer than five people in 10,000 [1]. Respective figures are fewer than 200,000 people in the United States of America (USA), fewer than 50,000 in Japan and fewer than one in 10,000 in Taiwan [2, 3].
Given low patient numbers, research and development of pharmaceuticals to treat rare diseases (so-called orphan medicines) has been promoted by governments. Thus, the EU, USA, Japan and further high-income countries offered incentives to pharmaceutical companies [4]. It has been argued that in response, manufacturers tend to focus on profitable areas such as oncology, and, as a result, orphan medicines have become non-affordable to public payers and patients as some tend to have (very) high price tags [5, 6]. Research confirmed high prices of orphan medicines [7,8,9,10,11,12] and limited access to orphan medicines, particularly in lower-resourced countries [7, 13,14,15,16]. Policymakers have to balance the objectives of access to effective medicines for the population, containment of pharmaceutical expenditure as well as long-term sustainability of the health care system and incentives for the industry. For their search for solutions, new policies to ensure access to premium-priced medicines, including orphan medicines, have been explored or are under discussion [17,18,19]. For instance, managed-entry agreements are frequently applied for orphan medicines [14, 20, 21]. As a standard, these agreements include a, usually confidential, discount granted to the public payer, and funding may, or may not, be linked to the health outcomes of a patient.
Concerns have been raised that a few orphan medicines may account for a comparably high share of the pharmaceutical budget of the public payers [2, 22, 23]. In addition to the high price tags, the increasing number of patients in rare diseases as well as the extension of indications are potential drivers for high shares in pharmaceutical budgets. Overall, rare diseases can be frequent, and it is assumed that 400 million people worldwide suffer from rare diseases [24]. Some studies, however, challenged the existence of a strong impact of orphan medicines on future pharmaceutical budgets [25, 26]. This debate is held against the backdrop of limited knowledge on spending for orphan medicines since expenditure for these medicines is not published as routine data in statistics in most countries.
To address this gap, the study aimed at identifying information on orphan medicine expenditure data that was globally published in literature and at possibly synthesising the shares of spending on orphan medicines as portion of the total public pharmaceutical expenditure across countries.
Methods
A systematic literature review was undertaken in September/October 2018 using Medline, the Orphanet Journal of Rare Diseases and Google Scholar in order to identify published information about pharmaceutical expenditure on rare diseases covered by public or other third-party payers. The following search terms were applied: rare disease(s), orphan disease(s), rare condition(s), orphan condition(s), orphan drug(s), orphan medicinal product(s), rare drug(s), pharmaceutical spending, drug cost(s), expenses, pharmaceutical expenditure and budget impact.
Literature was considered eligible for further analysis if it contained information on expenditure for orphan medicines that was retrospective, not disease specific (rare diseases in total) and referred to the year 2001 and later (1 year after the Orphan Medicinal Products Regulation in the European Union had come into force). No restriction with regard to geographical scope or language was applied.
The literature selection was performed in a two-step process: first, the title and the abstract of studies were screened with regard to their compliance with the defined inclusion/exclusion criteria, and in a second step, full texts of the selected pieces of literature were studied to assess whether or not they qualified for possible inclusion.
Upon selection of eligible articles, relevant information of the included publications was summarized in an extraction matrix. The matrix contained the following information: author(s), title, year of publication, journal information, language, aim/purpose/study question, study design, country/countries of the study, methodology parameters of the expenditure data reported (sector, type of analysis, year of data, data description, data source, price level) and scope of expenditure information (e.g. on orphan medicines, public pharmaceutical expenditure, total pharmaceutical expenditure).
Results
Selection of the studies
Six hundred forty four articles were identified in Medline, including one duplicate. An additional search in the Orphanet Journal of Rare Diseases and a search in Google Scholar were not successful in identifying further relevant pieces of literature.
Six hundred forty three articles were screened on the basis of title and abstract, thereof full texts of 28 articles were analysed. Eventually, 13 articles qualified for the in-depth analysis (see the literature review process in Fig. 1).
Characteristics of included studies
The 13 included articles were published between 2010 and 2018, thereof three in 2018, three in 2016, one in 2015, three in 2014, one in 2011 and two in 2010. In some cases, investigated data in these studies were related to earlier periods of time, such as the years 2006 and 2007 and even 2003 in one study. Survey periods varied between 1 year and 12 years. The number of countries analysed ranged from 1 to 22; however, most of the studies were related to a single country.
Spending data were mainly reported from countries in the EU and also from Canada, Taiwan, Turkey and the USA. The majority of studies (8) investigated retrospective spending data on orphan medicines, whereas the remaining (5) reported both retrospective and prospective orphan medicine expenditure. In five of the 13 studies, expenditure data were actually available, compared with eight studies with ‘expenditure proxies’: six studies were based on sales data, and two studies provided some type of cost data. In four studies, information sourced from a public data provider was analysed, while data were obtained from private providers in five studies and from a combination of public and private data providers in three studies (in one study, the data source was not traceable). Pharmaceutical expenditure data were indicated for both inpatient and outpatient sectors in three articles; in nine studies, the sectors were not defined. None of the studies was based on a primary data collection; thus, solely secondary data were analysed. In all but one study, national annual spending data were expressed either in absolute terms or as a share of total public pharmaceutical expenditure (frequently called ‘total pharmaceutical expenditure’ even if they were related on the public sector only), or both; one study provided a cumulative percentage rate over all surveyed countries.
Synthesis of included studies’ results
Taiwan experienced a nine-fold increase in spending on orphan medicines in absolute terms in a period of 12 years, and the Netherlands a 3.3-fold growth in the outpatient sector and a 4.2-fold increase in the inpatient sector during 7 years. Public spending on orphan medicines more than doubled in Bulgaria from 2011 till 2014. In comparison, the growth rates for Latvia (a 20% increase from 2010 to 2014) seemed to be rather low.
In terms of total public expenditure, reported public spending on orphan medicines ranged from less than 1% in Taiwan in 2003 to more than 9% in Bulgaria in 2014.
The authors of the analysed studies reported different methodologies and limitations of their research, including short observation periods, possible over- or underestimation due to methodological issues and uncertainties were related to the projections due to calculations based on historical data and the scope of medicines in the studies (Table 1).
Discussion
The study reviewed existing evidence in literature on the availability of public spending data on orphan medicines, with a view to understanding the relevance of pharmaceutical spending for these medicines.
Overall, the number of studies that were eligible for inclusion in the analysis was rather limited, but some spending data on orphan medicines were published in literature in the last decade. Improved availability of such information might be attributable to an increased interest in these medicines, given the market entry of several high-priced medicines in recent years and concerns of policymakers [36,37,38,39]. Also, apart from the USA that introduced legislation for orphan medicines 35 years ago, orphan medicine legislation in other countries and regions was mostly introduced in the last decades (e.g. Singapore 1991 [40], Japan in 1993 [4], Australia in 1997 [41], the EU in 2000 [1], Taiwan 2000 [42]). Legislation for orphan medicines has mainly been implemented in high-income countries [4], and data on spending for orphan medicines were predominantly reported for these countries. This may also be linked to other reasons such as publication and manuscript selection bias with regard to lower-income countries [43,44,45], advanced statistical data and frameworks available in higher-income countries (e.g. the System of Health Accounts methodology [46] developed by the Organisation for Economic Co-operation and Development) and availability of a higher number of medicines that have been defined as orphan medicines in higher-income countries [15, 47, 48].
In the light of current developments (i.e. a growing number of orphan medicines being authorised and also marketed, e.g. [49]), it could be expected that spending on orphan medicines would have increased over the years. In fact, almost all included studies that provided data for a longer period of time confirmed a growth in spending on orphan medicines.
There is variation in the shares of pharmaceutical spending on orphan medicines between the analysed countries. For the year 2013, studies reported shares of public spending on medicines that ranged from 1.83% in Latvia [31] and 2.06% in Taiwan [2] to 5.6% in Canada [33] and 8.9% in the USA [32]. Two studies reported different findings on 2014 data for Bulgaria (7.8% [29] and 9.39% [30]) even if the data had been retrieved from the same source. A study published after the authors had finalised their search showed similar figures for eight European countries: spending shares on orphan medicines as a portion of total pharmaceutical expenditure ranged from 1.95 to 6.18% in 2013 and from 2.5 to 6.84% in 2014 [13]. While the majority of the articles included in this review reported shares of orphan medicine expenditure of around 2–3%, some studies identified shares that exceeded 5% and even 7–8%. Such figures could be considered challenging and even alarming, given the tendency of growth in pharmaceutical expenditure for these medicines. Even if parts of the variations might be attributable to the methodological design of the analysed studies, a significant and rising share of pharmaceutical expenditure for comparatively low consumption urges policymakers to develop appropriate action to balance the aims to ensuring access to medicines and guaranteeing long-term sustainability of the health care system. In any case, the arguments made by some authors that orphan medicines would not have a strong impact on future pharmaceutical budgets [21, 22] cannot be confirmed by the data retrieved in this literature review.
The review identified methodological limitations in the existing evidence of public spending on orphan medicines published in literature. This also limits cross-country comparability of the data.
First, different national definitions of orphan medicines were applied, so the scope of medicines included as orphan medicines into national data varied between countries.
Second, some studies [32, 33, 35] included all medicines with a designation as an orphan medicine, independent from actual validity of the orphan status, whereas other research [31] considered solely orphan medicines with a valid orphan status (e.g. awarded by the European Medicines Agency in the EU). Apart from one study [25], it was not clear for the articles relating to EU Member States whether or not medicines for treating rare diseases before the introduction of the Orphan Medicinal Products Regulation in 2000 had been included.
Third, comparability was impaired by the reporting of national spending data for different price types (e.g. ex-factory price, consumer price), and some articles did not even provide any information on the price type at which spending data was measured.
Fourth, not all studies reported spending data but some considered sales data as kind of proxy for pharmaceutical spending.
Fifth, some studies did not provide any information if their data were related to public or private expenditure. In general, given the character of these medicines (high prices, specific application that may require inpatient use) and the data mainly sourced from solidarity-based high-income countries, public funding of orphan medicines is highly likely. This assumption is also supported by the fact that some studies (e.g. on EU Member States) surveyed and analysed data from sources managed by public payers.
Limitations
The study has some limitations. It focused on articles in peer-reviewed journals because there was interest to see what has been published. It is, however, acknowledged that further studies and statistics of primary data might be available publicly (e.g. on a website), but they are not published in peer-reviewed literature. Furthermore, the study investigated spending data (including proxies for spending) but did not consider information on contributing factors for expenditure, such as the number of authorised or marketed orphan medicines (reported in some articles [22, 23, 27, 32,33,34,35]) and the number of treated patients [22, 23, 29]. Finally, the literature search was performed in September/October 2018, and the authors are aware that after completion of the literature review, at least one article on orphan medicines and spending [13] was published.
Call for further action
The review identified a range of shares related to public spending for orphan medicines from a rather limited body of literature. The relevance of the findings is challenged by the diversity of the definitions and measurement methodologies applied to the underlying data. Further research is needed. As a first step, it is suggested to complement this literature review by a search of primary public expenditure data on orphan medicines in national statistics. In this context, it is advised to analyse national definitions of these spending data and to explore cross-country comparability. Furthermore, to bridge gaps in cases of non-reporting in publicly accessible sources, it is recommended to address experts in public authorities (such as medicine agencies and public payers’ institutions) to provide these data, ideally in accordance with definitions pre-specified by researchers, in order to allow for follow-up international comparisons. Researchers are advised to pilot these surveys in countries with good statistics and then roll out globally. It should be aimed for the inclusion of low- and middle-income countries in these studies, though researchers should also take into account that the statistical basis for pharmaceutical expenditure data in general might need to be installed first in some countries. The methodology development could benefit from cross-country collaboration, with a possible involvement of international institutions.
The study findings do not only call for further research but also for action of policymakers. While research can offer exploratory work to generate evidence and can contribute to methodology development, also with a view to ensuring international comparability, it would be subsequently up to the public authorities to decide on a framework that provides for regular survey, analysis and publication of public spending data for orphan medicines. This would allow establishing an internationally comparable system of orphan medicines spending account that could, in the longer run, be implemented globally, including in middle- and low-income countries.
Conclusions
The study stressed the limited body of evidence on public spending for orphan medicines, as only few studies had been published, and some research used proxy data such as sales figures to inform on public spending. Some single-country studies pointed to a steady increase in the spending portion on orphan medicines over the years. While some studies reported a share of around 2–3% of pharmaceutical spend on these medicines, there was considerable variation in the figures (0.35%, Taiwan, 2003 and 9.39%, Bulgaria, 2014).
Despite methodological limitations in the retrieved literature which may be one explanatory factor for the diversity in outcome data, the comparatively high shares on spending for orphan medicines in some countries and their increases are key findings of policy relevance. They call for further monitoring of orphan medicine expenditure, analysing their causes and taking policy action if required.
As a prerequisite, the evidence base would need to be established in numerous countries. Overall, this review highlights the need for generating further information. More research to survey national primary spending data is required. Policymakers and researchers are called upon to collaborate on developing a robust methodology to survey and publishing public spending on orphan medicines as routine data.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- EU:
-
European Union
- USA:
-
United States of America
References
Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. Brussels; 2000. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000R0141.
Hsu JC, Wu H-C, Feng W-C, Chou C-H, Lai EC-C, Lu CY. Disease and economic burden for rare diseases in Taiwan: a longitudinal study using Taiwan’s National Health Insurance Research Database. PLoS One. 2018;13(9):e0204206.
Richter T, Nestler-Parr S, Babela R, Khan ZM, Tesoro T, Molsen E, et al. Rare disease terminology and definitions—a systematic global review: report of the ISPOR Rare Disease Special Interest Group. Value Health. 2015;18(6):906–14.
Gammie T, Lu CY, Babar ZU-D. Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. PLoS One. 2015;10(10):e0140002.
Cote A, Keating B. What is wrong with orphan drug policies? Value Health. 2012;15(8):1185–91.
Kanavos P, Nicod E. What is wrong with orphan drug policies? Suggestions for ways forward. Value Health. 2012;15(8):1182–4.
Picavet E, Dooms M, Cassiman D, Simoens S. Drugs for rare diseases: influence of orphan designation status on price. Appl Health Econ Health Policy. 2011;9(4):275–9.
Castaneda-Sanabria J, Hajage D, Le Jouan M, Perozziello A, Tubach F. Off-label use of the expensive orphan drug eculizumab in France 2009-2013 and the impact of literature: focus on the transplantation field. Eur J Clin Pharmacol. 2016;72(6):737–46.
Picavet E, Morel T, Cassiman D, Simoens S. Shining a light in the black box of orphan drug pricing. Orphanet J Rare Dis. 2014;9:62.
Young KE, Soussi I, Toumi M. The perverse impact of external reference pricing (ERP): a comparison of orphan drugs affordability in 12 European countries. A call for policy change. J Mark Access Health Policy. 2017;5(1):1369817.
Simoens S. Pricing and reimbursement of orphan drugs: the need for more transparency. Orphanet J Rare Dis. 2011;6:42.
Michel M, Toumi M. Access to orphan drugs in Europe: current and future issues. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):23–9.
Szegedi M, Zelei T, Arickx F, Bucsics A, Cohn-Zanchetta E, Furst J, et al. The European challenges of funding orphan medicinal products. Orphanet J Rare Dis. 2018;13:184.
Malinowski KP, Kawalec P, Trabka W, Czech M, Petrova G, Manova M, et al. Reimbursement legislations and decision making for orphan drugs in Central and Eastern European countries. Front Pharmacol. 2019;10:487.
Kamusheva M, Stoimenova A, Doneva M, Zlatareva A, Petrova G. A cross-country comparison of reimbursed orphan medicines in Bulgaria, Greece and Romania. Biotechnol Biotechnol Equip. 2013;27(5):4186–92.
Malinowski KP, Kawalec P, Trabka W, Sowada C, Pilc A. Reimbursement of orphan drugs in Europe in relation to the type of authorization by the European Medicines Agency and the decision making based on health technology assessment. Front Pharmacol. 2018;9:1263.
Vogler S, Paris V, Ferrario A, Wirtz VJ, de Joncheere K, Schneider P, et al. How can pricing and reimbursement policies improve affordable access to medicines? Lessons learned from European countries. Appl Health Econ Health Policy. 2017;15(3):307–21.
OECD. New health technologies: managing access, value and sustainability. Paris: OECD Publishing; 2017.
Vogler S, Paris V, Panteli D. Ensuring access to medicines: how to redesign pricing, reimbursement and procurement? European Observatory on Health Care Systems. Copenhagen: WHO Regional Office for Europe; 2018.
Morel T, Arickx F, Befrits G, Siviero P, van der Meijden C, Xoxi E, et al. Reconciling uncertainty of costs and outcomes with the need for access to orphan medicinal products: a comparative study of managed entry agreements across seven European countries. Orphanet J Rare Dis. 2013;8:198.
Morel T, Arickx F, Befrits G, Siviero PD, van der Meijden CMJ, Xoxi E, et al. Managed entry agreements and orphan drugs: a European comparative study (2006-2012). Value Health. 2013;16(7):A391.
Denis A, Mergaert L, Fostier C, Cleemput I, Simoens S. Budget impact analysis of orphan drugs in Belgium: estimates from 2008 to 2013. J Med Econ. 2010;13(2):295–301.
Kanters TA, Steenhoek A, Hakkaart L. Orphan drugs expenditure in the Netherlands in the period 2006-2012. Orphanet J Rare Dis. 2014;9:154.
Graf von der Schulenburg JM, Frank M. Rare is frequent and frequent is costly: rare diseases as a challenge for health care systems. Eur J Health Econ. 2015;16(2):113–8.
Schey C, Milanova T, Hutchings A. Estimating the budget impact of orphan medicines in Europe: 2010 - 2020. Orphanet J Rare Dis. 2011;6:62.
Schlander M, Adarkwah CC, Gandjour A. Budget impact analysis of drugs for ultra-orphan non-oncological diseases in Europe. Expert Rev Pharmacoecon Outcomes Res. 2015;15(1):171–9.
Orofino J, Soto J, Casado MA, Oyaguez I. Global spending on orphan drugs in France, Germany, the UK, Italy and Spain during 2007. Appl Health Econ Health Policy. 2010;8(5):301–15.
Hutchings A, Schey C, Dutton R, Achana F, Antonov K. Estimating the budget impact of orphan drugs in Sweden and France 2013–2020. Orphanet J Rare Dis. 2014;9(1):22.
Iskrov G, Jessop E, Miteva-Katrandzhieva T, Stefanov R. Budget impact of rare diseases: proposal for a theoretical framework based on evidence from Bulgaria. Georgian Med News. 2015;5(242):46–53.
Iskrov GG, Jakovljevic MM, Stefanov RS. Budgetary impact of medicinal therapies for rare diseases in Bulgaria. Folia Med. 2018;60(1):79–91.
Logviss K, Krievins D, Purvina S. Impact of orphan drugs on Latvian budget. Orphanet J Rare Dis. 2016;11:59.
Divino V, DeKoven M, Kleinrock M, Wade R, Kaura S. Orphan drug expenditures in the United States: a historical and prospective analysis, 2007-18. Health Aff. 2016;35(9):1588–94.
Divino V, DeKoven M, Kleinrock M, Wade RL, Kim T, Kaura S. Pharmaceutical expenditure on drugs for rare diseases in Canada: a historical (2007-13) and prospective (2014-18) MIDAS sales data analysis. Orphanet J Rare Dis. 2016;11:68.
Kockaya G, Wertheimer AI, Kilic P, Tanyeri P, Mert Vural I, Akbulat A, et al. An overview of the orphan medicines market in Turkey. Value Health Reg Issues. 2014;4C:47–52.
Deticek A, Locatelli I, Kos M. Patient access to medicines for rare diseases in European countries. Value Health. 2018;21:553–60.
OECD. Health at a glance 2015: OECD indicators. Paris: OECD Publishing; 2015.
The Council of the European Union. Council conclusions on strengthening the balance in the pharmaceutical systems in the EU and its Member States. 2016.
Vogler S, Paris V, Pantelli D. Policy Brief 30: ensuring access to medicines: How to redesign pricing, reimbursement and procurement? European Observatory; 2018.
OECD. Pharmaceutical innovation and access to medicines. Paris: OECD Publishing; 2018.
Singapore Statutes Online. Medicines Act (Chapter 176,Section 9) Medicines (Orphan Drugs), (Exemption) Order,G.N. No. S 470/1991, Revised Edition; 2005. https://sso.agc.gov.sg/SL/MA1975-OR12?DocDate=20050331&ValidDate=20160122&TransactionDate=20160122. Accessed 24 Aug 2020.
Therapeutic goods regulations (Amendment), 399 (1997). https://www.legislation.gov.au/Details/F1997B02888.
Health Promotion Administration. The Rare Disease and Orphan Drug Act. 2000. Available from: https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=1058&pid=6031. Accessed 31 May 2019.
Yousefi-Nooraie R, Shakiba B, Mortaz-Hejri S. Country development and manuscript selection bias: a review of published studies. BMC Med Res Methodol. 2006;6:37.
Harris M, Macinko J, Jimenez G, Mullachery P. Measuring the bias against low-income country research: an implicit association test. Global Health. 2017;13:80.
Smith E, Hunt M, Master Z. Authorship ethics in global health research partnerships between researchers from low or middle income countries and high income countries. BMC Med Ethics. 2014;15:42.
OECD, Eurostat, and World Health Organization. A system of health accounts 2011: revised edition. Paris: OECD Publishing; 2017.
Pavlović N, Stanimirov B, Stojančević M, Paut-Kusturica M, Stoimenova A, Goločorbin-Kon S, et al. An insight on differences in availability and reimbursement of orphan medicines among Serbia, Bulgaria and Sweden. Biotechnol Biotechnol Equip. 2012;26(5):3236–41.
Sarnola K, Ahonen R, Martikainen JE, Timonen J. Policies and availability of orphan medicines in outpatient care in 24 European countries. Eur J Clin Pharmacol. 2018;74(7):895–902.
Zamora B, Garau M, Maignen F, O'Neill P, Mestre-Ferrandiz J. OP138 access to orphan drugs in the United Kingdom and other European countries. Towards an HTA ecosystem: from local needs to global opportunities; 2017.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MG conducted the literature review by screening titles and abstracts, reading all relevant full texts and compiling the extraction matrix. Additionally, she drafted the manuscript, table and figure. SV studied all articles whose exclusion and inclusion were difficult to decide and reviewed the extraction table. Additionally, she reviewed and finalised the draft manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gombocz, M., Vogler, S. Public spending on orphan medicines: a review of the literature. J of Pharm Policy and Pract 13, 66 (2020). https://doi.org/10.1186/s40545-020-00260-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40545-020-00260-0