Abstract
BLU-945, a new-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), is a potential drug candidate for the treatment of non-small-cell lung cancer (NSCLC) in patients with mutations that are resistant to previous generations of EGFR TKI. This compound has been investigated in preclinical and phase 1 dose-escalation studies that require a bioanalytical method for drug quantitation. In this study, an LC–MS/MS method was developed and validated for the quantitation of BLU-945 in rodent plasma and was applied to pharmacokinetic studies. The compound was extracted from plasma samples using a simple protein precipitation method. The method was validated in the linearity range of 1–1000 ng/mL with acceptable accuracy and precision, no matrix effects, and complete extraction recovery. BLU-945 was stable in the plasma quality control samples under various handling and storage conditions. The compound was stable after 4-h incubation in human, mouse, and rat plasma but was extensively metabolized in the microsomal fractions of these species. Furthermore, the validated analytical method was applied to a pharmacokinetic study in rats, revealing that BLU-945 had a high oral bioavailability range (55.91–105.6%) with a nonlinear pharmacokinetic profile up to an oral dose of 20 mg/kg. The validated bioanalytical method and findings of our study represent valuable assets for future investigations and clinical studies of BLU-945.
Similar content being viewed by others
Introduction
Non-small-cell lung cancer (NSCLC) accounts for the majority (80–85%) of lung cancer, which is the leading cause of cancer mortality, with 1.8 million deaths (18%) globally in 2020 (Sung et al. 2021). Over the past few decades, substantial advancements have been made in the development of targeted therapies and immunotherapies for NSCLC (Li et al. 2023a, b). Nevertheless, the emergence of treatment resistance remains a significant challenge in effectively managing the disease, especially in patients with activating epidermal growth factor receptor (EGFR) mutations. These mutations play a critical role in the development of NSCLC, with a particularly high prevalence in adenocarcinoma, the most common subtype of NSCLC (Midha et al. 2015).
The most prevalent EGFR-activating mutations (EGFR+) observed in NSCLC include exon 19 deletions and a single point mutation in exon 21 (L858R) of EGFR (Gazdar 2009). Patients with EGFR-mutated NSCLC frequently exhibit positive responses to first- and second-generation EGFR tyrosine kinase inhibitors (TKIs) such as afatinib, erlotinib, and gefitinib (Chang et al. 2023). The T790M mutation is a secondary alteration that can arise in NSCLC patients who initially respond to first- and second-generation EGFR TKIs but subsequently develop resistance to these medications by restoring a few of the EGFR protein activities (Belani 2010; Santoni-Rugiu et al. 2019). Third-generation TKI, such as osimertinib and lazertinib, can effectively inhibit EGFR+/T790M-positive tumors (Li et al. 2022). However, acquired resistance still emerges over time with C797S, another secondary mutation within the EGFR gene, limiting the clinical efficiency of the third-generation TKI (Lategahn et al. 2019; Li et al. 2023a, b). Recent reports indicate that approximately 40% of patients develop C797S mutations following treatment with third-generation EGFR TKIs (Duggirala et al. 2022). Pharmaceutical companies worldwide have focused on developing small molecules to target the EGFR+/T790M/C797S triple mutant NSCLC. Several compounds with varying scaffolds and binding interactions have been developed as fourth-generation small-molecule EGFR-TKIs (Mansour et al. 2023). Among these, BLU-945 is a highly potent drug candidate designed to selectively target and inhibit the EGFR T790M/C797S and T790M resistance mutations (Schalm et al. 2020a, b).
In enzymatic assays, BLU-945 exhibits excellent inhibitory activity against EGFR+(L858R, ex19del), EGFR+/T790M, and EGFR+/T790M/C797S mutants (Schalm et al. 2020a, b). In preclinical pharmacokinetic studies involving rats, dogs, and cynomolgus monkeys, BLU-945 exhibited low-to-moderate clearance, a moderate volume of distribution, and good oral bioavailability (Eno et al. 2022). Significant tumor regression was observed in mice carrying xenografts of NCI-H1975 (a resistant NSCLC cell line), mice with engineered Ba/F3 EGFR L858R/T790M/C797S and Ba/F3 ex19del/T790M/C797S osimertinib-resistant tumors, and in mice bearing a patient-derived cell line (osimertinib-resistant EGFR ex19del/T790M/C797S). Preclinical toxicity studies in rats and non-human primates have shown suitable safety margins for BLU-945. In a phase 1 dose-escalation study (NCT04862780), BLU-945 was evaluated in patients with EGFR-mutated NSCLC who had previously undergone at least one EGFR-targeted TKI treatment. The plasma concentration–time profile of BLU-945 (25 mg) showed low clearance and an extended plasma half-life (T1/2) of the candidate drug (Eno et al. 2022).
Liquid chromatography-tandem mass spectrometry (LC–MS/MS) was used to analyze BLU-945 in in vivo PK studies (Eno et al. 2022). However, details of the method, including compound extraction from plasma and MS/MS parameters, have not been reported, and the method has not been validated. The objective of this study was to develop and validate a bioanalytical method for quantifying BLU-945 using LC–MS/MS. The developed method was used to investigate the in vitro metabolic stability and in vivo oral pharmacokinetic profile of the compound in rats. These data will be valuable for future preclinical and clinical evaluations of BLU-945.
Materials and methods
Materials
BLU-945 and ND207937 (internal standard, IS) were synthesized indoors. High-performance liquid chromatography (HPLC)-grade acetonitrile and methanol were purchased from Honeywell Burdick and Jackson (Ulsan, Korea). Formic acid, dimethyl sulfoxide (DMSO), PEG400 (Polyethylene glycol 400), and Kolliphor EL were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents were of the highest quality or HPLC grade.
Instrumentation and analytical conditions
The LC–MS/MS system used for the BLU-945 determination consisted of a 4500 Triple Quad mass spectrometer and an Exion LC AD instrument (Applied Biosystems, Framingham, MA, USA). Electrospray ionization was performed in positive mode. The separation of compounds was performed using a Synergi Polar-RP 80 Å column (150 × 2.0 mm, 4 µm; Phenomenex (Torrance, CA, USA)) with an isocratic mobile phase comprising 0.1% formic acid in distilled water and 0.1% formic acid in acetonitrile (30:70, v:v). The flow rate of the mobile phase was set at 0.35 mL/min for 3 min of running time, and the injection volume was 1 µL. The autosampler was kept at 4 °C, while the column was maintained at 25 °C. The mass spectrometry parameters, including ion spray voltage, the temperature of the ion source, curtain gas, nebulizing gas, turbo gas, and entrance potential, were set at 5500 V, 550 °C, 35 psi, 50 psi, 40 psi, and 10 V, respectively, for both BLU-945 and IS. Other parameters were optimized for each compound: declustering potentials of 12 and 36 V, collision energies of 47 and 49 V, and collision cell exit potentials of 18 and 34 V for BLU-945 and IS, respectively. The multiple reaction monitoring (MRM) transitions were m/z 557.3 → 423.3 for BLU-945 and m/z 543.2 → 423.2 for IS (ND207937). The analytical data were processed using the Analyst software version 1.6.2 (Applied Biosystems-SCIEX).
Sample preparation
A primary stock solution of BLU-945 was prepared at a concentration of 1 mg/mL in DMSO and serially diluted with methanol to obtain working standards and quality control (QC) samples. Two µL of working standard solution or QC sample was spiked to 18 µL of blank plasma, followed by the addition of 130 µL acetonitrile containing IS (200 ng/mL ND207937) for the protein precipitation. After vortexing for 5 min and centrifuging at 4 °C for 5 min at 14,000 rpm, the aliquots of the supernatant were injected into the LC–MS/MS system for BLU-945 analysis. The standard concentrations were 1, 2, 5, 10, 20, 50, 100, 200, and 1000 ng/mL. QC samples were prepared at concentrations of 1 (lower limit of quantitation, LLOQ), 3 (Low QC, LQC), 30 (Middle QC1, MQC1), 500 (Middle QC2, MQC2), and 800 ng/mL (High QC, HQC).
Assay validation
Full validation of the analytical method, including selectivity, linearity, accuracy, precision, matrix effect, recovery, process efficiency, stability, and dilution integrity, was performed on rat and mouse plasma according to the guidelines of the United States Food and Drug Administration and European Medicines Agency (US-FDA 2018; ICH 2019).
Selectivity was evaluated by analyzing plasma from six individual sources (mouse or rat), including blank plasma (double blank), blank plasma spiked with IS only (zero), blank plasma spiked with the BLU-945 working solution and IS, and plasma samples after oral administration. The chromatographic retention times of BLU-945 and the IS were compared across samples to confirm the absence of interference from endogenous substances or impurities in the plasma.
Calibration curves for BLU-945 in rat and mouse plasma were obtained by calculating the peak ratios of the analyte to IS versus the nominal concentrations of the standards. Calibration curves were fitted using linear least-squares regression with a weighting factor of 1/x2. Linearity was assessed using correlation coefficients (r), with values ≥ 0.990 considered acceptable (Yoon et al. 2020).
QC samples (1, 3, 30, 500 and 800 ng/mL) were used to assess the accuracy and precision of the method. Intraday accuracy and precision were estimated using six replicates on the same day, whereas interday values were calculated using experiments conducted over three days. Precision was expressed as the relative standard deviation (CV%), and accuracy was calculated as the difference between the calculated and nominal values divided by the nominal value (RE%). The acceptance criteria for precision and accuracy were CV ≤ ± 15% and RE ≤ ± 15%, respectively, except for the LLOQ, the acceptance value was ≤ 20%.
The matrix effect, extraction recovery, and process efficiency were investigated for BLU-945 at four QC levels (LQC, MQC1, MQC2, and HQC) and IS at a concentration of 200 ng/mL. Three sets of samples were prepared with four replicates for each concentration. Set 1 was defined as the response of the analyte from the extracted spiked plasma, whereas set 2 was the response of the analyte from the extracted blank plasma spiked with BLU-945 (post-extraction samples). Set 3 was defined as the response of the analyte to the neat standard solution. The matrix effect was calculated by dividing the mean peak area of set 2 by that of set 3. Extraction recovery was computed by dividing the data of set 1 by that of set 2, and process efficiency was assessed by comparing the data of sets 1 and 3 (Hyun et al. 2017).
The stock solution stability of BLU-945 (300 ng/mL) and the IS (200 ng/mL) was conducted by comparing the peak response from a freshly prepared solution in methanol and that of solutions subjected to short-term storage conditions (approximately 25 °C for 6 h) and long-term storage conditions (− 20 °C for 6 months). The stability of BLU-945 in rat and mouse plasma was evaluated by analyzing three replicates of LQC (3 ng/mL) and HQC (800 ng/mL). The short-term stability study was performed at room temperature (25 ℃) for 1 h before processing, while samples stored at − 20 °C for 8 weeks were analyzed to assess long-term stability. The processed sample stability was determined by analyzing samples in an autosampler at 4 °C for 12 h. Samples subjected to three freeze–thaw cycles were analyzed for freeze–thaw stability. All stability samples were evaluated in comparison to the freshly prepared ones, and a sample was considered stable with a difference in peak response of ≤ 15%.
Dilution integrity was investigated using dilution quality controls (DQC) at 4000 ng/mL (DQC1) and 5000 ng/mL (DQC2), which were further diluted five times with blank rat/mouse plasma to obtain samples at 800 ng/mL and 1000 ng/mL, respectively (Lee et al. 2022). Six replicates were tested in one run to determine accuracy and precision. The acceptance criteria for diluted samples were precision and accuracy within ± 15%.
In vitro metabolic stability
The liver microsomal and plasma stabilities of BLU-945 were investigated in humans, rats, and mice, as previously reported (Doan et al. 2020; Nguyen et al. 2022; Le et al. 2023). For liver microsomal stability, a mixture of liver microsomes (final protein concentration, 0.5 mg/mL) and 1 mM nicotinamide adenine dinucleotide phosphate in phosphate buffer (pH 7.4) was pre-incubated in a thermal shaker (200 rpm, 37 °C, 5 min). The reaction was initiated by adding 5 µL of BLU-945 (final concentration of 1 µM) to the pre-incubated mixtures. Aliquots of the reaction mixture were collected at 0, 15, 30, 60 and 120 min of incubation in a thermomixer. Verapamil and buspirone were used as controls in the microsomal stability assay. The plasma stability of BLU945 was investigated by spiking the compound into blank plasma (final concentration of 1 µM). Aliquots of plasma were collected at 0, 15, 30, 60, 120 and 240 min of incubation (37 °C, 200 rpm). Procaine was used as the control in the plasma stability assay. At each sampling point, 20 µL of the microsomal or plasma reaction mixture was pipetted into a microtube containing 130 µL of ice-cold IS in acetonitrile to terminate the reaction. The tubes were vortexed for 5 min and centrifuged at 14,000 rpm for 5 min. The supernatant was used to determine the amount of BLU-945 remaining using LC–MS/MS analysis. The area ratio of BLU-945 to IS was plotted against time to calculate the metabolic rate constant (ke). The elimination half-life (T1/2) was calculated using the following equation: T1/2 = ln2/ke (Maeng et al. 2019; Ji et al. 2020).
Application in pharmacokinetics studies
Male Sprague Dawley (SD) rats, aged 7 weeks and weighing 240–270 g, were purchased from Orient Bio Inc. (Seongnam, Republic of Korea) and allowed to acclimatize before the experiments. Animal studies were performed following the Guidelines for Animal Care and Use of Gachon University (approval number: GUI-2023-IA0040-00). The animals were provided ad libitum access to both food and water in plastic cages under the specific conditions: a 12/12 h light/dark cycle, room temperature maintained at 20–25 °C, and relative humidity set at 40–60%. Before oral administration, the rats were fasted for 14 h. Pharmacokinetic studies were performed as reported previously (Yoon et al. 2020; Vo et al. 2022; Le et al. 2023). Anesthesia was performed via intraperitoneal injection with Rompun® (Bayer AG, Leverkusen, Germany) and Zoletil® (Vibrac, Westlake, TX, USA). For blood sampling, the rat femoral arteries were catheterized using polyethylene tubes (Clay Adams, Parsippany, NJ, USA). BLU-945 was dissolved in 70% saline (2.5 mg/mL) and administered intravenously to rats at 5 mg/kg. The blood sampling times were 1, 2, 5, 15, 30, 60, 120, 240, 480, 720 and 1440 min. For oral administration, a vehicle mixture containing 5% DMSO, 20% PEG400, 5% Kolliphor EL, and 70% distilled water was prepared to dissolve the drug. The rats were orally administered doses of 5, 10 and 20 mg/kg. The blood sampling times were 15, 30, 60, 120, 240, 480, 720 and 1440 min. Samples above the upper limit of quantitation were diluted with blank plasma using the same dilution factor used in the dilution integrity test. Plasma obtained after centrifugation of blood (14,000 rpm, 15 min) was stored at − 20 °C for further LC–MS/MS analyses. For sample preparation, 130 µL of IS in acetonitrile was added to 20 µL of rat plasma for deproteinization. After vortexing and centrifugation, the supernatants were analyzed for drug quantification. The plasma peak concentration (Cmax) and time to reach Cmax (Tmax) after oral administration were determined directly from the plasma concentration–time profile. Other pharmacokinetic parameters, including terminal elimination half-life (T1/2), area under the time curve (AUC) from zero to the last time (AUClast) and to infinity (AUCinf), mean residence time (MRT), total clearance (CL), and apparent distribution volume at steady state (Vss), for each rat were analyzed by non-compartmental analysis using Phoenix® 8.2 software (Certara L.P., Princeton, NJ, USA).
Statistical analyses
Statistical significance was assessed using the analysis of variance (ANOVA) tests followed by Duncan’s test. A p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism v. 8.4.2 software (San Diego, CA, USA).
Results and discussion
LC–MS/MS method development
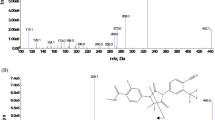
We have developed a highly sensitive LC–MS/MS method to quantify BLU-945 in rat and mouse plasma samples. This method employs a straightforward protein precipitation technique for compound extraction. BLU-945 and the IS were protonated in the positive electrospray ionization mode. The protonated molecular ions ([M+H]+) of the target and IS were detected at m/z 557.3 and 543.2, respectively. As shown in Fig. 1, the most prominent fragments in the product ion spectra were at m/z 423.2 for both BLU-945 and ND207937. These product ions were formed, likely due to the breaking of the azetidine ring of the precursor ions. Quantitative analyses were performed in MRM mode with m/z 557.43 → 423.2 and 543.23 → 423.4 for BLU-945 and ND207937, respectively. The operational parameters of the LC–MS/MS system, including the declustering potential, collision energy, and collision cell exit potential, were fine-tuned to maximize the responses of both BLU-945 and IS. The chromatographic separation was optimized by evaluating the mobile phase composition and column. The final LC conditions involved the use of a polar reversed-phase column with a typical mobile phase consisting of 0.1% formic acid in distilled water and 0.1% formic acid in acetonitrile (30:70, v/v).
Method validation
Under optimized LC–MS/MS conditions, successful separation of BLU-945 and the IS from the endogenous components of blank rat and mouse plasma was achieved (Figs. 2 and 3). No interference was observed in the blank rat and mouse plasma at the retention times of BLU-945 and the IS. In the case of the zero-calibrator, only the IS peak was observed. In the chromatogram of LLOQ, symmetrical peaks were observed at about 1.9 and 1.7 min for BLU-945 and IS, respectively. The retention times of BLU-945 and the IS were consistent and identical to those of rat plasma samples from the pharmacokinetic study. These results confirmed the specificity of the validated analysis method for BLU-945 in rat and mouse plasma samples.
The calibration curves of BLU-945 were established within the range of 1–1000 ng/mL in the rat and mouse plasma samples. Using a 1/x2 weighting factor, the resulting linear equations were determined to be y = 0.00140x + 0.00057 (r2 = 0.9973) and y = 0.00126x + 0.00077 (r2 = 0.9971) for the rat and mouse plasma, respectively, where y denotes the peak area ratio and x refers to the concentration ratio of the target and IS. In rat plasma, the accuracy and precision of all standards were 0.267–11.33% and 2.834–7.654%, respectively. Meanwhile, these values ranged from 0.600 to 9.867% for accuracy and from 1.868 to 7.399% for precision in mouse plasma. Thus, the peak ratios of BLU-945 and the IS were directly proportional to their plasma concentration ratios with acceptable accuracy and precision, indicating the linearity of the assay in the investigated range.
The intra- and inter-day accuracy and precision of the QC samples are presented in Table 1. These values for the rat plasma ranged from 0.438 to 7.900% for accuracy and 2.916–8.018% for precision. In mouse plasma, the intra- and inter-day accuracy and precision values ranged from 0.667 to 4.878% and 3.576 to 10.19%, respectively. The dilution integrity, as indicated by the accuracy and precision values of DQC after five dilutions in blank rat and mouse plasma, is also displayed in Table 1. In rat plasma, the accuracy and precision values were 3.217–5.650% and 1.230–2.260%, respectively. Meanwhile, in mouse plasma, the accuracy ranged from 4.967 to 8.483%, and the precision ranged from 1.267 to 1.813%. These data indicate the reliability of the validated method in analyzing samples exceeding the upper limit of quantification after dilution. Overall, these results satisfied all criteria, indicating the reliability of the developed method for BLU-945 analysis in rat and mouse plasma.
Matrix effect, extraction recovery, and process efficiency were investigated for the QC and IS samples (Table 2). The matrix effect values in rat and mouse plasma ranged from 100.2 to 106.9% and 101.8 to 109.3%, respectively. The CV% for the matrix effect met the required criterion of < 15% across all samples. These data suggest the absence of matrix effects for BLU-945 and the IS in both rat and mouse plasma. For the extraction recovery, values for BLU-945 and IS in rat and mouse plasma ranged from 94.48 to 102.0% and 85.91 to 95.05%, respectively. In addition, the overall process efficiency values in rat and mouse plasma were within the acceptable range, 97.22–103.1% and 92.71–100.4%, respectively. These results demonstrated that the protein precipitation method is suitable for compound extraction from rat and mouse plasma samples.
The short-term and long-term stabilities of stock solution were 93.03 ± 0.907% and 103.4 ± 2.030% for BLU-945 and 99.35 ± 0.812% and 100.6 ± 2.207% for the IS, respectively. The stability of BLU-945 at LQC and HQC levels in rat and mouse plasma is shown in Table 3. BLU-945 was stable after short- and long-term storage, with a bias in the peak area of stored samples and freshly prepared samples of < 15%. The stability of the compound in processed samples stored in the autosampler for 24 h or in QC samples subjected to three freeze–thaw cycles was within the acceptance criteria. These data show that BLU-945 remained stable under specific sample handling and storage conditions.
In vitro stability
BLU-945 was stable in human, rat, and mouse plasma after 4 h of incubation (Fig. 4A), suggesting that the drug was not metabolized by enzymatic degradation in the plasma of these species. In contrast, BLU-945 was unstable in a microsomal stability study. Following After 2-h incubation, the drug’s residual percentage (%remaining) gradually decreased to 42.60, 26.97, and 2.17% in human, rat, and mouse microsomes, respectively (Fig. 4B). Moreover, the calculated half-life, derived from the slopes of the corresponding curves were 110.0, 66.00, and 23.41 min for humans, rats, and mice, respectively. These data suggest that the primary metabolic route for BLU-945 is a phase I reaction involving hepatic enzymes, particularly CYP450, which exists in liver microsomal fractions.
In vivo pharmacokinetic studies
The established bioanalytical method was subsequently used in a rat pharmacokinetic study. The average plasma concentration–time profiles of BLU-945 following intravenous (5 mg/kg) and oral (5, 10, and 20 mg/kg) administration are shown in Fig. 5. The pharmacokinetic parameters obtained from these profiles are summarized in Table 4. The drug concentration was readily measurable in the rat plasma collected up to 24 h after drug administration. The ratios of AUClast to AUCinf were 99.2% for intravenous and 88.2–93.5% for oral administration, indicating that the sensitivity of the developed method is adequate to measure the exact terminal phase concentration of BLU-945. The drug was slowly eliminated from the rat plasma, with a relatively long half-life (4.6 h) and a moderate CL of 1506.6 mL/h/kg, compared to the regular hepatic blood flow rate (3300 mL/h/kg) (El-Kattan 2017). Moreover, it showed 2.584 h of MRT and moderate volume distribution (3751.3 mL/kg) (Smith et al. 2015).
After a single oral dose of 5, 10, and 20 mg/kg to the rats, BLU-945 rapidly reached maximum concentration (Cmax) in plasma at 1.4–2.8 h. Cmax and AUC elevated more than the increase in the administered dose. The dose-normalized AUCinf values differed among the three dose groups, suggesting that BLU-945 may follow nonlinear pharmacokinetics up to an oral dose of 20 mg/kg. When increasing the dose from 5 to 10 mg/kg, the AUCinf/Dose increased, but without reaching statistical significance (p > 0.05). However, the differences were significant when the dose was further increased to 20 mg/kg. In particular, the p-value was < 0.001 when comparing doses of 5 mg/kg and 20 mg/kg, indicating a highly significant difference. Similarly, the p-value for comparing doses of 10 mg/kg and 20 mg/kg was < 0.05, indicating a statistically significant difference. The absolute bioavailability increased with dose (55.91%, 70.35%, and 105.6% for 5, 10, and 20 mg/kg doses, respectively), indicating that BLU-945 may exhibit a high oral bioavailability in rats.
Overall, our findings suggest that the developed LC–MS/MS assay is appropriate for determining BLU-945 levels in pharmacokinetic studies.
Conclusion
This study describes a simple and sensitive LC–MS/MS method for quantifying BLU-945 in rat and mouse plasma. The developed bioanalytical method was fully validated according to the guidelines of the United States Food and Drug Administration and the European Medicines Agency and showed good reproducibility and reliability. BLU-945 is stable in human, rat, and mouse plasma. Moreover, it was moderately or completely metabolized in the human, rat, and mouse hepatic microsomal fractions after 2-h of incubation. Finally, the developed method was successfully applied to the pharmacokinetic study of BLU-945 to determine its drug concentration in rat plasma. The established analytical method and the outcomes of our research will be helpful for further investigations and clinical studies of BLU-945.
Availability of data and materials
The datasets generated or analyzed in this study are available from the corresponding author upon reasonable request.
Abbreviations
- AUCinf :
-
Area under the time curve from zero to infinity
- AUClast :
-
Area under the time curve from zero to the last time
- CL:
-
Total clearance
- DMSO:
-
Dimethyl sulfoxide
- EGFR:
-
Epidermal growth factor receptor
- HQC:
-
High-quality control
- IS:
-
Internal standard
- LC–MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- LLOQ:
-
Lower limit of quantitation
- LQC:
-
Low-quality control
- MQC:
-
Middle-quality control
- MRM:
-
Multiple reaction monitoring
- MRT:
-
Mean residence time
- NSCLC:
-
Non-small-cell lung cancer
- QC:
-
Quality control
- T1/2 :
-
Terminal elimination half-life
- TKI:
-
Tyrosine kinase inhibitor
- UHQC:
-
Ultra-high quality control
- Vss :
-
Apparent distribution volume at steady state
References
Belani CP. The role of irreversible EGFR inhibitors in the treatment of non-small cell lung cancer: overcoming resistance to reversible EGFR inhibitors. Cancer Invest. 2010;28:413–23.
Chang HC, Huang KT, Tseng CC, Chen YM, Lai CH, Chang YP, Chen YC, Chuang HY, Wang CC. Survival outcomes of east Asian patients with advanced non-small cell lung cancer treated with first-line EGFR tyrosine kinase inhibitors: a network meta-analysis of real-world evidence. Thoracic Cancer. 2023;14:3217–25.
Doan TNK, Vo D-K, Kim H, Balla A, Lee Y, Yoon I-S, Maeng H-J. Differential effects of 1α,25-dihydroxyvitamin D3 on the expressions and functions of hepatic CYP and UGT enzymes and its pharmacokinetic consequences in vivo. Pharmaceutics. 2020;12:1129.
Duggirala KB, Lee Y, Lee K. Chronicles of EGFR tyrosine kinase inhibitors: targeting EGFR C797S containing triple mutations. Biomol Ther. 2022;30:19–27.
El-Kattan AF. in Oral bioavailability assessment: basics and strategies for drug discovery and development Wiley series on pharmaceutical science and biotechnology: practices, applications, and methods. Wiley; 2017. pp. 1–34.
Eno MS, Brubaker JD, Campbell JE, De Savi C, Guzi TJ, Williams BD, Wilson D, Wilson K, Brooijmans N, Kim J, Özen A, Perola E, Hsieh J, Brown V, Fetalvero K, Garner A, Zhang Z, Stevison F, Woessner R, Singh J, Timsit Y, Kinkema C, Medendorp C, Lee C, Albayya F, Zalutskaya A, Schalm S, Dineen TA. Discovery of BLU-945, a reversible, potent, and wild-type-sparing next-generation EGFR mutant inhibitor for treatment-resistant non-small-cell lung cancer. J Med Chem. 2022;65:9662–77.
Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28:S24–31.
Hyun H-C, Jeong J-W, Kim H-R, Oh J-H, Lee J-H, Choi S, Kim Y-S, Koo T-S. Development and validation of a liquid chromatography–tandem mass spectrometry method for the assay of tafamidis in rat plasma: application to a pharmacokinetic study in rats. J Pharm Biomed Anal. 2017;137:90–5.
ICH (2019) ICH guideline M10 on bioanalytical method validation and study sample analysis https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (European Medicines Agency, International Council for Harmonization). Accessed 1 Dec 2023.
Ji Y-G, Shin Y-M, Jeong J-W, Choi H-I, Lee S-W, Lee J-H, Lee K-R, Koo T-S. Determination of motolimod concentration in rat plasma by liquid chromatography-tandem mass spectrometry and its application in a pharmacokinetic study. J Pharm Biomed Anal. 2020;179:112987.
Lategahn J, Keul M, Klövekorn P, Tumbrink HL, Niggenaber J, Müller MP, Hodson L, Flaßhoff M, Hardick J, Grabe T, Engel J, Schultz-Fademrecht C, Baumann M, Ketzer J, Mühlenberg T, Hiller W, Günther G, Unger A, Müller H, Heimsoeth A, Golz C, Blank-Landeshammer B, Kollipara L, Zahedi RP, Strohmann C, Hengstler JG, WaL VO, Bauer S, Rauh D. Inhibition of osimertinib-resistant epidermal growth factor receptor EGFR-T790M/C797S. Chem Sci. 2019;10:10789–801.
Le Q-D, Duong V-A, Lee S-H, Nguyen T-T-L, Maeng H-J. Bioanalytical method validation, biopharmaceutical and pharmacokinetic evaluation of GSK-650394, a serum- and glucocorticoid-regulated kinase 1 inhibitor. Arab J Chem. 2023;16:104462.
Lee S, Kim M-S, Jeong J-W, Chae J-W, Koo T-S, Maeng H-J, Chung S-J, Lee K-R, Chae Y-J. Bioanalysis of alpelisib using liquid chromatography–tandem mass spectrometry and application to pharmacokinetic study. J Anal Sci Technol. 2022;13:31.
Li L, Huang Q, Sun J, Yan F, Wei W, Li Z, Liu L, Deng J. Efficacy and safety of osimertinib for patients with EGFR-mutated NSCLC: a systematic review and meta-analysis of randomized controlled studies. Acta Oncol (Stockholm, Sweden). 2022;61:1347–53.
Li MSC, Mok KKS, Mok TSK. Developments in targeted therapy & immunotherapy-how non-small cell lung cancer management will change in the next decade: a narrative review. Ann Transl Med. 2023a;11:358.
Li Y, Mao T, Wang J, Zheng H, Hu Z, Cao P, Yang S, Zhu L, Guo S, Zhao X, Tian Y, Shen H, Lin F. Toward the next generation EGFR inhibitors: an overview of osimertinib resistance mediated by EGFR mutations in non-small cell lung cancer. Cell Commun Signal. 2023b;21:71.
Maeng H-J, Doan TNK, Yoon I-S. Differential regulation of intestinal and hepatic CYP3A by 1α,25-dihydroxyvitamin D3: effects on in vivo oral absorption and disposition of buspirone in rats. Drug Dev Res. 2019;80:333–42.
Mansour MA, Aboulmagd AM, Abbas SH, Abdel-Rahman HM, Abdel-Aziz M. Insights into fourth generation selective inhibitors of (C797S) EGFR mutation combating non-small cell lung cancer resistance: a critical review. RSC Adv. 2023;13:18825–53.
Midha A, Dearden S, Mccormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5:2892–911.
Nguyen T-T-L, Kim JW, Choi H-I, Maeng H-J, Koo T-S. Development of an LC–MS/MS method for ARV-110, a PROTAC molecule, and applications to pharmacokinetic studies. Molecules. 2022;27:1977.
Santoni-Rugiu E, Melchior LC, Urbanska EM, Jakobsen JN, Stricker K, Grauslund M, Sørensen JB. Intrinsic resistance to EGFR-tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: differences and similarities with acquired resistance. Cancers (Basel). 2019;11:923.
Schalm SS, Dineen T, Lim SM, Park CW, Hsieh J, Woessner R, Zhang Z, Wilson K, Eno M, Wilson D, Williams B, Campbell J, De Savi C, Stevison F, Utt C, Guzi T, Dorsch M, Hoeflich K, Cho BC. 384P BLU-945, a highly potent and selective 4th generation EGFR TKI for the treatment of EGFR T790M/C797S resistant NSCLC. Ann Oncol. 2020a;31:S1391.
Schalm SS, Dineen T, Lim SM, Park CW, Hsieh J, Woessner R, Zhang Z, Wilson K, Eno M, Wilson D, Williams B, Campbell J, De Savi C, Stevison F, Utt C, Guzi T, Dorsch M, Hoeflich K, Cho BCC. 1296P BLU-945, a highly potent and selective 4th generation EGFR TKI for the treatment of EGFR T790M/C797S resistant NSCLC. Ann Oncol. 2020b;31:S839.
Smith DA, Beaumont K, Maurer TS, Di L. Volume of distribution in drug design. J Med Chem. 2015;58:5691–8.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J Clin. 2021;71:209–49.
US-FDA (2018) Guidance for Industry: bioanalytical method validation https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf. Accessed 1 Dec 2023.
Vo D-K, Nguyen T-T-L, Maeng H-J. Effects of 1α,25-dihydroxyvitamin D3 on the pharmacokinetics and biodistribution of ergothioneine, an endogenous organic cation/carnitine transporter 1 substrate, in rats. J Pharm Investig. 2022;52:341–51.
Yoon J-H, Nguyen T-T-L, Duong V-A, Chun K-H, Maeng H-J. Determination of KD025 (SLx-2119), a selective ROCK2 inhibitor, in rat plasma by high-performance liquid chromatography–tandem mass spectrometry and its pharmacokinetic application. Molecules. 2020;25:1369.
Acknowledgements
Not applicable.
Funding
This work was supported by Grants from the National Research Foundation of Korea (NRF) Funded by the Korean government Ministry of Education (2020R1A6A1A03043708) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) Funded by the Ministry of Science, ICT, and Future Planning (NRF-2021R1F1A1060378).
Author information
Authors and Affiliations
Contributions
J-HO, T-T-LN and H-JM conceived and designed the study. J-HO performed the experiments and analyzed the data. J-HO and T-TLN performed data curation and visualization; J-HO, T-T-LN, and H-JM wrote the manuscript; H-JM reviewed and edited the manuscript. H-JM supervised this study. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oh, JH., Nguyen, TTL. & Maeng, HJ. Development and validation of an LC–MS/MS method for the determination of BLU-945, a fourth-generation EGFR tyrosine kinase inhibitor, in rat and mouse plasma: application to a pharmacokinetic study in rats. J Anal Sci Technol 15, 37 (2024). https://doi.org/10.1186/s40543-024-00452-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40543-024-00452-2