Abstract
Background
Paper mulberry has been considered as a high-quality protein feedstuff to cope with the shortage of feed and the development of livestock. In addition, the features of high moisture and low water-soluble carbohydrate concentration in fresh paper mulberry make it difficult to ensile. Therefore, it is important to find an optimal way to improve the paper mulberry silage quality. In this study, we aimed to investigate the application of Lactobacillus plantarum (LP) and wheat bran (WB) on the fermentation characteristics, chemical composition and microbial community of paper mulberry silage.
Results
The objective of this study was to evaluate the effects of Lactobacillus plantarum and wheat bran alone or combination (LP + WB) addition on the fermentation quality and bacterial community of paper mulberry silage. After 60 days of ensiling, the employed three treatments had higher crude protein contents compared with control (P < 0.05). More importantly, WB and LP + WB treatments significantly reduced the pH value and NH3-N concentration, and increased lactic acid content (P < 0.05). Microbial analysis indicated that the bacterial community in WB and LP + WB treatments showed distinct difference with LP and control. Lactobacillus was the dominant genera in all treatments. However, at the species level, Lactobacillus farciminis became the most dominant bacteria in control and LP treatments while the dominant bacteria in WB and LP + WB were Lactobacillus brevis and Lactobacillus farciminis. In addition, Lactobacillus brevis was positively correlated to crude protein and lactic acid and negatively correlated to pH and NH3-N. Overall, this study revealed that ensiling paper mulberry with WB or combination LP could improve silage quality through altering microbial community, which provided a practical approach for enhancing paper mulberry silage quality.
Conclusion
Wheat bran and combinations of Lactobacillus plantarum and wheat bran additions could reduce pH, NH3-N and increase LA content. The application of WB and LP + WB shifted the dominant bacteria species to Lactobacillus brevis. In summary, the addition of wheat bran and combinations of lactic acid bacteria and wheat bran were effective ways to enhance paper mulberry silage fermentation.
Graphical Abstract

Similar content being viewed by others
Introduction
Paper mulberry (Broussonetia papyrifera) has been considered as a pioneer source to cope with the shortage of feed and the development of livestock [1]. The crude protein content of whole-plant paper mulberry could be equivalent to alfalfa [2]. Besides, paper mulberry contains abundant biologically active compounds, amino acids, vitamins, digestible crude fiber and mineral elements [3, 4]. Due to these excellent features, paper mulberry can be a new type of high-quality protein feedstuff for ruminants.
Paper mulberry usually grows in the high humidity and rainy area in south of China, which is not conducive to production and storage of hay. Ensiling is a reliable way to effectively preserve fresh forage due to its good palatability and low nutrient loss [5, 6]. Ensiling has been proved to be an efficient method for preserving paper mulberry [7]. However, it has been found that high temperature and humidity conditions lead to the propagation of harmful microorganisms, which subsequently leads to the deterioration of silage. In addition, the features of high moisture and low water-soluble carbohydrate (WSC) concentration in fresh paper mulberry make it difficult to ensile, revealed by unpleasant smelling and low fermentation quality [8,9,10,11]. Therefore, it is critical to find the optimal way to improve the paper mulberry silage quality.
In China, more than 20 million tons of wheat bran (WB) are produced annually [12]. As a traditional animal feed, WB has a high dry matter content, rich in carbohydrates and other nutrients [13]. Adding WB is an effective method to reduce feed moisture content. During ensiling, WB could provide fermentation substrate for the growth of lactic acid bacteria (LAB) and accelerate the succession process to become dominant bacteria. The exogenous LAB has the characteristics of high utilization rate of sugar, fast acid production and pH reduction [14], which can speed up the ensiling process and inhibit the growth of harmful microorganisms [15]. In this regard, adding LAB inoculant and/or WSC substrates might be critical for enhancing paper mulberry silage quality.
The purpose of our research was to evaluate the application of LAB and WB on the fermentation quality of paper mulberry silage. We investigated the fermentation characteristics, chemical composition and microbial community of paper mulberry silage.
Materials and methods
Silage preparation
The experiment was conducted on 16 September, 2020 at Shandong hengda coal industry co. LTD (E116.87°, N35.88°) in Taian, Shandong, China. The industry is located in a monsoon climate of medium latitudes, with a mean annual temperature of 15.5 °C, relative humidity of 81% and annual rainfall of 1250–1440 mm.
Paper mulberry (Broussonetia papyrifera L.-Zhongke No.1), cultivated at the experimental station, was approximately 1.2 m in height and harvested from May to October in a year. Paper mulberry was harvested in the third cutting and grew approximately 4 months. The whole paper mulberry was obtained at the second round of cutting, leaving stubble of 15 cm. Before ensiling, the harvested paper mulberry was directly chopped into 1–2 cm pieces and then mixed manually.
The L. plantarum strain was isolated from paper mulberry silage in our laboratory, named of Forage Production and Processing Lab. The bacterial inoculants were added at a level of 106 cfu g−1 of fresh material (FM). Wheat bran (WB, applied at 30% rate of FM) was purchased from China Oil and Foodstuffs Corporation Organization, Zhengzhou Haijia Food Co. Ltd.
A single factor completely randomized design was used in this experiment. The silage treatments were as followed: (i) no additives (control group, CK); (ii) application of L. plantarum (LP silage); (iii) application of wheat bran additives (WB silage); (iv) combination of L. plantarum and wheat bran (LP + WB silage). Then, 500 g of materials with different additives were mixed homogenously and packed into polyethylene bags (30 cm × 20 cm). The silage bags were vacuum-sealed and stored at room temperature (20–30 °C) for 60d. After 60d of ensiling, three bags for each treatment were opened to analyze the fermentation characteristics, chemical composition and bacterial communities [16].
Fermentation quality, microbial population and chemical composition analysis
Twenty grams of samples were homogenized in 180 mL distilled water and stored at 4 °C for at least 4 h and then filtered by qualitative filter paper [17]. The pH, lactic acid (LA), acetic acid (AA), propionic acid (PA), butyric acid (BA) and NH3-N were immediately measured with filtrate. The pH was measured with a glass pH meter (PHS-3C, INESA Scientific Instrument, Shanghai, China). The NH3-N content was accessed by the phenol–sodium hypochlorite method, as described by Guan et al. [18]. The contents of organic acid (LA, AA, PA and BA) were measured by high-performance liquid chromatography method (column: Shodex RS Pak KC-811; detector: DAD, 210 nm, SPD-20A; Shimadzu Co., Ltd., Kyoto, Japan; 3 mmol L−1 HClO4) [19]. The microbial count of all samples was determined by the plate culture method. The filtrate was serially diluted from 10–1 to 10–6 and each diluted suspension was spread. The populations of LAB, coliform bacteria, yeasts and molds were determined on de Man Rogosa Sharpe (MRS) agar (AOBOX Biotechnology Co. Ltd., Beijing China), Blue Light (BL) agar and Rose Bengal (RB) agar, respectively, after incubation at 30 °C for 48–72 h [20].
On days 0 and 60, a total of 42 fresh and silage of paper mulberry samples were dried to determine the DM concentration in an air oven at 65 °C for at least 48 h. All samples were milled to pass through a 1.0 mm size of the sieve for chemical composition analysis. Ether extract (EE) were estimate by AOAC [21]. The content of total nitrogen (TN) was determined by the Kjeldahl procedure and the crude protein (CP) content was calculated by multiplying TN by 6.25 [21]. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were measured by the method of Van Soest et al. [22]. The content of WSC was analyzed by the methodology of Murphy et al.[23].
Microbial diversity analysis
The method of DNA extraction of each sample was according to Liu et al. [24] with slight modifications. After monitored the quality and concentration of DNA, the full-length 16S ribosomal RNA (rRNA) gene was amplified with the polymerase chain reaction (PCR), with the primer 27F (5′-AGRGTTTGATYNTGGCTCAG-3′) and the reverse primer 1492R (5′-TASGGHTACCTTGTTASGACTT-3′). The PCRs program was: 95 °C for 2 min, 25 cycles at 98 °C for 10 s, 55 °C for 30 s, 72 °C for 90 s and a final extension at 72 °C for 2 min [25].
After extraction, purification and qualification, the PCR products were sequenced on PacBio Sequel platform (Biomarker Technologies, Beijing, China). The raw sequences were selected by Single Molecule Real Time (SMRT) according to Li et al. [26]. We clustered the unique sequence set into operational taxonomic units (OTUs) based on a 0.97 threshold identity using UCLUST [27]. The high-quality sequences were annotated based on Silva132 database [28].
Statistical analysis
Analysis of variance (ANOVA) was performed using generalized linear modeling in the Statistical Package for the Social Sciences (SPSS version 21.0, SPSS Inc., Chicago, IL, USA) to examine the chemical composition, fermentation quality and microbial counts data among samples. Duncan’s multiple range methods were used to determine the significant difference between means with three replicates per treatment. The significance was declared at P < 0.05.
After the OTUs analysis, QIIME software (version 2) [29] was operate to calculated alpha diversity indices. Principal component analysis (PCA) was performed to access the differences in community composition and structure of microbiota, basing on the beta-diversity analysis. Heatmap analysis was operated to analysis the relationship between pH, AA, NH3-N, CP, WSC, LA. LA/AA and microbial community of paper mulberry silage using R-based statistics tool. Linear discriminant analysis effect size (LEfSe) analyses were conducted using a free online platform [30].
Accession number
The raw sequencing data including all samples have been deposited into the NCBI Sequence Read Archive (SRA) database under the Accession Number of PRJNA972621.
Results
Chemical and microbial composition of raw materials
The chemical characteristics and microbial composition of fresh paper mulberry and wheat bran are listed in Table 1. The DM contents of paper mulberry and wheat bran were 259.29 and 917.70 g kg−1 FM, respectively. In addition, the CP, NDF and ADF of raw paper mulberry were 183.84 g kg−1 DM, 442.52 g kg−1 DM and 251.03 g kg−1 DM, respectively. Paper mulberry had low WSC (25.79 g kg−1 DM) and LAB (5.09 log10 cfu g−1 FM).
Chemical composition of paper mulberry silage
The chemical composition of paper mulberry silage is shown in Table 2. There was no difference in NDF contents between control and treatments (P > 0.05). The WB and LP + WB treatments significantly increased (P < 0.05) DM and WSC contents and decreased (P < 0.05) ADF and EE contents compared with control and LP treatments. The CP content of treatments was significantly higher than control while the CP in LP and WB treatments was highest (Table 2).
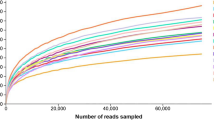
The OTU information and Shannon index of paper mulberry added with lactic acid bacteria and wheat bran after 60 days of ensiling. a OTU; b Shannon index. CK, no additives; LP, Lactobacillus plantarum additives; WB, 30% wheat bran additives; LP + WB: Lactobacillus plantarum additives plus 30% wheat bran; p < 0.01 means obviously difference significant, 0.01 < p < 0.05 means difference significant; p > 0.05 means no significance
Dynamic changes of before and after ensiling
The dynamic change of chemical composition paper mulberry silage at the before and after ensiling process is shown in Table 3. The dynamic change of ADF content before and after ensiling in WB and LP + WB treatments was significantly higher (P < 0.05) than control and LP treatments. The WB and LP + WB treatments had lower (P < 0.05) DM, CP and WSC losses compared with control. No difference in NDF content was observed among treatments (P > 0.05).
Fermentation characteristics and microbial population of silage
As shown in Table 4, the treatments had a significant influence on pH, NH3-N, LA, AA, PA, BA contents and LAB counts (P < 0.05). The WB and LP + WB treatments significantly increased (P < 0.05) LA content and decreased pH, the contents of NH3-N, AA, BA and the amount of LAB compared with control and LP treatments. There was no difference in yeast, molds and coliform between control and treatments (P > 0.05).
Bacterial community indices of paper mulberry silage
The coverage values of all samples were beyond 0.995, indicating the credible analysis for sequencing depth of the microbial composition of paper mulberry silage. A total of 155 OTUs were clustered at 97% sequenced similarity. Shannon index ranged from 1.11 to 2.43. Compared with control, inoculations with treatments significantly increased (P < 0.05) Shannon, while the highest was 2.43 of LP + WB treatment (Fig.1).
As shown in Fig. 2, the variance of bacterial community was observed by principal component analysis (PCA) on the OTUs levels. The bacterial community in control were clearly separated from the other groups, which suggested that bacterial community changed significantly after the addition of LP and WB and combinations of LP and WB.
The bacterial community at genus (A) and species (B) levels are shown in Fig. 3, using a threshold of > 0.1% of total. At the genus levels, Lactobacillus was the dominant genus in all silages, and the proportion of Lactobacillus in LP, WB and LP + WB were 84.45%, 89.27% and 86.10%, respectively, which were lower than control (P < 0.05). In addition, the proportion of Weissella in LP was higher than other treatments.
At the species levels, L. farciminis and L. ginsenosidimutans were the dominant species in control. In LP treatment, L. farciminis, and W. cibaria were the representative bacterium. Interestingly, treatments with wheat bran shifted the dominant bacteria to L. brevis and L. farciminis. The relative abundance of L. brevis and L. farciminis in WB treatment were 57.55% and 30.03%, while the relative abundance in LP + WB treatment were 47.75% and 36.97%, respectively. L. brevis can be used as a biomarker in WB treatment by the LEfSe algorithm (Fig. 4). These findings clearly indicated lactic acid bacteria and wheat bran influenced bacterial community significantly.
Linear discriminant analysis effect analysis of bacterial community (LDA > 3.0) at species level in paper mulberry silages compared by different additives. CK, no additives; LP, Lactobacillus plantarum additives; WB, 30% wheat bran additives; LP + WB: Lactobacillus plantarum additives plus 30% wheat bran
Correlation between bacterial community and fermentation products
Lactobacillus brevis was negatively correlated to pH (r = − 0.91), AA content (r = − 0.86) and NH3-N (r = − 0.69) (P < 0.05), but positively correlated to CP content (r = 0.68), LA content (r = 0.89), WSC content (r = 0.64) and ratio of LA and AA (r = 0.86), respectively (P < 0.05). In contract, L. farciminis, L. ginsenosidimutans, L. maninotivorans, and L. dextrinicus were positively correlated to pH, AA content and AN/TN, but were negatively correlated to LA, WSC content and ratio of LA and AA (P < 0.05).
Discussion
Previous studies have confirmed that paper mulberry is difficult to be well ensiled due to its low WSC and high moisture content [31]. Additives, such as LAB and bran, are often widely utilized to effectively improve the silage fermentation quality [32,33,34,35]. WB and LP + WB treatments contained lower AA and NH3-N contents (P < 0.05), indicating less protein degradation in silage with WB and LP + WB. In addition, L. brevis, with high acid production capacity, is the dominant in WB and LP + WB treatment. Therefore, these results verified that ensiling with WB and LP + WB could enhance paper mulberry silage quality (Table 4, Fig. 3B and Fig. 5).
Dendro-heatmap visualized interrelationship between bacterial community and fermentation products at species levels in paper mulberry silage. Corresponding value of middle heat map is Spearman correlation coefficient r, which ranges between 1 and − 1, r < 0 indicates a negative correlation (blue) and r > 0 indicates a positive correlation (red). * significance at P < 0.05; ** significance at P < 0.01; *** significance at P < 0.001. CK, no additives; LP, Lactobacillus plantarum additives; WB, 30% wheat bran additives; LP + WB: Lactobacillus plantarum additives plus 30% wheat bran
In this experiment, the CP content of fresh paper mulberry was much lower than that determined by Dong et al. [36], but much higher than that in our previous study [37]. ADF and NDF contents of fresh paper mulberry were lower than that reported by Li et al. [38], but comparable to those reported by Li et al. [19]. These discrepancies might be due to the factors such as growth period, harvest time, fertilization and climate conditions [39, 40]. The DM content of paper mulberry was 259.29 g kg−1 FM, which was not conducive to well-quality fermentation [41]. Higher WSC (> 50 g kg−1 DM) and LAB (> 5 log10 cfu g−1 FM) of raw materials are necessary to obtain well-preserved silage [42]. In this study, WSC content and LAB number of fresh paper mulberry were below the minimal requirement, which was not conducive to silage fermentation [10].
All treatments showed higher CP content and lower CP loss compared with control, which could be due to LAB and WB limiting the growth of undesirable microbes [43]. The WB and LP + WB treatments had higher ADF content changes rate than control and LP treatment, which could be due to acidolysis during ensiling process [44]. The WB and LP + WB treatments showed lower ADF content than control and LP treatments, which was due to lower ADF in wheat bran. After 60 days of ensiling, the WSC contents in silages were lower than that in fresh paper mulberry, which was possible due to LAB converting WSC into organic acids and ethanol during ensiling process. The lower WSC content changes in WB and LP + WB treatment than that control and LP treatment could be due to the different fermentation pattern, but the exact reason needs to be further classified in the future [45].
The low pH value around 4.2, a critical criterion for well-preserved silage, was important to inhibit harmful Clostridia [46]. In this experiment, the pH of WB and LP + WB treatment was around 4.2 while the pH of LP treatment was beyond 4.2, indicating that the effect of wheat bran was better than L. plantarum in regulating fermentation quality of paper mulberry silage. This might be because high moisture and low WSC are not conducive to propagation and role of LAB [47]. Generally, lactic acid bacteria are often employed to convert WSC into LA to reduce pH and inhibit harmful microorganisms [48]. In our study, silage treated with LP showed lower LA content and higher NH3-N, AA and BA content, indicating that low WSC in paper mulberry cannot provide a good growth environment for LAB. As a typical indicator of proteolysis [49], silage treated with WB and LP + WB contained lower NH3-N content than LP treatment, indicating less proteolysis occurred with WB and LP + WB. The above results indicated that it is important to regulate the moisture content promote fermentation quality of paper mulberry.
Lactobacillus and Weissella are beneficial to improve silage quality [50]. The dominant genus involved in all treatments were Lactobacillus and Weissella. Lactobacillus is known as a predominant microbial species in high-quality silage [51], which is consistent with our experimental results. LAB plays an important role in producing LA and reducing pH to inhibit harmful bacteria, while as a hetero-fermentative bacteria with weak acid-producing ability [52], Weissella is difficult to become the dominant genus in silage. Interestingly, there was a sharp increase in the relative abundance of Weissella species only in LP treatment. Similar result was also reported by Guo et al. [30]. The high abundance of Weissella in LP treatment would explain its relatively poor fermentation quality compared with WB and LP + WB treatments.
At species level, the addition of L. plantarum did not significantly increase the relative abundance of L. plantarum but increased the relative abundance of L. farciminis and W. cibaria in LP treatment, which might be due to incompatibility between the inoculants and plant materials [53]. L. brevis and L. farciminis were the dominant species in WB and LP + WB treatments while L. farciminis was the dominant species in control and LP treatment. In addition, L. ginsenosidimutans and W. cibaria were the secondary dominant species in control and LP treatment, respectively. Generally, desirable LAB, such as L. plantarum, L. farciminis, L. buchneri and L. brevis, play an essential role in increasing LA content and reducing pH value [54]. In addition, the homo-fermentative L. plantarum and L. farciminis were overtaken by hetero-fermentative L. buchneri and L. brevis in later stage of high-quality silage [55, 56]. At the later of stage of fermentation, lower WSC content was insufficient to support the fermentation of homo-fermentative LAB while hetero-fermentative LAB use lactic acid for growth, resulting in fermentation mode changes [57]. In addition, the anaerobic conditions promoted the growth of LAB that produced organic acids inhibiting other bacteria [58, 59]. However, the role of L. farciminis in silage fermentation remains unclear. In this study, L. brevis was positively relative to LA and negatively to pH, indicating that the production of LA was largely attributable to L. brevis. Similar results have been reported by a previous study [60]. In this study, L. ginsenosidimutans in control could be isolated from fermented foods, such as Korean fermented pickle, and used as probiotic strains to biotransform ginsenosides and improve the taste of functional foods [61]. However, the role of L. ginsenosidimutans in silage needs further study.
Conclusion
Paper mulberry silages with the addition of inoculant had lower NDF, ADF and WSC content than fresh paper mulberry. The addition of LP had no impact in improving the fermentation quality of paper mulberry silage. However, wheat bran (WB) and its combination with L. plantarum (LP + WB) additions could reduce pH, NH3-N and increase LA content. The application of WB and LP + WB shifted the dominant bacteria species to L. brevis. Thus, the addition of wheat bran or combined with lactic acid bacteria were effective ways to enhance paper mulberry silage fermentation.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Abbreviations
- AA:
-
Acetic acid
- ADF:
-
Acid detergent fiber
- ANOVA:
-
Analysis of variance
- BA:
-
Butyric acid
- BL:
-
Blue light
- cfu:
-
Colony forming unit
- CP:
-
Crude protein
- DM:
-
Dry matter
- EE:
-
Ether extract
- FM:
-
Fresh matter
- HPLC:
-
High-performance liquid chromatography
- LA:
-
Lactic acid
- LAB:
-
Lactic acid bacteria
- LEfSe:
-
Linear discriminant analysis effect size
- MRS:
-
De Man Rogosa Sharpe
- NDF:
-
Neutral detergent fiber
- NH3-N:
-
Ammonia nitrogen
- OTU:
-
Operational taxonomic units
- PA:
-
Propionic acid
- PacBio:
-
Pacific Biosciences
- PCA:
-
Principal component analysis
- PCR:
-
Polymerase chain reaction
- RB:
-
Rose Bengal
- SEM:
-
Standard error of the mean
- SMRT:
-
Single-molecule real-time sequencing
- SPSS:
-
Statistical Package for the Social Sciences
- SRA:
-
Sequence Read Archive
- TN:
-
Total nitrogen
- WB:
-
Wheat bran
- WSC:
-
Water-soluble carbohydrates
- LP:
-
Lactobacillus plantarum
References
Zhang YC, Li DX, Wang XK, Lin YL, Zhang Q, Chen XY, Yang FY. Fermentation dynamics and diversity of bacterial community in four typical woody forages. Ann Microbiol. 2019;69(3):233–40.
Du Z, Sun L, Lin Y, Yang F, Cai Y. The use of PacBio SMRT technology to explore the microbial network and fermentation characteristics of woody silage prepared with exogenous carbohydrate additives. J Appl Microbiol. 2021;131(5):2193–211.
Peng X, Liu H, Chen P, Tang F, Hu Y, Wang F, Pi Z, Zhao M, Chen N, Chen H, Zhang X, Yan X, Liu M, Fu X, Zhao G, Yao P, Wang L, Dai H, Li X, Xiong W, Xu W, Zheng H, Yu H, Shen S. A chromosome-scale genome assembly of paper mulberry (Broussonetia papyrifera) provides new insights into its forage and papermaking usage. Mol Plant. 2019;12(5):661–77.
Xiong Y, Wang X, Li X, Guo L, Yang F, Ni K. Exploring the rumen microbiota of Hu lambs in response to diet with paper mulberry. Appl Microbiol Biotechnol. 2023;107:4961–71.
Li D, Ni K, Zhang Y, Lin Y, Yang F. Fermentation characteristics, chemical composition and microbial community of tropical forage silage under different temperatures. Asian Australas J Anim. 2019;32(5):665–74.
Herrmann C, Idler C, Heiermann M. Improving aerobic stability and biogas production of maize silage using silage additives. Bioresource Technol. 2015;197:393–403.
Guo L, Wang Y, Wang X, Li X, Xiong Y, Lin Y, Ni K, Yang F. Dynamic fermentation quality and bacterial community structure of paper mulberry silage from three regions of China. Chem Biol Technol Ag. 2023. https://doi.org/10.1186/s40538-023-00414-7.
Borreani G, Tabacco E, Schmidt RJ, Holmes BJ, Muck RE. Silage review: factors affecting dry matter and quality losses in silages. J Dairy Sci. 2018;101(5):3952–79.
Cheng Q, Chen Y, Bai S, Chen L, You M, Zhang K, Li P, Chen C. Study on the bacterial community structure and fermentation characteristics of fresh and ensiled paper mulberry. Anim Sci J. 2021. https://doi.org/10.1111/asj.13656
Ni K, Zhao J, Zhu B, Su R, Pan Y, Ma J, Zhou G, Tao Y, Liu X, Zhong J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresource Technol. 2018;265:563–7.
Wang W, Nie Y, Tian H, Quan X, Li J, Shan Q, Li H, Cai Y, Ning S, Santos BR, He W. Microbial community, co-occurrence network relationship and fermentation lignocellulose characteristics of Broussonetia papyrifera ensiled with wheat bran. Microorganisms. 2022. https://doi.org/10.3390/microorganisms10102015
Wu X, Li F, Wu W. Effects of rice bran rancidity on the oxidation and structural characteristics of rice bran protein. LWT-Food Sci Tehnol. 2020;120: 108943.
Apprich S, Tirpanalan Ö, Hell J, Reisinger M, Böhmdorfer S, Siebenhandl-Ehn S, Novalin S, Kneifel W. Wheat bran-based biorefinery 2: valorization of products. LWT Food Sci Technol. 2014;56(2):222–31.
Yang F, Wang Y, Zhao S, Wang Y. Lactobacillus plantarum inoculants delay spoilage of high moisture alfalfa silages by regulating bacterial community composition. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.01989
Oliveira AS, Weinberg ZG, Ogunade IM, Cervantes AAP, Arriola KG, Jiang Y, Kim D, Li X, Gonçalves MCM, Vyas D, Adesogan AT. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J Dairy Sci. 2017;100(6):4587–603.
Ding Z, Bai J, Xu D, Li F, Zhang Y, Guo X. Microbial community dynamics and natural fermentation profiles of ensiled alpine grass Elymus nutans prepared from different regions of the Qinghai-Tibetan plateau. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.00855
Tian J, Li Z, Yu Z, Zhang Q, Li X. Interactive effect of inoculant and dried jujube powder on the fermentation quality and nitrogen fraction of alfalfa silage. Anim Sci J. 2017;88(4):633–42.
Guan H, Yan Y, Li X, Li X, Shuai Y, Feng G, Ran Q, Cai Y, Li Y, Zhang X. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour Technol. 2018;265:282–90.
Li X, Chen F, Xu J, Guo L, Xiong Y, Lin Y, Ni K, Yang F. Exploring the addition of herbal residues on fermentation quality, bacterial communities, and ruminal greenhouse gas emissions of paper mulberry silage. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2021.820011.
He L, Lv H, Wang C, Zhou W, Pian R, Zhang Q, Chen X. Dynamics of fermentation quality, physiochemical property and enzymatic hydrolysis of high-moisture corn stover ensiled with sulfuric acid or sodium hydroxide. Bioresource Technol. 2020;298: 122510.
AOAC. Official methods of analysis. Association of official analytical chemists. Arlington, VA: Association of Official Analytical Chemists; 1990. p. 3583–3597.
Van Soest PJ, Robertson JB, Lewis BA, Abbasi M, Abbasi M. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–97.
Murphy RP. A method for the extraction of plant samples and the determination of total soluble carbohydrates. J Sci Food Agr. 1958;9(11):714–7.
Liu B, Huan H, Gu H, Xu N, Shen Q, Ding C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresource Technol. 2019;273:212–9.
Yan Y, Li X, Guan H, Huang L, Ma X, Peng Y, Li Z, Nie G, Zhou J, Yang W, Cai Y, Zhang X. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresource Technol. 2019;279:166–73.
Li X, Chen F, Wang X, Sun L, Guo L, Xiong Y, Wang Y, Zhou H, Jia S, Yang F, Ni K. Impacts of low temperature and ensiling period on the bacterial community of oat silage by SMRT. Microorganisms. 2021;9(2):274.
Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–1.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41(D1):D590–6.
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodriguez AM, Chase J, Cope EK, Da SR, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille M, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MN, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft J, Vargas F, Vazquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson C, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7.
Guo L, Wang X, Lin Y, Yang X, Ni K, Yang F. Microorganisms that are critical for the fermentation quality of paper mulberry silage. Food Energy Secur. 2021. https://doi.org/10.1002/fes3.304.
Hao Y, Huang S, Liu G, Zhang J, Liu G, Cao Z, Wang Y, Wang W, Li S. Effects of different parts on the chemical composition, silage fermentation profile, in vitro and in situ digestibility of paper mulberry. Animals. 2021;11(2):413.
Bernardes TF, Daniel JLP, Adesogan AT, McAllister TA, Drouin P, Nussio LG, Huhtanen P, Tremblay GF, Bélanger G, Cai Y. Silage review: Unique challenges of silages made in hot and cold regions. J Dairy Sci. 2018;101(5):4001–19.
Gül S. The impact of wheat bran and molasses addition to caramba mix silage on feed value and in vitro organic matter digestibility. J King Saud Univ-Sci. 2023;35(1): 102400.
Okoye CO, Wang Y, Gao L, Wu Y, Li X, Sun J, Jiang J. The performance of lactic acid bacteria in silage production: a review of modern biotechnology for silage improvement. Microbiol Res. 2023;266: 127212.
You J, Zhang H, Zhu H, Xue Y, Cai Y, Zhang G. Microbial community, fermentation quality, and in vitro degradability of ensiling caragana with lactic acid bacteria and rice bran. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2022.804429.
Dong L, Zhang H, Gao Y, Diao Q. Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass. Bioresource Technol. 2020;310: 123396.
Wang N, Xiong Y, Wang X, Guo L, Lin Y, Ni K, Yang F. Effects of Lactobacillus plantarum on fermentation quality and anti-nutritional factors of paper mulberry silage. Fermentation. 2022;8(4):144.
Li M, Yu Q, Xu J, Sun H, Cheng Q, Xie Y, Wang C, Li P, Chen C, Zheng Y. Effect of different organic acid additives on the fermentation quality and bacterial community of paper mulberry (Broussonetia papyrifera) silage. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2022.1038549.
Van Soest PJ, Mertens DR, Deinum B. Preharvest factors influencing quality of conserved forage. J Anim Sci. 1978;47(3):712–20.
Zhang Q, Yu Z, Yang H, Na RS. The effects of stage of growth and additives with or without cellulase on fermentation and in vitro degradation characteristics of Leymus chinensis silage. Grass Forage Sci. 2016;71(4):595–606.
Yuan XJ, Wen AY, Wang J, Desta ST, Dong ZH, Shao T. Effects of four short-chain fatty acids or salts on fermentation characteristics and aerobic stability of alfalfa (Medicago sativa L.) silage. J Sci Food Agr. 2018;98(1):328–35.
Cai Y, Benno Y, Ogawa M, Kumai S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J Dairy Sci. 1999;82(3):520–6.
Arriola KG, Kim SC, Adesogan AT. Effect of applying inoculants with heterolactic or homolactic and heterolactic bacteria on the fermentation and quality of corn silage. J Dairy Sci. 2011;94(3):1511–6.
Zhang Y, Wang M, Usman S, Li F, Bai J, Zhang J, Guo X. Lignocellulose conversion of ensiled Caragana korshinskii Kom. facilitated by Pediococcus acidilactici and cellulases. Microb Biotechnol. 2023;16(2):432–47.
Muck RE, Nadeau EMG, McAllister TA, Contreras-Govea FE, Santos MC, Kung L. Silage review: recent advances and future uses of silage additives. J Dairy Sci. 2018;101(5):3980–4000.
Zheng ML, Niu DZ, Jiang D, Zuo SS, Xu CC. Dynamics of microbial community during ensiling direct-cut alfalfa with and without LAB inoculant and sugar. J Appl Microbiol. 2017;122(6):1456–70.
Yitbarek MB, Tamir B. Silage additives: review. Open J Applied Sci. 2014;04(05):258–74.
Cai Y, Du Z, Jethro DB, Nignan M, Yamasaki S. Analysis of main factors affecting silage fermentation of sorghum prepared with whole crop and stover in semiarid west Africa. Afr J Range Sci. 2021;38(2):169–78.
Scherer R, Gerlach K, Südekum KH. Biogenic amines and gamma-amino butyric acid in silages: formation, occurrence and influence on dry matter intake and ruminant production. Anim Feed Sci Tech. 2015;210:1–16.
Driehuis F, Wilkinson JM, Jiang Y, Ogunade I, Adesogan AT. Silage review: animal and human health risks from silage. J Dairy Sci. 2018;101(5):4093–110.
Mu L, Xie Z, Hu L, Chen G, Zhang Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresource Technol. 2020;315: 123772.
Tohno M, Kobayashi H, Nomura M, Uegaki R, Cai Y. Identification and characterization of lactic acid bacteria isolated from mixed pasture of timothy and orchardgrass, and its badly preserved silage. Anim Sci J. 2012;83(4):318–30.
Koc F, Aksoy SO, Okur AA, Celikyurt G, Korucu D, Ozduven ML. Effect of pre-fermented juice, Lactobacillus plantarum and Lactobacillus buchneri on the fermentation characteristics and aerobic stability of high dry matter alfalfa bale silage. J Anim Plant Sci. 2017;27(5):1426–31.
Fu Z, Sun L, Hou M, Hao J, Lu Q, Liu T, Ren X, Jia Y, Wang Z, Ge G. Effects of different harvest frequencies on microbial community and metabolomic properties of annual ryegrass silage. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2022.971449.
Wang T, Zhang J, Shi W, Sun J, Xia T, Huang F, Liu Y, Li H, Teng K, Zhong J. Dynamic changes in fermentation quality and structure and function of the microbiome during mixed silage of Sesbania cannabina and sweet sorghum grown on saline-alkaline land. Microbiol Spect. 2022. https://doi.org/10.3389/fmicb.2022.971449.
Dunière L, Sindou J, Chaucheyras-Durand F, Chevallier I, Thévenot-Sergentet D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim Feed Sci Tech. 2013;182(1–4):1–15.
Ogunade IM, Jiang Y, Pech Cervantes AA, Kim DH, Oliveira AS, Vyas D, Weinberg ZG, Jeong KC, Adesogan AT. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: effects of Escherichia coli O157:H7 and silage additives. J Dairy Sci. 2018;101(3):2048–59.
Axelsson L T. Lactic acid bacteria: classification and physiology, p1–64. In Salminen S, von Wright A (ed), Lactic acid bacteria.: New York, NY: Marcel Dekker; 1993.
Xu Z, Zhang S, Zhang R, Li S, Kong J. The changes in dominant lactic acid bacteria and their metabolites during corn stover ensiling. J Appl Microbiol. 2018;125(3):675–85.
Zhao M, Wang Z, Du S, Sun L, Bao J, Hao J, Ge G. Lactobacillus plantarum and propionic acid improve the fermentation quality of high-moisture amaranth silage by altering the microbial community composition. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2022.1066641.
Jung H, Liu Q, Kim J, Lee S, Kim S, Im W. Lactobacillus ginsenosidimutans sp. nov., isolated from kimchi with the ability to transform ginsenosides. Antonie van Leeuwenhoek. 2013;103(4):867–76.
Acknowledgements
Not applicable.
Funding
This work was supported by funds from the National Natural Science Foundation of China (Grant Nos. 32171686) and the Research and Demonstration of Key Technologies for Efficient Planting, Processing and Utilization of Hybrid Paper Mulberry in Hills and Mountains (CSTC2020NGZX0000).
Author information
Authors and Affiliations
Contributions
NW designed the study and wrote the manuscript. NW and YW performed the experiments. NW and YW conducted the statistical and bioinformatics analysis. FY and NK contributed to conceptualization and funding acquisition. YL, GX and NK were involved in the revision of the manuscript. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors listed have read the complete manuscript and have approved submission of the paper.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, N., Wang, Y., Lin, Y. et al. Effect of lactic acid bacteria and wheat bran on the fermentation quality and bacterial community of Broussonetia papyrifera silage. Chem. Biol. Technol. Agric. 10, 130 (2023). https://doi.org/10.1186/s40538-023-00481-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00481-w