Abstract
Background
Anthocyanins are the secondary metabolites of flavonoids in plants. As a key enzyme in the biosynthetic pathway of anthocyanin, dihydroflavonol 4-reductase (DFR) act as an important regulatory point, but DFR family genes has not been systematically characterized in apple (Malus domestica Borkh.).
Methods
The members of DFR genes in apple were identified and their gene structure, chromosome distribution, evolutionary relationships, collinearity, cis-component and protein interaction relationships were predicted using bioinformatics methods. The expression patterns of MdDFRs in various organs, such as leaves, fruit flushes, fruits, ripe fruit peels, flowers and stems were analyzed using GeneChip expression array analysis. qRT-PCR was employed to analyze the expression levels of MdDFRs in different apple varieties with varying levels of fruit skin at maturity.
Results
The apple database revealed 96 DFR genes, which are distributed on 17 chromosomes and can be divided into 3 subfamilies. These 96 DFR genes were mostly composed of α-helix and random coil according to secondary structure prediction, and were mainly expressed in chloroplasts and cytoplasm. MYB binding site involved in flavonoid biosynthetic genes regulation element (MBSI) was identified in the promoter of MdDFR15/76/81/89/90/91/93/94. Lignin/flavonoid synthesis-related elements of MYB recognition site and MYB-binding site were identified in the promoters of MdDFR05/09/13/19/22/24/26/30/31/33/34/46/50/52/54/64/65/69/75/76/79/86. The internal collinearity analysis of the apple MdDFR genome revealed a total of 34 pairs of duplicated gene pairs. Interspecific collinearity analysis showed that there were 66 and 57 homologous gene pairs in apple/tomato and apple/grape, respectively. GeneChip expression array analysis showed that MdDFR72 and MdDFR96 were higher expressed in ripe fruit fleshes and peel, MdDFR01/06/67/49/54/91 were higher expressed in flowers, MdDFR64 was higher expressed in ripe fruit peels and flowers than those of other tissues. Besides, 75 MdDFR proteins interacted directly or indirectly with anthocyanidin synthesis related proteins MdANS, MdF3H, MdMYB1, MdMYBPA1 to form a protein interaction network. Interestingly, MdDFR69 and MdDFR87 had direct interactions with these four proteins, MdDFR64 had direct interactions with MdANS and MdF3H. qRT-PCR analysis showed that the expression levels of MdDFR01/05/31/53/64/69/73/84/87/94/96 were up-regulated with the accumulation of anthocyanins.
Conclusions
This study lays a foundation for further research on the function of DFR genes in apple.
Graphical Abstract

Similar content being viewed by others
Background
Apple (Malus domestica Borkh.) is a perennial woody plant of the Rosaceae family and is one of the most popular fruits in China, with a wide cultivation area and high yield throughout the country. Apple fruit is rich in minerals and vitamins, and its nutrient components are soluble, easy to be absorbed by the human body, with the role of calming the nerves, helping sleep, and reducing cholesterol [1]. Apple fruits also contain a certain amount of anthocyanins, which has the function of anti-aging and preventing cardiovascular and cerebrovascular diseases [2]. Anthocyanidin and proanthocyanidin are two end products of flavonoid biosynthesis pathway, which are believed to be synthesized and modificated in cytoplasm and endoplasmic reticulum before being sequestered into vacuoles [3]. Anthocyanins are synthesized by phenylalanine via phenylalanine lyase (PAL), cinnamate-4-hydroxylase (C4H), 4-coumaryl CoA ligase (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H), and flavonoid 3′5′-hydroxylase (F3′5′H), dihydroflavonol 4-reductase (DFR), and anthocyanin synthase (ANS). DFR catalyzed stereospecific reduction reaction of dihydroflavonol at the C4 position, which enables dihydroflavonol to generate colorless proanthocyanidins, and is one of the most critical enzymes in anthocyanin synthesis [4]. Notably, DFR enzymes exhibit striking substrate specificities, resulting in accumulation of distinct patterns of anthocyanins, catechins and proanthocyanidins [5].
F3H, as the upstream of DFR, can catalyze the synthesis of dihydroflavonols from flavonoids [6]. DFR further catalyzes catalyze three different colorless dihydroflavonols, including dihydro kaempferol (DHK), dihydro quercetin (DHQ) and dihydromyricetin (DHM), to generate different anthocyanidin precursors, and then synthesize various anthocyanin under the action of ANS [7]. It can be seen that DFR is the first enzyme to convert dihydroflavonol into anthocyanin. DFR can use DHK, DHQ, and DHM as substrates, respectively. The functional expression of Saussurea medusa gene SmDFR in yeast (Saccharomyces cerevisiae) confirms that SmDFR has a reducing effect on DHQ and DHK, but not on DHM [8]. The heterologous expression of grape hyacinth (Muscari spp.) gene MaDFR in tobacco (Nicotiana tabacum) resulted in increasing anthocyanin accumulation, leading to a darker flower color [9]. However, due to the different substrate selectivity of different species of DFR, different anthocyanins are synthesized, presenting different plant colors. Structural genes encoding enzymes of the anthocyanin pathways are regulated at the transcriptional level by a MYB-bHLH-WD40 (MBW) ternary complex of R2R3-MYB, basic helix-loop-helix (bHLH), and tryptophan aspartic (WD40)-repeat proteins [10, 11]. Among these regulatory factors, the R2R3-MYB transcription factors, which play a crucial role in determining the activation of specific downstream genes to control the spatiotemporal accumulation of anthocyanins, have been widely reported in apple, including MdMYBPA1 and MdMYB1 [12, 13]. The regulatory interaction between transcription factors related to anthocyanin synthesis and structural genes has been a focus of research [14,15,16].
Our precious study reported that the expression of DFR was upregulated with the increase of anthocyanin accumulation revealed by comprehensive transcriptome analyses [17]. Due to the substrate specificity of DFR, different members of its gene family have different catalytic efficiency for each substrate and different expression levels in different tissues, which causes different tissues or organs of plants show various colors [18]. Given the importance of DFRs in the regulation of flavonoid synthesis and the production and accumulation of anthocyanidin, the expression and functional characterization of DFR family members in anthocyanidin biosynthesis of apple have not been systematically investigated, especially in different apple organs and varieties, as well as the relationships with other identified anthocyanidin synthesis related genes or transcription factors. Consequently, in light of the discovery of the apple DFR gene members, this experiment performed a bioinformatics analysis of the MdDFR gene members and predicted the interaction network between the MdDFRs and the anthocyanidin synthesis related proteins MdANS, MdF3H, MdMYB1, MdMYBPA1. The role of DFR genes in apple anthocyanin synthesis was examined through the analysis of GeneChip expression array of different apple tissues and expression levels of apples with different coloring degrees, which provided theoretical reference for the study of the regulatory network of apple anthocyanin synthesis.
Materials and methods
Plant materials
Apple varieties ‘Golden Delicious’, ‘Ruixue’, ‘Chengji No.1’ and ‘Astar’ with different coloring degrees at maturity were selected as research materials, and named G1, G2, G3 and G4, respectively. Fifteen fruits were collected from each variety, with every five fruits being a duplicate. Fruit skin samples were accurately weighed and quickly frozen in liquid nitrogen for 2 h, and then stored in a refrigerator at − 80 °C for RNA extraction.
Identification of DFR gene members in apple
The FASTA sequence of apple DFR gene CDS was obtained by searching gene names in NCBI network database (https://www.ncbi.nlm.nih.gov/). InterPro number (IPR001509) of this gene was obtained from the Pfam website (https://pfam.xfam.org/) using the obtained FASTA sequence. Collect all the genes containing InterPro number in Phytozome v13 (https://phytozome-next.jgi.doe.gov/). The mRNA and CDS sequences of genes were downloaded from the apple reference genome website (https://iris.angers.inra.fr/gddh13/) using the accession number, and the corresponding protein sequences were obtained from the translation website (https://web.expasy.org/translate/). Chromosome localization of DFR gene members of apple was performed using TBtools (Version 1.108) software.
Bioinformatics analysis of DFR gene members in apple
ProtParam tool in Expasy database (https://web.expasy.org/protparam/) was used to analyze the basic physicochemical properties of protein, such as the number, relative molecular weight and instability coefficient. ClustalX, MEGA7.0 and EvolView (https://www.evolgenius.info/) were used to construct a phylogenetic tree using the Neighbor-Joining method (NJ) with the boot-strap value of 1000. The gene structure was analyzed by GSDS 2.0 (http://gsds.cbi.pku.edu.cn/). The conserved motifs of MdDFRs were determined using the MEME online tool (http://meme-suite.org/tools/meme) the number of motifs output was 10. The motif analysis and gene structure were visualized using the TBtools software. The subcellular localization prediction of DFR gene members was performed online using WoLF PSORT (http://www.genscript.com/wolf-psort.html). The secondary structure prediction of DFR proteins was carried out using PredictProtein (https://predictprotein.org/). The chromosomal location information of MdDFRs was obtained from the apple genome website (https://iris.angers.inra.fr/gddh13/) and was drawn a picture by MG2C (http://mg2c.iask.in/mg2c_v2.0/).
Promoter cis-acting element analysis
The 2000 bp upstream promoter sequences of MdDFR genes were submitted to the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) and PLACE (https://www.dna.affrc.go.jp/PLACE/) to predict cis-elements and to subsequently screen cis-elements manually, then visualized at TBtools (Version 1.108).
Intraspecific and interspecific collinearity analysis of MdDFR genes
Download the genomes and annotated files of tomato and grape using Phytozome v13. The intraspecific collinearity of MdDFR gene pairs as well as the interspecific collinearity of DFR members from Malus domestica/Solanum lycopersicum, Malus domestica/Vitis vinifera L. was calculated and visualized by MCScanX method at TBtools (Version 1.108).
Expression pattern and protein interaction analysis of DFR genes in apple
The FPKM values of 96 MdDFRs in different organs, including leaves, young leaves, fruit flushes, fruits, ripe fruit peels, flowers and stems, were obtained through the apple MDO multidimensional omics database (http://bioinformatics.cau.edu.cn/AppleMDO/gene_detail.php) and standardized with log10. The expression heat maps were drawn using TBtools. The protein interaction network was predicted by STRING Version 11 (https://string-db.org/).
Quantitative RT-PCR (qRT-PCR) analysis
Total RNA was extracted from 100 mg of frozen tissue using an RNA plant (Real Times, Beijing, China) basing on the manufacturer's instructions. The first strand cDNA fragment was synthesized from 1.0 μg of DNase created RNA in a reaction solution (total volume of 20 μL) by using the Evo M-MLV RT kit (Accurate Biology, Changsha, China). Gene specific primers (Additional file 1: Table S1) were designed and synthesized by Sangon Biotech Co., Ltd. (China). Gene expression was detected using qRT-PCR using SYBR® Green Premix Pro Taq HS qPCR Kit (Accurate Biology). Using apple GADPH (GenBank access NO. CB973647) gene as internal reference. Light Cycler® 96 RealTime PCR System (Roche, Basel, Switzerland) was used to determine the expression levels of MdDFRs in the peel of four apple cultivars. The reaction system is 20 μL, including 2 mL cDNA, 1 μL upstream and downstream primers, 10 μL SYBR, and 20 μL ddH2O complement. The PCR reaction was as follows: 95 ℃ for 30 s, 40 cycles at 95 ℃ for 5 s and 60 ℃ for 1 min, 95 ℃ for 5 s, 55 ℃ for 30 s and 72 ℃ for 1 min. The relative expression levels of genes were calculated using the 2− △△CT method [19].
Results
Physicochemical properties and subcellular localization analysis of DFR genes in apple
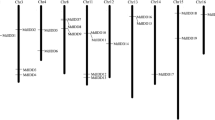
A total of 96 DFR genes, named MdDFR01-MdDFR96, were obtained through the homologous search on the apple reference genome website, and distributed on 17 apple chromosomes (Fig. 1, Table 1). Among them, chromosome 2 (chr2) and chr11 were the most distributed with 12 genes, followed by chr7 with 9 genes, chr6 and chr8 with 8 genes, chr0 and chr14 with 1 gene each. The number of amino acids encoded by this gene members was ranged from 75 aa (MdDFR10) to 1204 aa (MdDFR70). The relative molecular weight ranged from 8344.64 D (MdDFR10) to 136,293.11 D (MdDFR70). The isoelectric point (PI) ranged from 5.02 (MdDFR78) to 9.96 (MdDFR47), including 28 basic proteins (PI > 7.5), 44 acidic proteins (PI < 6.5), and 24 neutral proteins (PI 6.5–7.5). The instability index ranged from 18.26 (MdDFR29) to 49.93 (MdDFR05). There were 23 hydrophilic proteins and 73 unstable hydrophobic proteins. The full length of the mRNA ranged from 353 bp (MdDFR40) to 10,524 bp (MdDFR01). The full length of the cDNA ranged from 228 bp (MdDFR10) to 3615 bp (MdDFR70). Among the 96 apple DFR genes, 47 were located in the chloroplast, 31 were located in the cytoplasm, and there were 4 genes each in cytoskeleton, extracellar matrix, and mitochondria. There were 3 genes each in nucleus and endoplasmic reticulum, and only 1 member in peroxisome (Table 1).
Secondary structure prediction analysis of DFR proteins in apple
The secondary structure of the protein encoded by the apple DFR genes was mainly α-helix and random coil, followed by extended strand, and β-corner structure was the least (Table 2). The proportion of α-helix structure was between 29.84% (MdDFR23) and 56.06% (MdDFR70). The proportion of irregular curly structures ranged from 26.67% (MdDFR10) to 48.17% (MdDFR01). Extended strand accounted for 10.71% (MdDFR40) to 23.00% (MdDFR77). β-corner accounted for the smallest proportion of 4.13% (MdDFR18) to 12.00% (MdDFR10). According to the above analysis, it can be concluded that α-helix and random coil were the main factors in the formation of spiral structure of apple DFRs.
Phylogenetic analysis of DFR genes in apple
To further understand the evolution and functional characteristics of apple DFR genes, the phylogenetic tree of apple DFR members was constructed and divided into three subfamilies (Clade I-III, Fig. 2). Among them, the Clade I subfamily includes 7 genes, including MdDFR19, MdDFR22, MdDFR54, MdDFR62, MdDFR79, MdDFR89, and MdDFR95. The Clade II subfamily includes 40 genes, and the Clade III subfamily includes 49 apple DFR genes (Fig. 2). In each branch, apple DFR genes have high homology and close evolutionary relationship, but there are some differences, which preliminarily indicates that their evolution is conservative, and it is speculated that the functions of the lineal homologous genes are similar.
Gene structure and motif sequence analysis of apple DFRs
To gain more insight into the evolutional and structural diversity of MdDFRs, the conserved motifs in MdDFR proteins were analyzed using the MEME online software. A total of 10 distinct and highly conserved motifs were captured (Fig. 3A). The motif distribution pattern in most MdDFR proteins is highly conserved. Clade I mainly contain motif 1, motif 2, motif 3, motif 4, motif 7 and motif 8. Clade II and III mainly contains motif 7 and motif 10. To further explore the structural diversity of MdDFRs, the intron–exon organization of each gene was analyzed. As shown in Fig. 3B, the exon number varied from 1 to 12, and most of the MdDFR genes have 1–7 exons. In addition, 6 genes contain 9 exons, 3 genes contain 8 exons, 4 genes contain 10 exons, and 4 genes contain 12 exons.
Prediction of cis-component analysis of MdDFRs
To explore the mechanism of MdDFR genes in plant growth and development, the cis-regulatory elements in the 2 kb upstream sequence of MdDFR genes were predicted by PlantCARE (Fig. 4). In the MdDFR genes’ promoter, a variety of hormones (ABA, Auxin, SA, MeJA, GA, ERF)-, stress (heat shock, drought, low temperature, defense and stress)-, MYB binding site involved in flavonoid biosynthetic genes regulation-, light responsiveness- and lignin/flavonoid biosynthesis-related cis-acting elements were identified. Among them, light responsive related cis-acting elements were the most at 751, followed by GA related at 542, MeJA related at 322, and lighting/flavonoid biosynthesis related at 211. Abscisic acid (ABA) response element (ABRE), auxin response element (TGA-element), salicylic acid (SA) response element (TCA-element), MeJA response element (CGTCA-motif and as-1), gibberellin (GA) response element (TGACG-motif, GARE-motif, P-BOX, GT1-motif, GA-motif), low temperature response element (LTR), ERF response element (ERE), drought response element (MBS), MYB binding site involved in flavonoid biosynthetic genes regulation element (MBSI), defense and stress responsiveness element (TC-rich repeats), light responsiveness element (G-box, Box4, Box4 II, ACE TCT-motif, TCCC-motif, I-box, chs-CMA1a/2a, and WUN-motif), and lignin/flavonoid biosynthesis-related cis-acting elements (W-BOX, MYB recognition site and MYB-binding site) were present in almost all MdDFR promoter sequences. MBSI element was identified in the promoter of MdDFR15/76/81/89/90/91/93/94. Lignin/flavonoid synthesis-related elements of MYB recognition site and MYB-binding site were both identified in the promoters of MdDFR 05/09/13/19/22/24/26/30/31/33/34/46/50/52/54/64/65/69/75/76/79/86.
Intraspecific and interspecific colinearity analysis of DFRs
The internal collinearity analysis of the apple MdDFR genome revealed a total of 34 pairs of duplicated gene pairs (Fig. 5A), which were located on chromosomes chr1, chr2, chr3, chr4, chr5, chr6, chr7, ch8, chr9 and chr13. Most of these duplicated gene pairs were located on chromosomes 2 and 3 with 8 and 9 pairs, respectively. This indicates that some MdDFR genes may be generated through gene duplication, which may have similar functions. However, no linked genes were found on chromosomes 10, 11, 12, 14, 15 and 16. To further elucidate the phylogenetic mechanism and homology relationship of the DFR genes in apple, synteny analysis was conducted between apple and two representative species, grape (Vitis vinifera L.) and tomato (Solanum lycopersicum). The results showed that there were 66 pairs of homologous genes in apple and tomato, mainly concentrated in apple chr3, chr7 and chr11, with 8, 10 and 8 pairs of homologous genes, respectively (Fig. 5B, Dataset1). There were 57 pairs of homologous genes in apple and grape, which were mainly concentrated in apple chr3, chr7 and chr11, with 7, 7 and 9 pairs of homologous genes, respectively. Therefore, it is speculated that the DFR gene is relatively conservative in evolution. In addition, some DFR genes were identified to be associated with 2–3 pairs of orthologs, whereas some had no homologous pairs, which may be due to differences in evolutionary speed and genetic specificity between species.
Collinearity analysis of DFRs. A Collinearity analysis of MdDFRs. B Collinearity analysis of DFRs in apple and two representative plants tomato (Solanum lycopersicum) and grape (Vitis vinifera L.). The gray lines in the background show collinearity between the apple and tomato/grape genomes. The blue lines show collinearity between the MdDFRs and tomato/grape genome
Expression pattern analysis and protein interaction prediction
The expression patterns of MdDFR gene members in different developmental organs were analyzed, including leaves, young leaves, fruits, fruit fleshes, fruit peels, flowers and stems. It was found that the expression levels in the same subfamily were similar (Fig. 6). The expression level of MdDFR72, MdDFR96 was higher in ripe fruit fleshes and peels, whereas lower in other tissues. The expression level of MdDFR01, MdDFR06, MdDFR67, MdDFR49, MdDFR54 and MdDFR91 was higher in flowers, whereas lower in other tissues. The expression level of MdDFR64 was higher in ripe fruit peels and flowers, whereas lower in other tissues.
The interactions among 96 MdDFR proteins were predicted by the STRING online website. The results showed that 75 out of 96 MdDFR proteins form a complex network structure (Fig. 7). The three-dimensional structure of the MdDFR05, MdDFR20, MdDFR28, MdDFR53, MdDFR62, MdDFR64, MdDFR69, MdDFR72, MdDFR76, MdDFR79, MdDFR83, MdDFR87, MdDFR88, MdDFR90, MdDFR95 and MdDFR96 proteins is known. Besides, these 75 MdDFR proteins interacted directly or indirectly with anthocyanidin synthesis related proteins MdANS (DQ381771.1), MdF3H (AF117270.1), MdMYB1 (JN315783.1), MdMYBPA1 (KJ909759.1) to form a protein interaction network. Among these interacting proteins, MdDFR21, MdDFR31, MdDFR35, MdDFR36, MdDFR37, MdDFR38, MdDFR39, MdDFR55, MdDFR58, MdDFR59, MdDFR64, MdDFR67, MdDFR69, MdDFR78, MdDFR84, MdDFR87 and MdDFR96 were directly interacted with MdANS. MdDFR21, MdDFR31, MdDFR53, MdDFR58, MdDFR59, MdDFR64, MdDFR66, MdDFR67, MdDFR69, MdDFR73, MdDFR78, MdDFR84, MdDFR87 and MdDFR96 were directly interacted with MdF3H. MdDFR53, MdDFR58, MdDFR69 and MdDFR87 were directly interacted with MdMYBPA1. MdDFR87 and MdDFR69 were directly interacted with MdMYB1. Interestingly, MdDFR69 and MdDFR87 interact directly with these four anthocyanidin synthesis related proteins, MdANS, MdF3H, MdMYBPA1 and MdMYB1.
Analysis of protein interaction of 96 DFRs, ANS (DQ381771.1), F3H (AF117270.1), MYB1 (JN315783.1) and MYBPA1 (KJ909759.1) in apple. Nodes indicate proteins. Empty nodes indicate the protein of unknown 3D structures, and filled nodes indicate that 3D structures are known or predicted. The connection between nodes indicates the interaction between proteins, and different colors correspond to different types of interaction
Expression analysis of DFR gene members in different apple cultivars
Based on the above results, 20 aforementioned MdDFRs were selected for expression level analysis in the fruit skin of four different colored apple varieties. Our previous research revealed that the degree of pigmentation and anthocyanin content in the fruit skin from G1 to G4 apple varieties were gradually increased [17]. The results of qRT-PCR analysis in this research showed that the expression levels of MdDFR01, MdDFR05, MdDFR31, MdDFR53, MdDFR64, MdDFR69, MdDFR73, MdDFR84, MdDFR87, MdDFR94 and MdDFR96 were up-regulated with the increase of fruit skin pigmentation and the accumulation of anthocyanins (Fig. 8). Among these 20 genes, the expression level of MdDFR87 was the highest. Besides, the expression levels of MdDFR06, MdDFR15, MdDFR24, MdDFR46, MdDFR50 and MdDFR78 were significantly higher in G3 than those of other three apple varieties. However, the expression levels of MdDFR34, MdDFR65, and MdDFR81 were all lower, and the expression levels in G1 and G3 were higher than those in G2 and G4.
Discussion
After being extracted from maize and snapdragon using the transposon tagging approach [20], the DFR gene was later discovered in other plants [21,22,23]. The 96 gene members of DFR were categorized into three subclades (Fig. 2, 3), and there were 66 and 57 pairs of homologous genes in apple/tomato and apple/grape (Fig. 5b), respectively, exhibiting significant sequence conservation in apple. DFR is a structural gene that regulates the phenylalanine metabolic pathway and has a significant impact on plant coloring [24]. In transgenic tobacco lines, overexpression of Hosta ventricose gene HvDFR enhanced anthocyanin accumulation and elevated critical genes, such as F3H, F3'H, ANS, and UFGT [25]. Heterologous expression of Ophiorrhiza japonica gene OjDFR1 in transgenic tobacco contributed to darker flower color via up-regulating the expressions of endogenous NtANS and NtUFGT [26]. Physicochemical property analysis showed that most MdDFR genes were acidic proteins, followed by basic proteins and neutral proteins (Table 1). Subcellular predictions found that 47 MdDFRs were located in the chloroplast, 31 MdDFRs were located in the cytoplasm (Table 1), which is consistent with the conclusion that anthocyanins are synthesized mainly in the cytoplasm [27]. Therefore, we speculate that DFR genes are involved in the synthesis of apple anthocyanins.
Gene duplication plays an important role in the evolution of organisms, including tandem duplication, local duplication and whole genome duplication [28]. Duplicate genes may be maintained through subfunctionalization and neofunctionalization at the level of expression or coding sequence [29]. Although 96 DFR genes were distributed on 17 apple chromosomes (Fig. 1, Table 1), chr2 and chr11 each had 12 genes, followed by chr7 with 9 genes, chr6 and chr8 with 8 genes each, which was speculated to be formed by tandem repeats. Internal collinearity analysis revealed a total of 34 DFR duplicate gene pairs, mostly located on chromosomes 2 and 3, with 8 and 9 pairs, respectively (Fig. 5a), suggesting that these genes may have similar functions. This suggests that tandem duplication may be the main reason for the amplification of DFR family in apple.
The different colors of flowers and fruits of higher plants are closely related to plant pigments, which are anthocyanins and carotenoids. Anthocyanin transport and accumulation are regulated by many factors, mainly internal factors, but also by environmental factors. DFR is also regulated by environmental factors, and many plants also synthesize anthocyanins to protect the organism under environmental stress, which is accompanied by the increase of DFR expression in vivo [22]. Previous studies have shown that in most plants, anthocyanin synthesis requires light induction [30,31,32]. Under the effect of strong light, the expression of structural genes and regulatory genes will be enhanced [33]. Natural variations of DFR were analyzed using an expanded population of 282 accessions belonging to the spiny Solanum group, and the single-nucleotide polymorphisms were found at the MYB recognition site in the promoter region, which causes differential expression of DFR and affects anthocyanin accumulation in fruits of the detected accessions [27]. In the present research, lignin/flavonoid biosynthesis-related cis-acting elements were present in almost all MdDFR promoter sequences (Fig. 4). The past decades have seen remarkable inroads made into our understanding of MYB proteins and their binding sites more specifically. The MBSI (aaaAaaC(G/C)GTTA) and MBSII (aaaAGTTAGTTA) were the binding sites of Ph3 in Petunia hybrid and involved in flavonoid biosynthetic genes regulation [34]. MBSI element was identified in the promoter of MdDFR15/76/81/89/90/91/93/94 in this study. MYB recognition site and MYB-binding site were both identified in the promoters of MdDFR 05/09/13/19/22/24/26/30/31/33/34/46/50/52/54/64/65/69/75/76/79/86. What’s more, the expression level of MdDFR54 and MdDFR91 was higher in apple flowers, and MdDFR64 was higher in ripe apple fruit peels and flowers, whereas lower in other tissues (Fig. 6). The expression levels of MdDFR05/31/64/69/94 were up-regulated with the increase of fruit skin pigmentation verified by qRT-PCR (Fig. 8). Therefore, the position and number of cis-acting elements related to anthocyanin synthesis in DFR promoter region are the causes for the changes in apple anthocyanin accumulation.
The biosynthetic pathway of anthocyanins has been defined, but its regulatory network still needs to be perfected. MYB plays an important role in promoting plant growth and development, secondary metabolism regulation, hormone regulation, and stress response [35,36,37,38]. These transcription factors can recognize and bind to structural gene promoters to enhance or inhibit anthocyanin synthesis [39]. In tree peony (Paeonia suffruticosa Andr.), PsMYB44 negatively regulates anthocyanin biosynthesis by directly binding to the PsDFR promoter and subsequently inhibiting blotch formation, which helps to elucidate the molecular regulatory network of anthocyanin-mediated blotch formation in plants [40]. The ectopic combined expression of transcription factors MYB, bHLH, and WDR in Lotus japonicus indicated that the DFR2 promoter was activated [41]. MdMYB1 appears to coordinately regulate structural genes in the anthocyanin pathway and segregates with red-skinned apples [42]. MdbHLH33 can directly binds to the MdMYBPA1 promoter and interacts with it to promote anthocyanidin biosynthesis in response to low temperatures red-fleshed apples [12]. Recent study shows that mdm-miR858 targets transcription factor genes MdMYB9 and MdMYBPA1 to participate in anthocyanin accumulation in apple [43]. In addition, F3H and ANS, as upstream and downstream structural genes of DFR, respectively, are crucial in the phenylalanine metabolic pathway [6, 7]. In the present research, 75 MdDFR proteins interacted directly or indirectly with anthocyanidin synthesis related proteins MdANS, MdF3H, MdMYB1 and MdMYBPA1 to form a protein interaction network (Fig. 7). Besides, MdDFR69 and MdDFR87 had direct interactions with these four proteins, MdDFR64 had direct interactions with MdANS and MdF3H (Fig. 7), and all of these three genes were up-regulated with the increase of fruit skin pigmentation (Fig. 8), suggesting that the expression and regulation of these genes and transcription factors were coordinated with the production of anthocyanin. Therefore, further research on the transcription factors regulated by DFR upstream and downstream is of great significance to improve the regulatory network of anthocyanidin synthesis.
Conclusions
In this study, a total of 96 members of the DFR genes were identified from the apple database and were mainly expressed in chloroplasts and cytoplasm. Lignin/flavonoid synthesis-related elements of MBSI, MYB recognition site and MYB-binding site were identified in the promoters of MdDFR05/09/13/15/19/22/24/26/30/31/33/34/46/50/52/54/64/65/69/75/76/79/81/86/89/90/91/93/94. There were 34 pairs of duplicated MdDFR gene pairs in apple, 66 and 57 homologous DFR gene pairs in apple/tomato and apple/grape, respectively. MdDFR64 were directly interacted with MdANS and MdF3H, and the expression level of MdDFR64 was higher in ripe apple fruit peels and flowers other than other tissues. Besides, MdDFR69 and MdDFR87 had direct interactions with anthocyanidin synthesis related proteins MdANS, MdF3H, MdMYB1, MdMYBPA1. The expression levels of MdDFR64, MdDFR69 and MdDFR87 were up-regulated with the accumulation of anthocyanins in apple verified by qRT-PCR.
Availability of data and materials
The data that support the finding of this study are available from the corresponding author upon reasonable request.
References
Chai SC, Houshmand S, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Daily apple versus dried plum: impact on cardiovascular disease risk factors in postmenopausal women. J Acad Nutr Diet. 2012;112(8):1158–68.
Hair R, Sakaki JR, Chun OK. Anthocyanins, microbiome and health benefits in aging. Molecules. 2021;26(3):537.
Wang R, Lu N, Liu C, Dixon RA, Wu Q, Mao Y, Yang Y, et al. MtGSTF7, a TT19-like GST gene, is essential for accumulation of anthocyanins, but not proanthocyanins in Medicago truncatula. J Exp Bot. 2022;73(12):4129–46.
Martens S, Teeri T, Forkmann G. Heterologous expression of dihydroflavonol 4-reductases from various plants. FEBS Lett. 2002;531(3):453–8.
Zhou H, Lin-Wang K, Wang F, Espley RV, Ren F, Zhao J, Ogutu C, et al. Activator-type R2R3-MYB genes induce a repressor-type R2R3-MYB gene to balance anthocyanin and proanthocyanidin accumulation. New Phytol. 2018;221(4):1919–34.
Li WF, Ning GX, Mao J, Guo ZG, Zhou Q, Chen BH. Whole-genome DNA methylation patterns and complex associations with gene expression associated with anthocyanin biosynthesis in apple fruit skin. Planta. 2019;250:1833–47.
Lim SH, You MK, Kim DH, Kim JK, Lee JY, Ha SH. RNAi-mediated suppression of dihydroflavonol 4-reductase in tobacco allows fine-tuning of flower color and flux through the flavonoid biosynthetic pathway. Plant Physiol Bioch. 2016;109:482–90.
Li H, Qiu J, Chen F, Lv X, Fu C, Zhao D, Hua X, et al. Molecular characterization and expression analysis of dihydroflavonol 4-reductase (DFR) gene in Saussurea medusa. Mol Biol Rep. 2012;39:2991–9.
Liu H, Lou Q, Ma J, Su B, Gao Z, Liu Y. Cloning and functional characterization of dihydroflavonol 4-reductase gene involved in anthocyanidin biosynthesis of grape hyacinth. Int J Mol Sci. 2019;20(19):4743.
Albert NW, Davies KM, Lewis DH, Zhang H, Montefiori M, Brendolise C, Boase MR, et al. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell. 2014;26:962–80.
Yan S, Chen N, Huang Z, Li D, Zhi J, Yu B, Liu X, et al. Anthocyanin Fruit encodes an R2R3-MYB transcription factor, SlAN2-like, activating the transcription of SlMYBATV to fine-tune anthocyanin content in tomato fruit. New Phytol. 2019;225(5):2048–63.
Wang N, Qu C, Jiang S, Chen Z, Xu H, Fang H, Su M, et al. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018;96(1):39–55.
Hu Y, Cheng H, Zhang Y, Zhang J, Niu S, Wang X, Li W, et al. The MdMYB16/MdMYB1-miR7125-MdCCR module regulates the homeostasis between anthocyanin and lignin biosynthesis during light induction in apple. New Phytol. 2021;231:1105–22.
Nakatsuka T, Saito M, Yamada E, Fujita K, Kakizaki Y, Nishihara M. Isolation and characterization of GtMYBP3 and GtMYBP4, orthologues of R2R3-MYB transcription factors that regulate early flavonoid biosynthesis, in gentian flowers. J Exp Bot. 2012;63(18):6505–17.
Peng Y, Thrimawithana AH, Cooney JM, Jensen DJ, Espley RV, Allan AC. The proanthocyanin-related transcription factors MYBC1 and WRKY44 regulate branch points in the kiwifruit anthocyanin pathway. Sci Rep. 2020;10:14161.
Wang S, Zhang Z, Li LX, Wang HB, Zhou H, Chen XS, Feng SQ. Apple MdMYB306-like inhibits anthocyanin synthesis by directly interacting with MdMYB17 and MdbHLH33. Plant J. 2022;110(4):1021–34.
Li WF, Mao J, Yang SJ, Guo ZG, Ma ZH, Dawuda MM, Zuo CW, et al. Anthocyanin accumulation correlates with hormones in the fruit skin of ‘Red Delicious’ and its four generation bud sport mutants. BMC Plant Biol. 2018;18:363.
Tian J, Chen MC, Zhang J, Li KT, Song TT, Zhang X, Yao C. Characteristics of dihydroflavonol 4-reductase gene promoters from different leaf colored Malus crabapple cultivars. Hortic Res. 2017;4:17070.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8.
O’Reilly C, Shepherd NS, Pereira A, et al. Molecular cloning of the a1 locus of Zea mays using the transposable elements En and Mu1. EMBO J. 1985;4(4):877–82.
Zhang P, Wen PF, Wen SB, Wang W, Pan QH, Zhan JC, et al. Molecular cloning of dihydroflavonol-4-reductase gene from grape berry and preparation of an anti-DFR polyclonal antibody. Vitis. 2008;3:47.
Ahmed NU, Park JI, Jung HJ, Yang TJ, Hur Y, Nou IS. Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene. 2014;550(1):46–55.
Zhao A, Ding R, Wang C, Chen C, Wang D. Insights into the catalytic and regulatory mechanisms of dihydroflavonol 4-reductase, a key enzyme of anthocyanin synthesis in Zanthoxylum bungeanum. Tree Physiol. 2023;43(1):169–84.
Shin DH, Choi MG, Kang CS, Park CS, Choi SB, Park YI. Overexpressing the wheat dihydroflavonol 4-reductase gene TaDFR increases anthocyanin accumulation in an Arabidopsis dfr mutant. Genes Genomics. 2016;38(4):333–40.
Qin S, Liu Y, Cui B, Cheng J, Liu S, Liu H. Isolation and functional diversification of dihydroflavonol 4-Reductase gene HvDFR from Hosta ventricosa indicate its role in driving anthocyanin accumulation. Plant Signal Behav. 2022;17(1):2010389.
Sun W, Zhou N, Feng C, Sun S, Tang M, Tang X, Ju Z, et al. Functional analysis of a dihydroflavonol 4-reductase gene in Ophiorrhiza japonica (OjDFR1) reveals its role in the regulation of anthocyanin. PeerJ. 2021;20(9): e12323.
Wang X, Chen X, Luo S, Ma W, Li N, Zhang W, Tikunov Y, et al. Discovery of a DFR gene that controls anthocyanin accumulation in the spiny Solanum group: roles of a natural promoter variant and alternative splicing. Plant J. 2022;111(4):1096–109.
Xu G, Guo C, Shan H, Kong H. Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci USA. 2012;109:1187–92.
Duarte JM, Cui L, Wall PK, Zhang Q, Zhang X, Leebens-Mack J, et al. Expression pattern shifts following duplication indicative of subfunctionalization and neofunctionalization in regulatory genes of Arabidopsis. Mol Biol Evol. 2006;23(2):469–78.
Yang T, Ma H, Li Y, Zhang Y, Zhang J, Wu T, Song T, et al. Apple MPK4 mediates phosphorylation of MYB1 to enhance light-induced anthocyanin accumulation. Plant J. 2021;106(6):1728–45.
Xing Y, Sun W, Sun Y, Li J, Zhang J, Wu T, Song T, et al. MPK6-mediated HY5 phosphorylation regulates light-induced anthocyanin accumulation in apple fruit. Plant Biotechnol J. 2023;21(2):283–301.
Zhao Y, Sun J, Cherono S, An JP, Allan AC, Han Y. Colorful hues: insight into the mechanisms of anthocyanin pigmentation in fruit. Plant Physiol. 2023;192(3):1718–32.
Cominelli E, Gusmaroli G, Allegra D, et al. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J Plant Physiol. 2008;165(8):886–94.
Solano R, Nieto C, Paz-Ares JMYB. Ph3 transcription factor from Petunia hybrida induces similar DNA-bending/distortions on its two types of binding site. Plant J. 1995;8:673–82.
Abe H. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15:63–78.
Song SS, Qi TC, Huang H, Ren QC, Wu DW, Chang CQ, Peng W, et al. The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell. 2011;23(3):1000–13.
Xie XB, Li S, Zhang RF, Zhao J, Chen YC, Zhao Q, Yao YX, et al. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012;35(11):1884–97.
Vimolmangkang S, Han YP, Wei GC, Korban SS. An apple MYB transcription factor, MdMYB3, is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol. 2013;13(1):176.
Yang YN, Yao GF, Zheng D, Zhang S, Wang C, Zhang M, Wu J. Expression differences of anthocyanin biosynthesis genes revea1l regulation patterns for red pear coloration. Plant Cell Rep. 2014;34(2):189–98.
Luan Y, Chen Z, Tang Y, Sun J, Meng J, Tao J, Zhao D. Tree peony PsMYB44 negatively regulates petal blotch distribution by inhibiting dihydroflavonol-4-reductase gene expression. Ann Bot. 2023;131(2):323–34.
Yoshida K, Iwasaka R, Shimada N, Ayabe S, Aoki T, Sakuta M. Transcriptional control of the dihydroflavonol 4-reductase multigene family in Lotus japonicus. J Plant Res. 2010;123:801–5.
Takos AM, Jaffé FW, Jacob SR, Bogs J, Robinson SP, Walker AR. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006;142:1216–32.
Li Z, Liu W, Chen Q, Zhang S, Mei Z, Yu L, Wang C, et al. Mdm-miR858 targets MdMYB9 and MdMYBPA1 to participate anthocyanin biosynthesis in red-fleshed apple. Plant J. 2023;113(6):1295–309.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the 2023 Gansu Province University Teacher Innovation Fund Project (2023A-055), Double First-Class Major Scientific Research Project of Gansu Provincial Department of Education (GSSYLXM-02), and the Science and Technology Major Project of Gansu Province (22ZD6NA045).
Author information
Authors and Affiliations
Contributions
BHC and WFL designed the experiments; WFL, JG, and ZHM performed the experiments; YJH, XL, MYC and JM performed data analysis; WFL wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1:
Sequence of primers used for qRT-PCR analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, WF., Gao, J., Ma, ZH. et al. Molecular evolution and expression assessment of DFRs in apple. Chem. Biol. Technol. Agric. 10, 98 (2023). https://doi.org/10.1186/s40538-023-00470-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00470-z