Abstract
The objective was to determine effects of cellulase, xylanase, and commercial fibrolytic enzymes on fermentation quality, aerobic stability, bacterial community, and in vitro degradation of mixed silages. Mixtures of alfalfa, wheat bran, and rice straw [80:15:5 on a fresh matter (FM) basis] were ensiled for 1, 3, 5, 7, 15, 30, and 45 d after treatment with: distilled water (control, C); cellulase (E); xylanase (X); or commercial fibrolytic enzymes (EX), with all enzyme preparations applied at 100 U/g FM. The 45-day silages were subjected to an in vitro degradation test. Each of the three enzyme-treated groups enriched relative abundance (RA) of Lactobacillus, Weissella, and Stenotrophomonas maltophilia, increased water soluble carbohydrate (WSC) concentrations, and extended aerobic stability over 384 h, but concurrently inhibited growth of undesirable microbes (i.e., Acinetobacter sp, Lelliottia amnigena, and Sphingomonas sp), reducing pH and concentrations of ammonia nitrogen (AN), butyric acid (BA) and propionic acid (PA). Compared to C, adding X or EX increased the RA of L. paralimentarius and L. parabrevis, enhanced accumulation of acetic acid (AA) and crude protein (CP), and reduced hemicellulose content. Furthermore, E group silage had the highest abundance of W. cibaria. In addition, EX enriched RA of Bacillus velezensis, reduced AN concentration, increased DM degradability, total VFA production, and gas production during in vitro incubation. In conclusion, addition of X or EX enhanced ensiling by enhancing concentrations of AA; however, EX was the most promising enzyme, based on reducing AN concentration and increasing DM content and DM degradability.
Graphical Abstract

Similar content being viewed by others
Background
In recent years, combining high-moisture forage and agricultural by-products to produce a mixed silage has great potential, as it not only provides optimal dry matter (DM) for ensiling but also promotes the feed industry [1, 2]. For example, ensiling high-moisture amaranth with rice straw improved fermentation quality and reduced undesirable disposal and environmental impacts of the straw [1]. Furthermore, paper mulberry was combined with wheat bran to produce high-quality silage [3]. With increasing cultivation of alfalfa in southern China, there has been unprecedented interest in ensiling alfalfa with crop by-products, which could ensure appropriate preservation by reducing effluent production, and provide high-quality animal feed [4]. Ensiling alfalfa with wheat bran or rice straw have great potential to use low-value crop residues and provide year-round roughages for ruminants.
Rice straw is not an optimal animal feed due to its low digestibility [5]. Fibrolytic enzymes are often used in silage production to degrade cell wall carbohydrates to fermentable sugars, enhancing production of lactic acid (LA) and improving degradability [6]. Cellulase and xylanase are specific for breaking internal β-1,4 linkages of cellulose and hemicellulose (xylan) to release soluble sugars [7]; applying cellulase inhibited undesirable bacteria, improved fermentation quality, and increased digestibility of soybean residue and corn stover mixed silage [2]. Cellulase increased abundance of Lactobacillus and decreased ammonia production in amaranth and rice straw mixed silage [1]. The addition of xylanase to sugarcane silage significantly increased acetic acid (AA) content and decreased pH [8]. However, few studies investigated the efficacy of xylanase for use on mixed silage. Cellulase and xylanase have distinct degradation products due to their different sites of action on the cell wall. Therefore, we hypothesized that addition of either xylanase or cellulase alter bacterial community succession in mixed silage, and furthermore, that the combination of cellulase and xylanase have synergistic effects on mixed silage fermentation.
The objective was to determine effects of cellulase and/or xylanase on fermentation and nutritive characteristics, bacteria community succession, in vitro digestibility, and aerobic stability of mixed silage prepared by combining alfalfa, wheat bran, and rice straw.
Methods
Silage preparation
Alfalfa was harvested at the 10% bloom stage from 3 randomly selected sites on a commercial farm in Chang De, China (longitude 112°06′58″, latitude 29°06′27″, altitude 30 m). Rice straw was post-harvest residue from the same farm. The alfalfa variety was 55V12 (Beijing Clover Seed & Turf Co., Cal, USA), second year alfalfa is planted and harvested in the second crop. The rice variety is Xiang early indica No. 45 (Yiyang Agricultural Institute, Hunan, China). Wheat bran was from Kangda Agricultural Products Co., Ltd. (Anhui, China). The alfalfa and rice straw were chopped into 1–2 cm lengths with a fodder cutter (93ZT-300; Xingrong Co., Ltd, Guangzhou, China). Alfalfa, wheat bran and rice straw were combined in a ratio of 80/15/5, based on preliminary experiments. A total of 126 silage mixtures (4 kg alfalfa, 0.75 kg wheat bran and 0.25 kg rice straw) were prepared, with 84 randomly selected (4 treatments × 7 sampling days × 3 replicates/treatment), as follows: distilled water (control, C); cellulase (E, 50,000 U/g, Rhawn Chemical Reagnt Co., Ltd, Shanghai, China); xylanase (X, 100,000 U/g, Rhawn Chemical Reagnt Co., Ltd, Shanghai, China); and commercial fibrolytic enzymes (EX, cellulase 20,000 U/g and xylanase 15,000 U/g, Guangdong VTR Bio-Tech Co., Ltd. Guangzhou, China). For all 3 enzyme-treated groups, the amount of enzymes applied was 100 U/g of fresh forage, based on the manufacturer’s recommendations.
Twenty-one silos were made for each treatment, with 3 silos per treatment opened after 1, 3, 5, 7, 15, 30, and 45 d of ensiling for fermentation quality analyses. Additives were dissolved in deionized water with constant mixing and 20 mL/kg was sprayed (Gan Ming Co., Ltd., Jingsu, China) as a fine mist on the material to be ensiled. All samples (600 g of raw material) were packed into vacuum-sealing nylon–polyethylene standard barrier bags and vacuum-sealed (Dafeng Machinery Co., Ltd., Zhejiang, China) and stored at room temperature (∼30 °C).
Chemical composition and fermentation characteristics
A sample of silage (20 g) was put in a blender with 180 mL distilled water, processed for 1 min, filtered through 2 layers of cheesecloth and pH measured (SI400 pH meter, Spectrum, Aurora, IL, USA). The filtrate was centrifuged (10 000 × g, 15 min, 4 °C) and the supernatant assessed for the following: volatile fatty acids (VFAs), lactic acid (LA), and ammonia nitrogen (AN), as described [9], using a phenol–hypochlorite reaction. Samples were dried at 65 °C for 48 h in a forced-draft for DM analysis and then ground in a knife mill with a 1-mm screen for chemical composition analyses. Crude protein (CP) and water soluble carbohydrate (WSC), neutral and acid detergent fiber (NDF and ADF, respectively) and hemicellulose (HC) contents were determined as described [9, 10], A nitrogen analyzer (Kjeltec 8400, FOSS, Sweden) was used to measure total nitrogen (TN); it was multiplied by 6.25 to estimate crude protein. The method of Van Soest was used to assess neutral and acid detergent fiber (NDF and ADF, respectively), with ADF subtracted from NDF to determined hemi-cellulose (HC) content. Heat-stable amylase and sodium sulphite were used for the NDF analytical procedure. The content of WSC was analyzed by the phenol/sulfuric acid method.
Aerobic stability
To determine aerobic stability, 48 piles were randomly chosen and ensiled as described for silage. After 45 d, bales were opened, mixed thoroughly, and loosely packed into 2-L sterile plastic boxes that were covered with 2 layers of gauze and held at 30–35 °C). Every 2 h, temperatures of air and silage (middle of bottle) were measured (Smowo MDL-1048A, Tianhe Automation Instrument Co., Ltd, Shanghai, China). Aerobic stability was defined as the interval for silage to become at least 2 °C temperature warmer than air. Twelve silos were made for each treatment, with 3 silos per treatment subjected to aerobic exposure and pH assessed after 4, 8, 12 or 16 d.
Sequence analyses of bacterial communities
Unfermented (fresh) silage and silage ensiled for 5, 15, and 45 days were selected for bacterial community analyses. The DNA was isolated (DNA kit, DP812, Tiangen, Beijing, China) according to manufacturer’s instructions, from frozen–thawed samples of silage. The DNA was quantified with a NanoDrop 2000 and quality determined with 1% agarose gels. Single molecule real-time (SMRT) sequencing was done, with primers 27F and 1492R to detect 16S rRNA genes, and polymerase chain reaction (PCR) was done as described [9]. All DNA assessments were done by Biomarker Technologies Corporation (Beijing, China). A PacBio Sequel (Pacific Biosciences, Menlo Park, CA, USA) was used for analyses, with sequences determined as described [9]. Alpha diversity used Shannon, Simpson's diversity, Chao1 and rarefaction estimators, principle component analysis (PCA), with R heatmaps prepared as described [1]. Comparisons of microbial variations during ensiling were done using the Latent Dirichlet allocation effect size (LEfSe) analysis with a Kruskal–Wallis test (P < 0.05) with a linear discriminant analysis (LDA) score > 4.0. The BMK Cloud Platform (http://www.biocloud.net/) was used for data analyses.
In vitro rumen fermentation trial
For in vitro batch culture, rumen fluid was collected from 3 lactating, ruminally cannulated Holstein cows approximately 2 to 3 h after the morning feeding. Cows were fed a TMR (DM basis) consisting of corn silage (38.2%), alfalfa hay (4.0%), dry rolled corn (27.3%), soybean meal (14.5%), citrus pulp (9.1%), and a mix of minerals and supplements (6.8%). Diets were offered twice daily (06:00 and 14:00 h).
Rumen fluid was manually collected, filtered through two-layer cheesecloth, and pooled in pre-warmed thermos flasks kept at 39 °C. Thermos flasks containing pooled rumen fluid were kept airtight until transported to the laboratory for final filtration with 2 more layers of cheesecloth (total of 4 layers). Rumen fluid inoculum was then added to a buffered pre-warmed (39 °C) media Goering and Van Soest [11] in a 1:2 ratio (rumen fluid:artificial saliva). The media was continuously infused with CO2 to maintain the anaerobic environment for the rumen fluid inoculum.
In vitro incubations were conducted on 2 separate days (runs) using silage samples after 45 d of ensiling that were ground to 4 mm, with 0.05 g placed in Ankom F57 filter bags (Ankom Technology, Macedon, NY). Bags were sealed and placed into serum bottles before incubation. Buffered rumen fluid (52 mL) was added to the 160-mL serum vials containing throughout Ankom bags, each 160-mL serum vials contains one Ankom bags, and a continuous stream of CO2 was flushed into the vials the inoculation process. Vials were closed with rubber stoppers and crimped with aluminum seals. Vials were immediately placed in an air-forced incubator at 39 °C with a shaking system for 48 h. Gas pressure was measured at 0, 4, 8, and 12, 24, and 48 h after incubation using a pressure transducer and subsequently converted to gas volumes (after correcting for gas volumes from blank bottles). Based on our lab conditions, pressure was converted to volume using the following equation:
The DM degradability was calculated after 48 h of incubation duration, with 72 samples (4 treatments × 3 individual silage samples × 3 replicates per sample × 2 runs) analyzed. Incubations were terminated by placing bottles on ice. Bags were taken out of serum vials, washed with tap water until effluent was clear, and dried in a forced-air oven set at 60 °C for 48 h. In vitro degradability of DM and of NDF were calculated as their weight loss after an in vitro test.
After 48 h incubation, rumen fluid from 160-mL serum vials was collected for pH, volatile fatty acids, and AN analysis. The pH of the rumen inoculum post-incubation was measured using a pH meter (Corning Pinnacle M530, Corning Inc., Corning, NY). An aliquot (10 mL) of ruminal fluid was pooled into 15 mL centrifuge tubes and acidified with 0.1 mL of 20% H2SO4. A water-based solution using ethyl acetate extraction was used to determine VFA concentrations in ruminal fluid samples. Samples were prepared as described [12] and assessed with a gas chromatograph (Agilent 7820A GC, Agilent Technologies) using a flame ionization detector and a capillary column (CP-WAX 58 FFAP 25 m × 0.53 mm, Varian CP7767; Varian Inc.). Column temperature was maintained at 110 °C, and the injector and detector temperatures were 200 and 220 °C, respectively [13]. Concentrations of AN in samples were measured as described [14]. Samples were thawed at room temperature and centrifuged at 10,000 × g for 15 min. The supernatant was analyzed using the phenol–hypochlorite method in a 96-well flat-bottom plate. Absorbance was measured with a spectrophotometer (SpectraMax Plus 384 Microplate Reader, Molecular Devices, San Jose, CA, USA) at 620 nm.
Statistical analyses
Fermentation quality and chemical composition were analyzed as a 4 (treatments) × 7 (ensiling days) factorial arrangement by the GLM procedure (SAS 9.3, SAS Institute Inc., Cary, NC, USA) with a model containing factors including the treatment, ensiling day, and their interaction. The experiment was conducted as a completely randomized design (4 treatments, with 7 durations of ensiling to assess fermentation and chemical compositions). Means among treatments were tested using the Tukey's test (P < 0.05). The same method as above was adopted for statistical analysis of pH during aerobic exposure days. Chemical compositions of raw materials data were subjected to one-way analysis of variance, bacterial diversity were subjected to one-way analysis of variance and polynomial contrast examine effects of the different silage times; differences were located with Tukey's; and P < 0.05 was considered significant.
For the in vitro rumen fermentation trial, data were analyzed for individual silage using the GLM procedure of SAS as a completely randomized block design using run as a blocking factor, treatment was used as a fixed effect and run as a random effect in the model. For gas production data, treatment, sampling hours, and interactions were tested in the model as fixed effects. For all analyses, P < 0.05 was considered significant and differences were located with Tukey's.
Results
Chemical compositions of fresh material
Prior to ensiling, mixture characteristics were: DM = 364 g/kg fresh weight (FW); WSC = 79.85 g/kg dry weight (DW); CP = 172.37 g/kg DW; NDF = 489.05 g/kg DW; ADF = 321.65 g/kg DW; and HC = 167.41 g/kg DW (Table 1).
Fermentation characteristics of mixed silages
There was an interaction between treatment and ensiling days for pH, LA, AA, propionic acid (PA), butyric acid (BA), and AN (Table 2). Both E and EX had higher pH than C on d 3, whereas X had higher pH than C on d 5. After 15 d of ensiling, the pH of E, X, and EX silages was lower (P < 0.05) than that of the C silage. The highest (P < 0.05) LA concentration was observed in C silage during the first 5 d of ensiling; thereafter, LA concentrations in cellulase-treated silages increased to the same level as in C. The AA concentration was higher (P < 0.05) in X silage than C silages throughout the entire ensiling period. At 3, 5, 7 and 30 d of ensiling, the concentration of AA was higher in EX silage than in C silage, with higher (P < 0.05) AA concentration in E vs. C silage after 7 d of ensiling. After 45 d of ensiling, enzymes lowered BA and PA concentrations as compared to C silage (P < 0.05), with X and EX silages having lower PA concentrations than other treatments. Compared to untreated silage, AN concentration was lower (P < 0.05) in enzyme-treated silages than in C silages at 1, 3, 7 and 45 d of ensiling, with the lowest (P < 0.05) AN concentration in EX silage after 3 d of ensiling.
Chemical composition of mixed silages
There was an interaction between treatment and ensiling days (P < 0.01) for DM, WSC, CP, NDF, ADF and HC (Table 3). The DM content in C silage was the lowest (P < 0.05) between d 3 and 15 of ensiling, and both E and EX silages had higher (P < 0.05) DM content than C silage after 45 d of ensiling. Adding enzymes increased WSC contents as compared to C silage over the entire ensiling periods, and the WSC contents in EX silage was highest from d 1 to 5. After 15 d of ensiling, the WSC contents in X and EX silages was higher than that of other treatments. The lowest CP content was in C silage after 7 d of ensiling, whereas X and EX silage had greater CP content than all other groups after 45 d of ensiling. Enzyme-treated silages had lower NDF contents than C silage during the initial 15 d of ensiling. There was a lower ADF content in EX silage vs. control after 45 d of ensiling. Compared to C silage, X and EX-treated silages had lower HC content than C during 30 d of the ensiling, and the lowest and highest HC content were observed in X and EX silages, respectively, after 45 d of ensiling.
Aerobic stability
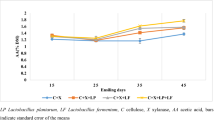
Applying E, X or EX extended the aerobic stability as compared to C silage (384 vs. 361 h). There was an interaction for pH during aerobic exposure (P < 0.01) (Fig. 1a, b); for C silage, there was a sharp rise in pH after 12 d of aerobic exposure, whereas E, X and EX silages still remained stable after 16 d.
Microbial community
Enzymes-treated silages had lower Shannon, Simpson index and Chao1 values as compared to C silage after 5, 15, and 45 d of ensiling (Table 4). According to the principal component (PCA; Fig. 2) analysis, the abscissa variation coefficient is 43.84%, and the ordinate variation coefficient is 19.90%, and there were differences in the bacterial community during ensiling, with 45-day silage separated from fresh, 5-, and 15-day silages. The relative abundances (RA) of bacteria on genus and species levels during the ensiling of mixed silages are shown in Figs. 3 and 4. Weissella, Acinetobacter, Pseudomonas, Stenotrophomonas, Sphingobacterium, and Chryseobacterium were the main epiphytic bacteria at the genus level in a mixture before ensiling. The main epiphytic bacteria at the species level were W. cibaria, Acinetobacter sp, Sphingobacterium sp, Pseudomonas fragi, Pseudomonas putida, and uncultured Stenotrophomonas. Dominant species changed from L. parabrevis, L. nodensis, L. paralimentarius, L. plantarum, and W. cibaria on d 5 and 15 of ensiling to Pseudomonas putida and Stenotrophomonas maltophilia on d 45 of ensiling. Enzyme-treated silages had higher RA of Lactobacillus and Weissella than C silage on d 5 and 15 of ensiling. The X and EX silages had higher RA of L. paralimentarius, whereas E silage had the highest RA of Weissella and W. cibaria on d 5 and 15 of ensiling. The RA of Lactobacillus and L. parabrevis in X and EX silages were higher than that of the other 2 treatments on d 15 of ensiling. Compared to C silage, enzyme-treated silages had a higher RA of Stenotrophomonas maltophilia on d 45 ensiling.
Based on linear discriminant analysis (LEfSe) (Fig. 5), at 5 d of ensiling, L. paralimentarius was enriched in X-treated silage, whereas L. nodensis and L. plantarum were enriched in E-treated silage. After 15 d of ensiling, L. parabrevis was enriched in EX silage, and W. cibaria was enriched in E silage, whereas Acinetobacter sp and L. nodensis were enriched in C silage. At 45 d of ensiling, Stenotrophomonas maltophilia was enriched in X silage; Bacillaceae, Bacillus, and Bacillus velezensis were enriched in EX silage; and L. parabrevis and Pediococcus pentosaceus were enriched in C silage.
Comparison of microbial variations during the ensiling using the Latent Dirichlet allocation effect size (LEfSe) analysis using the Kruskal–Wallis test (P < 0.05) with a linear discriminant analysis (LDA) score > 4.0. 5 d (A), 15 d (B) and 45 d (C) of ensiling. C, control; E, cellulase; X, xylanase; EX, commercial fibrolytic enzymes
Heatmaps (species level) of main bacterial communities in mixed silage are shown (Fig. 6). Acinetobacter sp, Lelliottia amnigena, and Sphingomonas sp, were positively correlated with C silage, whereas there were negative correlations between enzyme-treated silages and these bacteria. However, E silage was positively correlated with W. cibaria, L. brevis, Pantoea agglomerans, and EX silage was positively correlated with Chryseobacterium indoltheticum.
In vitro degradability, rumen fermentation parameters, and gas production
In vitro DM degradability in E, X, and EX silages was higher than that of C (P = 0.07), whereas there was a tendency of increase in NDF degradability for E and EX silages compared to C. Total VFA production tended to increase by 4.57, 5.06, and 13.03% with E, X, and EX-treated silage, respectively, resulting in lower pH in EX silage compared to C silage. There were no significant differences among treatments for concentrations of acetate, propionate, butyrate, iso-butyrate, valerate, iso-valerate, and AN (P > 0.05) (Table 5). Furthermore, gas production was higher (P < 0.01) in enzyme-treated silages than C silage from 4 to 24 h of incubation (Fig. 7).
Discussion
After mixing alfalfa with rice straw and wheat bran, the DM and WSC content was adjusted to 364 g/kg FW and 79.85 g/kg DW, which met the requirements for ideal DM (300–400 g/kg FW) and WSC (> 50 g/kg DM) content [15].
Effects of additives on fermentation and aerobic stability of alfalfa mixed silage
Enzyme-treated silages had higher pH and lower LA concentrations than the control during the initial 5 d of ensiling. Perhaps fibrolytic enzymes indirectly provided fermentable sugars, which were degraded from cell wall polysaccharides after a short lag [16]. In the previous study, applying cellulase in mixed silage of high-moisture amaranth and rice straw silage did not markedly accelerate LA fermentation during the initial 5 d of ensiling [1]. In mixed silage (corn and hulless–barley straw), LA accumulation was less efficient in cellulase- or xylanase-treated silages than in silages with added molasses [6]. However, after 15 d of ensiling, there was lower pH and higher LA concentrations in the E, X, and EX silages than C silage. Similarly, in a previous study, adding cellulase promoted production of LA, reducing pH in hybrid Pennisetum silage compared to the control after 60 d of ensiling [17]. A similar reduction in pH was obtained in xylanase-treated sugarcane silage after 60 d of ensiling [8]. Cellulase and xylanase hydrolyzed β-1,4-glycosidic linkages in major plant polysaccharides, cellulose, and xylan, making WSC available for silage fermentation by LAB [18, 19]. In the present study, X silage had higher AA concentrations than C silages throughout ensiling. Furthermore, adding xylanase to sugarcane silage increased AA concentration as compared to the control [8]. We speculate that more xylose production is beneficial to heterofermentative LAB production. Both homo- and heterofermentative LAB can ferment various pentose sugars; the xylose is taken up by specific permeases and converted to D-xylose-5-phosphate which is then fermented to a mixture of LA and AA [20].
All 3 enzyme-treated silages had a significantly lower AN content than C silage on d 45 of ensiling, attributed to lower pH and higher AA concentration in enzyme-treated silages than in C silage. Furthermore, AN is usually an indicator of clostridia and enterobacteria, which are inhibited by low pH [21]. As AA reduces pH and inhibits growth of organisms that promote spoilage [8], it may have improved silage quality in this study. The EX silage had the lowest AN concentration after 3 d of ensiling. The superior effect of EX treatment in this study was attributed in part to synergistic effects of cellulase and xylanase on mixed silage. In this study, X and EX silages had lower PA concentrations than other treatments after 45 d of ensiling. Similarly, application of Pediococcus acidilactici and cellulases decreased PA in Caragana korshinskii Kom. silage after 60 d of ensiling. In general, clostridial secondary fermentation can metabolize LA to propionic acid [22]. Reduced PA concentrations from application of cellulases may contribute to inhibited secondary fermentation, with better nutrient preservation [23], consistent with higher WSC and CP contents in X and EX silages after 45 d of ensiling.
In the study, greater WSC in EX silages than C silage was attributed to direct hydrolyzation of lignocellulose by cellulase and xylanase, releasing additional fermentable substrate. Cellulase accelerated LA fermentation and the decrease in pH, which were attributed to indirect supplementation of WSC by degradation of lignocellulose [24]. That C silage had the lowest DM content in this study may have been due to heterogeneous fermentation [25], consistent with Zhang et al. [26] who reported that adding cellulase markedly increased DM recovery in Caragana korshinskii Kom. silage. In this study, the CP content in C was lower than in X and EX silages, indicating more intensive proteolysis.
The lower NDF content in E, X, and EX treated silages than C silage during the initial 15 d of ensiling were attributed to: first, cellulase and xylanase effectively degrading cell wall carbohydrates in forage, thus reducing NDF and HC content in silage [27]; and second, the low pH promoting hydrolysis of the cell wall fraction, thereby reducing NDF and HC [28]. In that regard, cellulase lowered NDF and HC content as compared to control in hulless–barley straw mixed silage [29]. Xylan is the main component of hemicellulose, and the most important enzymes involved in hemicellulose degradation are xylanases and β-1-4 xylanases, which contributed to the lower HC contents in X silage than C silage.
Enzymes improved aerobic stability of silage. Perhaps the high AA concentration inhibited the growth of yeasts and molds to improve aerobic stability [30]. Similarly, cellulase and xylanase improved aerobic stability in bermudagrass silage [31].
Effects of additives on microbial community dynamics during ensiling
The lower alpha diversity in enzyme-treated silages than C silage after 5, 15, and 45 d of ensiling was attributed to the dominance of LAB and lower pH in enzyme-treated groups. In that regard, there was low alpha diversity when the dominant LAB became relatively simple during ensiling [32]. The dominance of LAB accompanied by a decreased pH inhibited proliferation of undesirable microbes, reducing microbial diversity in alfalfa silage [33]. In the study, 45-day silages were separated from fresh, 5-, and 15-day silages, indicating clear differences in bacterial communities.
Before ensiling, Weissella, Acinetobacter, Pseudomonas, Stenotrophomonas, Sphingobacterium, and Chryseobacterium were the main epiphytic bacteria. All of those epiphytic bacteria were also detected (at the genus level) in fresh Italian ryegrass, corn stover, and paper mulberry [34,35,36]. In this study, the most dominant genus changed from Lactobacillus after 5 and 15 d of ensiling, and to Pseudomonas and Stenotrophomonas after 45 d of ensiling. It is well-known that Lactobacillus is a rod-shaped LAB, with crucial roles in producing LA production and decreasing pH and it became the dominant bacteria in the natural fermentation of corn silage at the early stage of ensiling [37]. However, roles of Pseudomonas and Stenotrophomonas is silage are not well-known.
Pseudomonas can inhibit pathogenic microorganisms during plant growth and continue to survive in an anaerobic environment [38, 39], whereas Stenotrophomonas maltophilia is a non-LAB related to lignocellulosic biomass degradation [40]. However, as Pseudomonas and Stenotrophomonas can degrade protein, they are considered undesirable bacteria in silage [41, 42]. In contrast, Ogunade et al. [43] and Ren et al. [44] reported negative correlations between AN concentration and relative abundance of Pseudomonas and Stenotrophomonas. Therefore, the roles of these 2 bacteria in silage need further study. Desirable bacteria Lactobacillus and Weissella were promoted soon after ensiling began, increasing LA production and lowering pH [45]. The RA of LA-producing bacteria (Lactobacillus and Weissella) was higher in enzyme-treated silages than C silage at d 5 and 15 of ensiling. The availability of WSCs provides ready fermentation substrates to enhance proliferation of Lactobacillus and Weissella [46]. In addition, molasses also enriched abundance of Lactobacillus and Weissella in soybean and amaranth silage, respectively [47, 48]. Cellulase addition expectedly increased the LAB counts as more WSC was available for microbial fermentation due to cell wall degradation [49]. Zhao et al. [2] reported that LAB was the dominant microorganism in mixed silage of soybean residue and corn stover with cellulase after 56 d of ensiling, and furthermore, that Lactobacillus dominated the bacterial community.
L. paralimentarius and L. parabrevis were higher in X and EX silages than in the other 2 treatments after 15 d of ensiling. L. paralimentarius are Gram-positive, catalase-negative, facultative heterofermenters, whereas L. paralimentarius was the main species observed in corn silage after 90 d of ensiling [32, 50]. L. parabrevis was also observed in whole-plant corn silage during 14 d of ensiling [51], and was positively related to AA concentration [52]. In the current study, the addition of xylanase increased AA concentration after 15 d of ensiling, and we speculate that L. paralimentarius and L. parabrevis used xylose to produce AA by heterofermentation. Weissella is obligative heterofermentative LAB that converts WSC into LA and AA during the early stage of ensiling [15]. In the current study, Weissella and W. cibaria had the highest abundance in E silage after 5 and 15 d of ensiling, which may have contributed to higher AA concentration in E silage than that in C silage after 15 d of ensiling. Bacilli, Bacillaceae, Bacillus, and Bacillus velezensis were higher in EX silage than in other 3 groups (based on LEfSe analysis) for 5- and 45-day silages. Bacillus improved fermentation quality and aerobic stability in alfalfa silage [53], can improve animal performance, and was defined as a fourth-generation silage inoculant [54]. Wang et al. [55] reported that adding pectinase increased the abundance of Bacillus in alfalfa silage. Bacillus velezensis had antagonistic properties towards toxigenic molds in silage conditions [56], indicating bioactive roles of EX in promoting growth of Bacillus velezensis to exert antifungal properties. Furthermore, C silage had high RA of spoilage-producing organisms (Acinetobacter sp, Lelliottia amnigena, and Sphingomonas sp). Lelliottia was reclassified as a novel genus from Enterobacter, an undesirable bacteria in silage [57]. Adding Moringa oleifera leaf to alfalfa silage could decrease the RA of Lelliottia [58]. Sphingomonas was considered to hydrolyze soluble protein in silage comprised of agricultural by-products [59].
Effects of additives on in vitro parameters
Cellulase and/or xylanase increased DM degradability, total VFA production, and gas production during in vitro fermentation of mixed silage. Cellulase reduced plant cell wall fractions and protein loss during ensiling, providing more digestible substrates for fermentation by rumen microbes and facilitating ruminal digestion [60]. According to Del Valle et al. [8], xylanase acts on the most digestible content of the NDF in sugarcane silage, increasing DM degradability. The in vitro DM degradability in cellulase-treated mixed silage of soybean residue and corn stover was significantly higher than control [2]. Volatile fatty acids produced by microbial fermentation in the rumen could be a main energy source for ruminants [61]. Furthermore, there was a strong positive relationship between in vitro DM degradability and total VFA production [62]. In this study, E, X, and EX-treated silage increased molar proportions of VFAs and resulted in high gas production, thereby decreasing pH, attributed to increased DM degradability. The lower value of ADF, the higher the digestibility of the feed and the higher the feeding value [63]. In the current study, EX silage had the highest DM degradability, total VFA production, and lowest pH, attributed to the lowest ADF content in EX silage after 45 d of ensiling. The type of fermented substrate, microbial population, and rumen environment influence the type of VFA produced in the rumen. Acetate, propionate and butyrate are key VFAs formed in the rumen, with small quantities of iso-butyrate, valerate, and iso-valerate [61]. In the study, the dominant VFA of ruminal fermentation was acetate, with no significant differences among treatments for concentrations of AA, PA, BA, iso-butyrate, valerate, or iso-valerate. However, this contradicts a report of high concentrations of AA, PA and BA in agricultural waste-based complete feed silage given a cellulase enzyme treatment [64]. This response may be attributable to the consistency of the composition of the experimental diets [61]. The AN concentration in the study ranged from 24.06 to 25.85 mg/dL, within the target range (8.5–30 mg/dL) to maximize microbial protein synthesis [65]. Furthermore, enzyme treatments did not have any significant effect on AN concentration in the rumen. Similarly, there was no significant effect on rumen AN concentration in complete feed silage treated with cellulase [64].
Conclusion
In this study, the addition of 3 enzymes released WSC, decreased pH, AN, BA, PA contents, and extended the aerobic stability over 384 h. Adding X and EX enhanced accumulation of AA and CP, and reduced HC; however, EX was the most promising enzyme for reducing AN concentration. Enzymes increased the RA of Lactobacillus, Weissella, and Stenotrophomonas maltophilia, and concurrently inhibited growth of undesirable microbes (Acinetobacter sp, Lelliottia amnigena, and Sphingomonas sp). EX-treated increased DM degradability, total VFA production, and gas production during in vitro fermentation.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Mu L, Xie Z, Hu L, Chen G, Zhang Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour Technol. 2020;315:123772.
Zhao C, Wang L, Ma G, Jiang X, Yang J, Lv J, Zhang Y. Cellulase interacts with lactic acid bacteria to affect fermentation quality, microbial community, and ruminal degradability in mixed silage of soybean residue and corn stover. Animals (Basel). 2021;11(2):334.
Du Z, Lin Y, Sun L, Yang F, Cai Y. Microbial community structure, co-occurrence network and fermentation characteristics of woody plant silage. J Sci Food Agric. 2021;102(3):1193–204.
Chen X. Economic potential of biomass supply from crop residues in China. Appl Energy. 2016;166:141–9.
Zhao J, Dong Z, Li J, Chen L, Bai Y, Jia Y, Shao T. Ensiling as pretreatment of rice straw: the effect of hemicellulase and Lactobacillus plantarum on hemicellulose degradation and cellulose conversion. Bioresour Technol. 2018;266:158–65.
Guo G, Yuan X, Li L, Wen A, Shao T. Effects of fibrolytic enzymes, molasses and lactic acid bacteria on fermentation quality of mixed silage of corn and hulless-barely straw in the Tibetan Plateau. Grassl Sci. 2014;60(4):240–6.
Elghandour MMMY, Kholif AE, MÁRquez-Molina O, VÁZquez-Armijo JF, Puniya AK, Salem AZM. Influence of individual or mixed cellulase and xylanase mixture on in vitro rumen gas production kinetics of total mixed rations with different maize silage and concentrate ratios. Turk J Vet Anim Sci. 2015;39:435–42.
Del Valle TA, Antonio G, Zenatti TF, Campana M, Zilio EMC, Ghizzi LG, Gandra JR, Osório JAC, de Morais JPG. Effects of xylanase on the fermentation profile and chemical composition of sugarcane silage. J Agric Sci (Camb). 2019;156(9):1123–9.
Mu L, Wang Q, Cao X, Zhang Z. Effects of fatty acid salts on fermentation characteristics, bacterial diversity and aerobic stability of mixed silage prepared with alfalfa, rice straw and wheat bran. J Sci Food Agric. 2022;102(4):1475–87.
Van Soest PV, Robertson J, Lewis B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74(10):3583–97.
Goering HK, Van Soest PJ. Forage fiber analyses (apparatus, reagents, procedures, and some applications): US Agricultural Research Service; 1970.
Henry DD, Ciriaco FM, Araujo RC, Fontes PLP, Oosthuizen N, Mejia-Turcios SE, Garcia-Ascolani ME, Rostoll-Cangiano L, Schulmeister TM, Dubeux JCB, Lamb GC, DiLorenzo N. Effects of bismuth subsalicylate and encapsulated calcium ammonium nitrate on ruminal fermentation of beef cattle. J Anim Sci. 2020;98(8):skaa199.
Ciriaco FM, Henry DD, Mercadante VR, Schulmeister TM, Ruiz-Moreno M, Lamb GC, DiLorenzo N. Effects of molasses and crude glycerol combined in a liquid supplement on ruminal fermentation in beef steers consuming bermudagrass hay. J Anim Sci. 2016;94(9):3851–63.
Broderick G, Kang J. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. 1980;63(1):64–75.
Cai Y, Benno Y, Ogawa M, Ohmomo S, Kumai S, Nakase T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl Environ Microbiol. 1998;64(8):2982–7.
Zhang J-G, Kumai S, Fukumi R. Effects of temperature, moisture and cellulases on the fermentation quality and chemical composition of naked barley (Hordeum vulgare L. emand Lam) straw silage. Grassl Sci. 1997;43(2):95–102.
Xiong H, Zhu Y, Wen Z, Liu G, Guo Y, Sun B. Effects of cellulase, Lactobacillus plantarum, and sucrose on fermentation parameters, chemical composition, and bacterial community of hybrid Pennisetum silage. Fermentation. 2022;8(8):1.
Gilbert HJ, Hazlewood GP. Bacterial cellulases and xylanases. Microbiology. 1993;139(2):187–94.
Khota W, Pholsen S, Higgs D, Cai Y. Natural lactic acid bacteria population of tropical grasses and their fermentation factor analysis of silage prepared with cellulase and inoculant. J Dairy Sci. 2016;99(12):9768–81.
McDonald P, Henderson A, Heron S. The biochemistry of silage: Chalcombe Publications; 1991.
Kung L Jr, Shaver RD, Grant RJ, Schmidt RJ. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J Dairy Sci. 2018;101(5):4020–33.
Kung L. Silage fermentation end products and microbial populations: their relationships to silage quality and animal productivity. In: Annual conference of the american association of bovine practitioners, September 25–27, Charlotte, NC. 2008.
Li F, Ke W, Ding Z, Bai J, Zhang Y, Xu D, Li Z, Guo X. Pretreatment of Pennisetum sinese silages with ferulic acid esterase-producing lactic acid bacteria and cellulase at two dry matter contents: fermentation characteristics, carbohydrates composition and enzymatic saccharification. Bioresour Technol. 2020;295:122261.
Sun ZH, Liu SM, Tayo GO, Tang SX, Tan ZL, Lin B, He ZX, Hang XF, Zhou ZS, Wang M. Effects of cellulase or lactic acid bacteria on silage fermentation and in vitro gas production of several morphological fractions of maize stover. Anim Feed Sci Technol. 2009;152(3–4):219–31.
Desta ST, Yuan X, Li J, Shao T. Ensiling characteristics, structural and nonstructural carbohydrate composition and enzymatic digestibility of Napier grass ensiled with additives. Bioresour Technol. 2016;221:447–54.
Zhang Y, Wang M, Usman S, Li F, Bai J, Zhang J, Guo X. Lignocellulose conversion of ensiled Caragana korshinskii Kom. facilitated by Pediococcus acidilactici and cellulases. Microb Biotechnol. 2022;2022:1.
Sheperd A, Kung L Jr. An enzyme additive for corn silage: effects on silage composition and animal performance. J Dairy Sci. 1996;79(10):1760–6.
Huisden CM, Adesogan AT, Kim SC, Ososanya T. Effect of applying molasses or inoculants containing homofermentative or heterofermentative bacteria at two rates on the fermentation and aerobic stability of corn silage. J Dairy Sci. 2009;92(2):690–7.
Wang S, Guo G, Li J, Chen L, Dong Z, Shao T. Improvement of fermentation profile and structural carbohydrate compositions in mixed silages ensiled with fibrolytic enzymes, molasses and Lactobacillus plantarum MTD-1. Ital J Anim Sci. 2018;18(1):328–35.
Li Ying H, Borjigin N, Yu Z. Effect of inoculants and fibrolytic enzymes on the fermentation characteristics, in vitro digestibility and aflatoxins accumulation of whole-crop corn silage. Grassl Sci. 2017;63(2):69–78.
Dean DB, Adesogan AT, Krueger N, Littell RC. Effect of fibrolytic enzymes on the fermentation characteristics, aerobic stability, and digestibility of bermudagrass silage. J Dairy Sci. 2005;88(3):994–1003.
Xu D, Wang N, Rinne M, Ke W, Weinberg ZG, Da M, Bai J, Zhang Y, Li F, Guo X. The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb Biotechnol. 2021;14(2):561–76.
Yang L, Yuan X, Li J, Dong Z, Shao T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour Technol. 2019;275:280–7.
Du Z, Sun L, Chen C, Lin J, Yang F, Cai Y. Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim Feed Sci Technol. 2021;275:114766.
Yan Y, Li X, Guan H, Huang L, Ma X, Peng Y, Li Z, Nie G, Zhou J, Yang W, Cai Y, Zhang X. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour Technol. 2019;279:166–73.
Ren H, Wang C, Fan W, Zhang B, Li Z, Li D. Effects of formic or acetic acid on the storage quality of mixed air-dried corn stover and cabbage waste, and microbial community analysis. Food Technol Biotechnol. 2018;56(1):71.
Guan H, Yan Y, Li X, Li X, Shuai Y, Feng G, Ran Q, Cai Y, Li Y, Zhang X. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour Technol. 2018;265:282–90.
Roberson EB, Firestone MK. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol. 1992;58(4):1284–91.
Sun R, Yuan X, Li J, Tao X, Dong Z, Shao T. Contributions of epiphytic microbiota on the fermentation characteristics and microbial composition of ensiled six whole crop corn varieties. J Appl Microbiol. 2021;131(4):1683–94.
Zhao XH, He X, Zhang T, Wang JH. Isolation and characterization of a Swainsonine degrading bacteria strain Stenotrophomonas maltophilia. Afr J Microbiol Res. 2012;6(1):112–9.
Lu Q, Wang Z, Sa D, Hou M, Ge G, Wang Z, Jia Y. The potential efects on microbiota and silage fermentation of alfalfa under salt stress. Front Microbiol. 2021;12:688695.
Zi X, Li M, Yu D, Tang J, Zhou H, Chen Y. Natural fermentation quality and bacterial community of 12 Pennisetum sinese varieties in southern China. Front Microbiol. 2021;12:627820.
Ogunade IM, Jiang Y, Pech Cervantes AA, Kim DH, Oliveira AS, Vyas D, Weinberg ZG, Jeong KC, Adesogan AT. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J Dairy Sci. 2018;101(3):2048–59.
Ren H, Sun W, Yan Z, Zhang Y, Wang Z, Song B, Zheng Y, Li J. Bioaugmentation of sweet sorghum ensiling with rumen fluid: Fermentation characteristics, chemical composition, microbial community, and enzymatic digestibility of silages. J Clean Prod. 2021;294:126308.
You L, Bao W, Yao C, Zhao F, Jin H, Huang W, Li B, Kwok L-Y, Liu W. Changes in chemical composition, structural and functional microbiome during Alfalfa (Medicago sativa) ensilage with Lactobacillus plantarum PS-8. Animal Nutr. 2022;2022:1.
Wu B, Nishino N. Identification and isolation of Lactobacillus fructivorans from wilted alfalfa silage with and without molasses. J Appl Microbiol. 2016;120(3):543–51.
Ni K, Wang F, Zhu B, Yang J, Zhou G, Pan Y, Tao Y, Zhong J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour Technol. 2017;238:706–15.
Mu L, Xie Z, Hu L, Chen G, Zhang Z. Lactobacillus plantarum and molasses alter dynamic chemical composition, microbial community, and aerobic stability of mixed (amaranth and rice straw) silage. J Sci Food Agric. 2021;101(12):5225–35.
He L, Zhou W, Wang Y, Wang C, Chen X, Zhang Q. Effect of applying lactic acid bacteria and cellulase on the fermentation quality, nutritive value, tannins profile and in vitro digestibility of Neolamarckia cadamba leaves silage. J Anim Physiol Anim Nutr. 2018;102(6):1429–36.
Xu D, Ding W, Ke W, Li F, Zhang P, Guo X. Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative Lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front Microbiol. 2018;9:3299.
Bai J, Franco M, Ding Z, Hao L, Ke W, Wang M, Xie D, Li Z, Zhang Y, Ai L, Guo X. Effect of Bacillus amyloliquefaciens and Bacillus subtilis on fermentation, dynamics of bacterial community and their functional shifts of whole-plant corn silage. J Anim Sci Biotech. 2022;13(1):7.
Tian H, Wang Y, Liu Z, Hu Z, Guo Y, Deng M, Liu G, Sun B. Effects of malic acid and sucrose on the fermentation parameters, CNCPS nitrogen fractions, and bacterial community of Moringa oleifera leaves silage. Microorganisms. 2021;9(10):2102.
Bai J, Xu D, Xie D, Wang M, Li Z, Guo X. Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour Technol. 2020;315:123881.
Zhang L, Ma Q, Ma S, Zhang J, Jia R, Ji C, Zhao L. Ameliorating effects of Bacillus subtilis ANSB060 on growth performance, antioxidant functions, and aflatoxin residues in ducks fed diets contaminated with aflatoxins. Toxins (Basel). 2016;9(1):1.
Wang B, Sun Z, Yu Z. Pectin degradation is an important determinant for alfalfa silage fermentation through the rescheduling of the bacterial community. Microorganisms. 2020;8(4):488.
Wambacq E, Audenaert K, Hofte M, De Saeger S, Haesaert G. Bacillus velezensis as antagonist towards Penicillium roqueforti s.l. in silage: in vitro and in vivo evaluation. J Appl Microbiol. 2018;125(4):986–96.
Yuk K-J, Kim Y-T, Huh C-S, Lee J-H. Lelliottia jeotgali sp. nov., isolated from a traditional Korean fermented clam. Int J Systematic Evolution Microbiol. 2018;68(5):1725–31.
He L, Lv H, Xing Y, Wang C, You X, Chen X, Zhang Q. The nutrients in Moringa oleifera leaf contribute to the improvement of stylo and alfalfa silage: Fermentation, nutrition and bacterial community. Bioresour Technol. 2020;301:122733.
Zhou Y-M, Chen Y-P, Guo J-S, Shen Y, Yang J-X. The correlations and spatial characteristics of microbiome and silage quality by reusing of citrus waste in a family-scale bunker silo. J Clean Prod. 2019;226:407–18.
Li M, Zhou H, Zi X, Cai Y. Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim Sci J. 2017;88(10):1531–7.
Fimbres H, Hernández-Vidal G, Picón-Rubio JF, Kawas JR, Lu CD. Productive performance and carcass characteristics of lambs fed finishing ration containing various forage levels. Small Rum Res. 2002;43(3):283–8.
Meale SJ, Chaves AV, Baah J, McAllister TA. Methane production of different forages in In vitro ruminal fermentation. Asian Aust J Anim Sci. 2012;25(1):86–91.
Kung L Jr, Taylor CC, Lynch MP, Neylon JM. The effect of treating alfalfa with Lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for lactating dairy cows. J Dairy Sci. 2003;86(1):336–43.
Santoso B, Widayati TW, Hariadi BT. Improvement of fermentation and the in vitro digestibility characteristics of agricultural waste-based complete feed silage with cellulase enzyme treatment. Adv Anim Vet Sci. 2020;8(8):873–81.
Satter LD, Slyter LL. Effect of ammonia concentration of rumen microbial protein production in vitro. Br J Nutr. 1974;32(2):199–208.
Acknowledgements
Special acknowledgements to Dr. Diwakar Vyas' s laboratory in the Department of Animal Science, University of Florida, USA, for the technical assistance during in vitro digestion experiment of this research.
Funding
This work was financially supported by Natural Science Foundation of Hunan Province (2021JJ30353).
Author information
Authors and Affiliations
Contributions
LM: conceptualization, methodology, and writing—review and editing. QW: resources and formal analysis. YW: investigation and data curation. ZZ: validation and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal handling and rumen fluid sampling procedures were performed according to protocol approved by the University of Florida Animal Care Research Committee; Protocol No. 202009849.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mu, L., Wang, Q., Wang, Y. et al. Effects of cellulase and xylanase on fermentative profile, bacterial diversity, and in vitro degradation of mixed silage of agro-residue and alfalfa. Chem. Biol. Technol. Agric. 10, 40 (2023). https://doi.org/10.1186/s40538-023-00409-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00409-4