Abstract
Straw return is a beneficial agricultural practice but mechanisms and information on microbial community alterations initiated by decomposed straw and promotion of soil nutritive content and conservation is still not adequately understood. Therefore, the study applied molecular, bioinformatics, chemical and plant yield analysis to determine the effect of wheat straw return and decomposing agent on soil, fungi and crop yield characteristics under wheat biomass return with and without the inclusion of the decomposition agent at varying times. We hypothesized no difference in fungal community diversity and composition, no changes in soil characteristics as well as no changes in cotton growth characteristics between three different conditions (i) no straw return, (ii) straw return under decomposition agent and (iii) straw return under no decomposition agent. It involved analysis of changes in fungal community diversity and composition, changes in soil characteristics as well as changes in cotton growth characteristics under the different treatments. The phyla Ascomycota and Basidiomycota were in relatively in highest abundance within the straw return under decomposition agent treatment than in both the straw return treatment and straw return under no decomposition agent treatment. The pathogenic genus Aspergillus as the most dominant under no straw return treatment, while genus Trechispora, Lulwaona and Dioszegia were most dominant under straw return under no decomposition agent treatment. Indeed, there was high fungal community dissimilarity between the three treatments. Additionally, there was increased rise in fungal diversity, soil nutrients and crop yield under the straw return within the shortest possible time, and the addition of decomposing agent further enhanced the high fungal species diversity.
Graphical Abstract

Similar content being viewed by others
Introduction
Straw return is a beneficial agricultural practice for handling and advancing soil quality preservation and crop productivity [11]. It plays a critical part in lowering the adoption of chemical fertilizer and advancing soil carbon sequestration. Through that, it therefore keeps up soil richness and reduces the amount of environmental contaminants, which results in altered bacterial communities richness in the soil [21]. The microbial community alterations actuated by deteriorated straw return can significantly promote soil wellbeing [40]. Without a doubt, the key variables affecting soil microbial community varieties is significant in soil wellbeing administration based on conditions of straw return, and long-term of decomposed straw return can give appropriate fertility and saltiness for a more beneficial advancement of soil microbial community, both in richness and structure, when compared to undecomposed straw return [27]. Be that as it may, there is still inadequate knowledge on the reactions of natural carbon divisions, chemicals action, and soil bacterial communities and differing qualities to distinctive straw return modes under intensive maize–wheat double-cropping frameworks [20]. In addition, higher sums of straw returns can further lead to higher soil hydrolytic chemicals activities. However, the polyphenol oxidase action gets higher within the no straw return treatment. On another viewpoint, compared with the fresh straw return, long-term decayed straw return has been reported to benefit soil microbial communities, with lower relative number of pathogenic fungal communities which promote higher crop productivity [1].
Wheat or oilseed rape straw returning into soil, either by mulching or by furrowing, results in a more reducible soil with lower redox potential which can ruin the development of rice [16]. Comparing with other straw returning strategies, oilseed-rape straw returning back to the rice field by furrowing is more appropriate for the rice-oil seed rape turn framework [30]. Research experiments have indicated that, compared to the cases within the no straw return practices, close to between 80 and 90% of groundwater nitrate can be eliminated in low and high-water seasons within the farmlands where straw return is practiced, an evidence of potential positive impact of straw return to groundwater quality [12, 17, 18]. Additionally, return of wheat straw has also affirmed to be suitable for promoting soil structure, soil natural carbon, and crop quality. Straw return equally promote carbon sequestration in an intensive agro-ecosystem and ought to be approved as a long-term practice in promoting soil health and to achieve quality yields [10, 37, 45].
Straw ditch-buried returning may be a rare straw returning mode that can be a better avenue for promoting soil quality [17,18,19, 44]. In fact, cotton straw and cotton straw biochar have been reported to control the volatilization of alkali for improved cotton productivity through expanded efficiency in fertilizer uptake. Additionally, based on low potassium condition, returned straw as potassium source can result to lower C/N proportion in the leaf and higher apportion of biomass to regenerative organs more than the inorganic potassium fertilizer [14]. Long-term nonstop cotton cultivation under straw return have equally brought about soil natural carbon structure aliphatic and have also advanced soil mineral adhesive for expanded conservation and stability of organic matter [3, 31]. In spite of all these advantageous aspects of straw return to soil richness as well as to crop yield, genotypic and phenotypic characteristics, there are still few challenges that emerge from the practice. For instance, it has been shown to prevent the physiology and may advance the retention of metals within the agricultural farm soils [28].
Straw return plays a critical part in diminishing the utilization of chemical fertilizer, advancing soil carbon sequestration, hence keeping up soil quality and reducing natural contamination [41]. Returning straw to the field can cause an increment in the amount of expansive agglomerates and decrease the micro-agglomerations, making conducive condition for enhancement of soil structure, supplement status and the quality crop yields [33]. Indeed, the microbial community alterations are initiated by decomposed straw return contributions to soil nutritive content which promote soil nutritive content conservation [27]. Therefore, the study aimed at determining the dynamics in microbial characteristics under wheat straw biomass return with and without the inclusion of the decomposition agent. We hypothesized no difference in fungal community diversity and composition, no changes in soil characteristics as well as no changes in cotton growth characteristics between three different conditions (i) no straw return, (ii) straw return under decomposition agent and (iii) straw return under no decomposition agent. It involved analysis of changes in fungal community diversity and composition, changes in soil characteristics as well as changes in cotton growth characteristics under the different treatments. The main experimental goal was to explore the effects of wheat straw return on microbial characteristics dynamics, soil quality improvement for improved cotton productivity. The no straw return (A), straw return under decomposition agent (B) and straw return under no decomposition agent.
Materials and methods
Experimental set-up and design
This study was undertaken in 2017 within Cao Tsui Village, Maojiagang, Public Security County. The region has moderate fertility, flat terrain, mild withering yellowing disease. The variety of crop involved in this study was cotton ZD2040, in which the effects of the three different treatments on the fungal community assemblage, soil quality and dynamics in cotton growth parameters were investigated. The study design was an experimental randomized design having three treatments and three replications. The plot area was approximately 30.4 m2. For each crop, experimental plots were set in triplicate and each crop subjected to the three different treatments involving: (i) no straw return and no decomposition agent (control); (ii) application of straw return and decomposition agent; (iii) application of straw return.
The straw return (SR) involved the crushing of the wheat into small pieces of approximately 1–1.5 cm in length and then were manually spread over the field. The SR was also added to the decomposition agent and also manually spread over the field. For purposes of control, other plots were neither subjected to SR nor decomposition agent. The wheat straw application rate of 9500 kg ha−1 was maintained across all the experimental treatment plots. Sampling of soil was done in October 2017 from two soil layers; 0–20 cm and 20–40 cm in October 2017 and homogeneously mixed together. The planting was undertaken on the experimental plot at a planting density 4500 plants/acre. Selection of five points was done from each of the plots where soil cores were obtained. Thereafter, removal of stones and plant residues from the samples was undertaken and subsequently divided into two portions; one sub-sample was stored at − 20 °C for DNA extraction, while the other was air-dried and passed through an 8-mm sieve for the other soil quality analyses.
Sampling of soil
Sampling of soil for fungal-biodiversity analysis and N, P, K content measurement was undertaken after 0d, 20d, 40d, 60d and 80d and 100d under every experimental treatment.
Extraction of soil DNA extraction and Illumina HiSeq sequencing
The soil DNA extraction was obtained from 0.5 g of the soil samples. Under this, Fast DNA SPIN Kit for soil was applied while strictly following the manufacturer’s instructions. The quantification of the DNA was undertaken on a Nanodrop spectrometer and then kept at − 80 °C before further use. The amplification of the fungal ITS1 region was achieved through the primers ITS1-F (5ʹ-CTTGGTCATTTAGAGGAAGTAA-3ʹ) and ITS2-R (5ʹ-GCTGCGTTCTTCATCGATGC-3ʹ). The sequencing of the ITS gene fragments was performed through the application of Miseq platform. Amplicons were then extracted from 2% agarose gels, and followed by the purification using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences) according to the manufacturer instructions. Their quantification was then performed with Quanti FluorTM-ST (Promega). The purified amplicons were then pooled in an equimolar manner, and were then paired end-sequenced (2*300) on a MiSeq platform. The entire MiSeq sequencing procedure was performed at Sanshu Biotechnology Company, Shanghai, China.
The QIIME software was applied in the sequence analysis, and in this procedure, the primers were removed, and any sequences with a length of < 220 or > 500 bp, mean quality score < 20, and the number of nitrogenous bases > 3 were omitted from further analysis. UPARSE (version 7.1) was then used to cluster Operational taxonomic units (OTUs) with a 97% similarity cutoff; USEARCH was used to filter chimeras, and the remaining sequences were clustered to generate OTUs at the 97% similarity level [6]. For the calculation of species diversity (Chao and Shannon indices), the Mothur software was used with 35,000 sequence reads randomly selected from each sample. A representative sequence of each OTU was assigned to a taxonomic level in the Ribosomal Database Project (RDP) using the RDP classifier [23].
Variation in samples based on alpha and beta diversity analysis
The complexity of species diversity within the samples were analyzed through Alpha diversity based on the 6 indices; observed-species, Chao1, Shannon, Simpson, ACE, Good-coverage. All the indices were calculated under the QIIME (Version 1.9.1) and displayed with R software (Version 2.15.3). Chao1 estimator and ACE estimator were selected and applied in the identification of community richness, the Good’s coverage was used to characterized sequencing depth while Shannon index and Simpson index were applied to analyze community diversity. Beta diversity analysis was used to evaluate samples differences based on species complexities. Venn analysis was applied to study the fungal gene overlap between the different treatments. Additionally, Beta diversity on both weighted and unweighted UniFrac were calculated by the QIIME software (Version 1.9.1). Cluster analysis was preceded by principal coordinate analysis (PCoA), which was applied to reduce the dimension of the original variables using the FactoMineR package and ggplot2 package in R software (Version 2.15.3).
Quantification of soil chemical and determination of plant growth characteristics
Measurement of the soil pH was undertaken on day 0, 20, 40, 60 and 90 based on soil-to-water ratio of 1:2.5 using a pH meter (F20, Shanghai Mettler-Toledo International Trading Co., Ltd., Shanghai, China). The soil available nitrogen (N), available phosphorus (P) and available potassium (K) contents were also measured. The N was measured using alkaline hydrolysis-diffusion method and titration with ferrous ammonium sulfate, while P and K were determined calorimetrically after extraction with 3% ammonium carbonate. Plant growth characteristics (height, number of branches per plant, number of balls per plant, single boll weight, lint percentage, seed cotton yield and lint yield) were determined at harvest period.
Data analysis
The SPSS version 22.0 analytical software was used for the statistical analyses. One-way analysis of variance (ANOVA) with a least significant difference (LSD) test was used to evaluate the differences of dependent variables. The P < 0.05 level was considered to be significant.
Results
Operational taxonomic unit (OTU) and community diversity
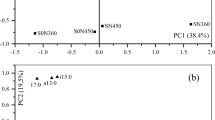
Result from principal coordinate analysis (PCoA) that followed Cluster analysis and applied to indicate the association in the operational taxonomic unit between different treatments and to reduce the dimension of the original variables is presented in Fig. 1. Based on this, it was indicated that there was distinct variation in the OTUs between no straw return treatment (A), straw return under decomposition agent treatment (B) and straw return under no decomposition agent treatment (C). Result on community diversity at phylum level across the three experimental treatments is provided in Fig. 2. Based on that, the most dominant phylum across all the treatments was Ascomycota, which was in relatively highest abundance within the straw return under decomposition agent treatment than in both the straw return treatment and straw return under no decomposition agent treatment. Additionally, phylum Basidiomycota was the second most abundance, though it occurred in relatively highest abundance under straw return under no decomposition agent treatment than under both the no straw return treatment and straw return under decomposition agent treatment. Other noted phyla, though in relatively low abundance were Chytridiomycota, Glomeromycota, Rozelomycota and Zygomycota. Result on community diversity at genus level across the three experimental treatments is provided in Fig. 3. Based on this, Aspergillus was the most dominant under no straw return treatment. Genus Trechispora, Lulwaona and Dioszegia were the most dominant under straw return under no decomposition agent treatment. Through weighted unifrac dissimilarity analysis (Fig. 4), it was shown that there was high fungal community dissimilarity between the three treatments, indicating that the different experimental treatments had different effects on fungal community diversity. To measure the fungal community network centrality and relationship across the treatments, the important nodes were identified based on the network analysis as provided in Fig. 5.
Dynamics in fungal community diversity
The diversity of the fungal communities across different treatments was undertaken through Simpson, Chao 1, ACE and Shannon diversity indices (Fig. 6). From the result, within the no straw return treatment, it was shown the relative high species diversity based on all the indices occurred at the 80d sampling while the lowest was at the 100d sampling period. Within the straw return under decomposition agent, highest relative species diversity based on all the indices occurred at 0d while the lowest was at 60d. Additionally, within the straw return under no decomposition agent, highest relative species diversity based on all the indices occurred at 0d while the lowest was at 20d.
Fungal diversity abundance through Simpson, Chao1, ACE and Shannon indices under; no straw return (A), straw return under decomposition agent (B) and straw return under no decomposition agent (C) from soil sampled at different periods; Time 1 (0d), Time 2 (20d), Time 3 (40d), Time 4 (60d), Time 5 (80d) and Time 6 (100d)
Soil fertility and cotton growth properties
The soil chemical properties under different treatments are presented in Table 1. From the result, it was noted that the pH generally increased from day 0 to day 40, then decreased to day 90 across all the treatments. At day 90, it was found that the straw return under decomposition agent treatment maintained the pH at near neutral. The value of N, P and K decreased from day 0 to 20, while the values of N and K decreased from day 20 to 60. The value of P increased in the no straw return (A) and straw return under decomposition agent (B), while decreased in the straw return under no decomposition agent (C) from day 20 to 60. Results on plant growth properties under different treatments are presented in Table 2. The finding indicated that plant height and the number of branches per plants were noted in the no straw return treatment (A). There were no significant differences in the single boll weight and lint percentage among the three treatments. The treatment of straw return under decomposition agent (B) has the highest number of balls and yield at harvest period.
Discussion
The study explored the effects wheat straw return on microbial characteristics dynamics, soil quality improvement for improved cotton productivity based on three different experimental treatments; the no straw return (A), straw return under decomposition agent (B) and straw return under no decomposition agent. Wheat return practice has been an important part of many arable rotations. However, studies have continued to record a tremendous decline in the uptake of the technology owing to anxieties around the risks, economic feasibility and pest prevalence (particularly cabbage stem flea beetle) that have resulted in large year-on-year reductions in its application hectarage. However, with a relatively favorable gross margin, compared with feed wheat, where oilseed rape can be grown well, it still performs competitively better in the rotation farming practice. Including oilseed rape in the rotation has also been shown to increase the yield of other crops in comparison to a cereal monoculture due to its greater ability to improve soil structure [13]. On this note, some studies have assessed the interactions that play a role in the plant-specific context in order to address relevant ecological interactions that need to be fully explored for its improved ecological roles [9]. Indeed, under increasing rape straw incorporation, the amount of root (number, weight and length) and root bleeding intensity of planted crop can increase significantly. Moreover, comparing with other straw returning methods, straw returning by ploughing method with the rape straw of 3.0 t/hm2 has been recorded to be more suitable in rotation farms [30].
The practice of incorporating wheat straw in soil has remained one of the effective methods applied with an aim of enhancing the use efficiency of agricultural resources in crop rotation practices, such as the rape–rice rotation system. However, the impacts of wheat straw incorporation on root growth and dynamic changes in soil have still remained unclear (Xuechun [36]. Under straw return, seeds characterized by lower bacterial diversity and lower number of beneficial taxa have been shown to be colonized by allochthonous cells in higher amounts than seeds with comparatively higher bacterial diversity. It is therefore suggested that the treatment of seeds showing lower bacterial diversity with beneficial bacterial strains may result in increased resistance towards pathogens. By contrast, under conditions in which seeds with higher bacterial diversity are treated with the same bacterial strains, the resistance of the seedlings to pathogens is less affected. Hence, such model is suggested for plant seeds with a tight bacterial network in which the introduction of new bacterial strains is rather challenging [25]. Indeed, the current study reported distinct variation in the OTUs between no straw return treatment, straw return under decomposition agent treatment and straw return under no decomposition agent treatment. This pointed out the effect brought about by the use of straw return and straw return with decomposing agent on the soil microbial quality. Indeed, this agree with the study that had indicated, and that the straw and its organic components decomposition proportion could be increased by adding straw decomposing agent for even a much better performance [32, 34]. Moreover, long term of decomposed straw return can provide suitable nutrient and salinity for healthier development of soil microbial community, both in abundance and structure, compared with fresh straw return [27].
The most dominant phylum across all the treatments was Ascomycota, which was in relatively highest abundance within the straw return under decomposition agent treatment than in both the straw return treatment and straw return under no decomposition agent treatment. Phylum Basidiomycota was the second most abundance, though it occurred in relatively highest abundance under straw return under no decomposition agent treatment than under both the no straw return treatment and straw return under decomposition agent treatment. Globally Ascomycota have been shown to dominate healthy soils [7]. Ascomycota fungi are important drivers in carbon and nitrogen cycling in arid ecosystems. These fungi play roles in soil stability, plant biomass decomposition, and endophytic interactions with plants. They may also form symbiotic associations with biocrust components or be latent saprotrophs or pathogens that live on plant tissues [2]. Moreover, land use type can promote Ascomycota, Basidiomycota, and Zygomycota to transition from Basidiomycota-dominant to Ascomycota-dominant community due to vegetation restoration [39]. In addition to the Ascomycota and Basidiomycota, other noted phyla, though in relatively low abundance were Chytridiomycota, Glomeromycota, Rozelomycota and Zygomycota.
There has been a push for shift from cataloging fungal species in different soil ecosystems toward a more global analysis based on functions and interactions between organisms and specific fungal species have been linked to particular beneficial contributions to soil and crop quality [8]. The current study indicated genus Aspergillus as the most dominant under no straw return treatment, while genus Trechispora, Lulwaona and Dioszegia were most dominant under straw return under no decomposition agent treatment. Indeed, the predominance of Aspergillus species in soil has shown a high risk of Aspergillus infections on exposure to fungi in agricultural fields, pointing to the beneficial contribution of oil seed straw return to the health of the soil and plant [24]. The Trechispora are considered as a soil-inhabiting fungus with biotrophic ability, processing beneficial soil remineralization potential [29]. The specific roles played by genus Lulwaona and Dioszegia have not been adequately reported, creating the need for their exploitation through research.
Significant correlation has been observed between fungal community compositional changes and carbon or nitrogen availability of different soil types, suggesting that few characteristics such as fungal richness and taxa abundance of rhizosphere and pneumatophore soil compartments may be significantly different based on the nature of soil conservation practice [26]. Indeed, the current study showed high fungal community dissimilarity between the three treatments, indicating that the different experimental treatments had different effects on fungal community diversity. This was also affirmed through network analysis which indicated fungal community network centrality and relationship across the treatments.
Cropping with wheat or oilseed rape can increase the microbial richness and diversity, alleviating the deleterious effect of the pollutants in soils, making oilseed rape to play a positive role in maintaining species diversity of microorganism from the side [35]. The current study showed a relative high species diversity based on all the indices at the 80d sampling while the lowest was at the 100d sampling period under no straw return, there was highest relative species diversity based on all the indices occurred at 0d while the lowest was at 60d within the straw return treatment. Additionally, within the straw return under no decomposition agent, highest relative species diversity based on all the indices occurred at 0d while the lowest was at 20d. This showed that under straw return, there was increased rise in fungal diversity within the shortest possible time, and the addition of decomposing agent further enhanced the high fungal species diversity.
Plant biomass return practice enhance soil fertility, and treatment applied continuously greatly promotes soil structure, raises soil nutrient decomposition, and increases microbial community composition [5, 32, 34]. Straw return can also promote the soil bacteria, fungal and nematode communities, resulting to increased levels of available nitrogen and phosphorus in the soil [38]. The contribution of exchangeable K towards plant K uptake has ben higher in the soil subjected to straw return. K fertilizer alongside straw return increase soil available K and this underlines the importance of the straw biomass return and its contribution for sustained K supplying ability of soils [42, 43]. Due to the slow decomposition of straw, N and K was mainly obtained from soil in the first 20 days of seedling stage, and the values of N and K content in soil decreased. Compared with straw return under no decomposition agent (C), the value of N in the soil with decomposition agent decreased significantly in the straw return under decomposition agent (B). Combined with the yield characteristics of cotton at harvest time (Table 2), it showed that straw returning and adding decomposition agent could promote the absorption of cotton, thus increasing the harvest index of cotton field. The differences of P content may indicate that different fungal types in soil of three treatments have different ability to promote the transformation of P into available P.
Changing of straw biomass into biochar and consolidating it into agricultural field was affirmed to be an applicable custom for maintaining quality cotton production within the barley–cotton rotation framework [22]. Straw incorporation under the conditions with deep tillage, irrigation and fertilization, has great potential to increase crop yield in the wheat–maize cropping system [15]. In fact, straw return coupled with appropriate tillage method can improve grain yield and nitrogen efficiency in most crops [4]. The improvement in cotton production was noted to be due to the enhanced contents of soil available N, P and K, root biomass, NPK uptake by cotton; apportionment percentage of NPK to bolls as well as canopy apparent photosynthesis rate [22]. Improved yield could also emanate from organic matter that supports the absorption of mineral nutrients by roots, thereby promoting the efficiency of fertilizer utilization [42, 43]. Indeed, the current study also showed highest quantities of the plant yield parameters being noted in the straw biomass return under decomposition agent treatment within the shortest possible time.
Conclusion
There was distinct variation in the OTUs between no straw return treatment, straw return under decomposition agent treatment and straw return under no decomposition agent treatment. The phyla Ascomycota and Basidiomycota were in relatively highest abundance within the straw return under decomposition agent treatment than in both the straw return treatment and straw return under no decomposition agent treatment. The pathogenic genus Aspergillus as the most dominant under no straw return treatment, while genus Trechispora, Lulwaona and Dioszegia were most dominant under straw return under no decomposition agent treatment. Indeed, there was high fungal community dissimilarity between the three treatments. Additionally, there was increased rise in fungal diversity, soil nutrients and crop yield under the straw return within the shortest possible time, and the addition of decomposing agent further enhanced the high fungal species diversity.
Availability of data and materials
The primary research data for this study are with the corresponding author and can be availed on reasonable request.
References
Cao Q, Li G, Yang F, Kong F, Cui Z, Jiang X, Lu Y, Zhang E. Eleven-year mulching and tillage practices alter the soil quality and bacterial community composition in Northeast China. Archiv Agron Soil Sci. 2021. https://doi.org/10.1080/03650340.2021.1890719.
Challacombe JF, Hesse CN, Bramer LM, McCue LA, Lipton M, Purvine S, Nicora C, Gallegos-Graves LV, Porras-Alfaro A, Kuske CR. Genomes and secretomes of ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genom. 2019. https://doi.org/10.1186/S12864-019-6358-X.
da Chang H, Wang J, Zhang FH. Effects of continuous cropping with straw return on particulate organic carbon and fourier transform infrared spectroscopy in cotton field Ying Yong Sheng Tai Xue Bao. J App Ecol. 2019. https://doi.org/10.3287/J.1001-9332.201904.007.
Jin C, Meng-jing Z, Dang-wei P, Yan-ping Y, Ming-ming H, Yan-xia L, Yong-li L, Xu X, Yong L, Zhen-lin W. Straw return and appropriate tillage method improve grain yield and nitrogen efficiency of winter wheat. J Integr Agric. 2017. https://doi.org/10.1016/S2095-3119(16)61589-7.
Cui H, Luo Y, Chen J, Jin M, Li Y, Wang Z. Straw return strategies to improve soil properties and crop productivity in a winter wheat-summer maize cropping system. Eur J Agron. 2022;133: 126436. https://doi.org/10.1016/J.EJA.2021.126436.
Edgar RC. Updating the 97% identity threshold for 16S ribosomal RNA OTUs. Bioinformatics. 2018;34(14):2371–5. https://doi.org/10.1093/BIOINFORMATICS/BTY113.
Egidi E, Delgado-Baquerizo M, Plett JM, Wang J, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK. A few ascomycota taxa dominate soil fungal communities worldwide. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-10373-z.
Frac M, Hannula SE, Belka M, Jȩdryczka M. Fungal biodiversity and their role in soil health. Front Microbiol. 2018. https://doi.org/10.3389/FMICB.2018.00707/BIBTEX.
Yang H, Ma J, Rong Z, Zeng D, Wang Y, Hu S, Ye W, Zheng X. Wheat straw return influences nitrogen-cycling and pathogen associated soil microbiota in a wheat–soybean rotation system. Front Microbiol. 2019;10:1811. https://doi.org/10.3389/FMICB.2019.01811/BIBTEX.
Gao X, Gu F, Hao W, Mei X, Li H, Gong D, Mao L, Zhang Z. Carbon budget of a rainfed spring maize cropland with straw returning on the Loess Plateau, China. Sci Total Environ. 2017;586:1193–203. https://doi.org/10.1016/j.scitotenv.2017.02.113.
Guo L, Zheng S, Cao C, Li C. Tillage practices and straw-returning methods affect topsoil bacterial community and organic C under a rice-wheat cropping system in central China. Sci Rep. 2016. https://doi.org/10.1038/srep33155.
Hao Z, Zhang X, Gao Y, Xu Z, Yang F, Wen X, Wang Y. Nitrogen source track and associated isotopic dynamic characteristic in a complex ecosystem: a case study of a subtropical watershed, China. Environ Pollut. 2018;236:177–87. https://doi.org/10.1016/j.envpol.2018.01.078.
Hegewald H, Wensch-Dorendorf M, Sieling K, Christen O. Impacts of break crops and crop rotations on oilseed rape productivity: a review. Eur J Agron. 2018;101:63–77. https://doi.org/10.1016/J.EJA.2018.08.003.
Hu W, Yu C, Zhao W, Liu R, Yang C, Zhou Z. The effects of straw-returning and inorganic K fertilizer on the carbon–nitrogen balance and reproductive growth of cotton. J Cotton Res. 2021;4(1):1–11. https://doi.org/10.1186/S42397-021-00106-3/FIGURES/7.
Islam MU, Guo Z, Jiang F, Peng X. Does straw return increase crop yield in the wheat-maize cropping system in China? a meta-analysis. Field Crops Res. 2022;279: 108447. https://doi.org/10.1016/J.FCR.2022.108447.
Jin Z, Shah T, Zhang L, Liu H, Peng S, Nie L. Effect of straw returning on soil organic carbon in rice–wheat rotation system: a review. Food Ene Sec. 2020. https://doi.org/10.1002/FES3.200.
Li R, Ruan X, Bai Y, Ma T, Liu C. Effect of wheat-maize straw return on the fate of nitrate in groundwater in the Huaihe River Basin, China. Sci Total Environ. 2017;592:78–85. https://doi.org/10.1016/J.SCITOTENV.2017.03.029.
Li S, Chen J, Shi J, Tian X, Li X, Li Y, Zhao H. Impact of straw return on soil carbon indices, enzyme activity, and grain production. Soil Sci Soc Am J. 2017;81(6):1475–85. https://doi.org/10.2136/SSSAJ2016.11.0368.
Li S, Li X, Zhu W, Chen J, Tian X, Shi J. Does straw return strategy influence soil carbon sequestration and labile fractions? Agron J. 2019;111(2):897–906. https://doi.org/10.2134/AGRONJ2018.08.0484.
Li Y, Tremblay J, Bainard LD, Cade-Menun B, Hamel C. Long-term effects of nitrogen and phosphorus fertilization on soil microbial community structure and function under continuous wheat production. Environ Microbiol. 2020;22(3):1066–88. https://doi.org/10.1111/1462-2920.14824.
Liu C, Lu M, Cui J, Li B, Fang C. Effects of straw carbon input on carbon dynamics in agricultural soils: a meta-analysis. Glob Change Biol. 2014;20(5):1366–81. https://doi.org/10.1111/gcb.12517.
Ma L, Kong F, Wang Z, Luo Y, Lv X, Zhou Z, Meng Y. Growth and yield of cotton as affected by different straw returning modes with an equivalent carbon input. Field Crop Res. 2019;243: 107616. https://doi.org/10.1016/J.FCR.2019.107616.
Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR, Farris RJ, Garrity GM, Olsen GJ, Schmidt TM, Tiedje JM. The RDP-II (ribosomal database project). Nucleic Acids Res. 2001;29(1):173–4. https://doi.org/10.1093/NAR/29.1.173.
Mousavi B, Hedayati MT, Hedayati N, Ilkit M, Syedmousavi S. Aspergillus species in indoor environments and their possible occupational and public health hazards. Current Med Mycol. 2016. https://doi.org/10.18869/ACADPUB.CMM.2.1.36.
Rybakova D, Mancinelli R, Wikström M, Birch-Jensen AS, Postma J, Ehlers RU, Goertz S, Berg G. The structure of the Brassica napus seed microbiome is cultivar-dependent and affects the interactions of symbionts and pathogens. Microbiome. 2017;5(1):104. https://doi.org/10.1186/S40168-017-0310-6/FIGURES/9.
Sanka Loganathachetti D, Poosakkannu A, Muthuraman S. Fungal community assemblage of different soil compartments in mangrove ecosystem. Sci Rep. 2017. https://doi.org/10.1038/S41598-017-09281-3.
Su Y, Lv JL, Yu M, Ma ZH, Xi H, Kou CL, He ZC, Shen AL. Long-term decomposed straw return positively affects the soil microbial community. J Appl Microbiol. 2020;128(1):138–50. https://doi.org/10.1111/JAM.14435.
Su Y, Kwong RWM, Tang W, Yang Y, Zhong H. Straw return enhances the risks of metals in soil? Ecotoxicol Environ Saf. 2021;207: 111201. https://doi.org/10.1016/J.ECOENV.2020.111201.
Vanegas-León ML, Sulzbacher MA, Rinaldi AC, Roy M, Selosse MA, Neves MA. Are Trechisporales ectomycorrhizal or non-mycorrhizal root endophytes? Mycol Prog. 2019;18(9):1231–40. https://doi.org/10.1007/S11557-019-01519-W.
Wang H, Yang G, Wang X, Zhao C, Muhammad N, Chen Y, Hu Y. Oilseed rape straw returning changes soil reducibility and affects the root and yield of rice in the rape-rice rotation field at Sichuan Basin area of China. Agron J. 2020;112(6):4681–92. https://doi.org/10.1002/AGJ2.20408.
Wang Hu, Wang XD, Tian XH. Effect of straw-returning on the storage and distribution of different active fractions of soil organic carbon. Chin J Appl Ecol. 2014;25(12):3491–8.
Wang L, Wang C, Feng F, Wu Z, Yan H. Effect of straw application time on soil properties and microbial community in the Northeast China Plain. J Soils Sed. 2021;21(9):3137–49. https://doi.org/10.1007/S11368-021-03009-7.
Wang SL, Liu YH, Song XL, Wei SB, Li JP, Nie JJ, Qin DL, Sun XZ. Effects of cotton straw returning on soil organic carbon, nitrogen, phosphorus and potassium contents in soil aggregates. Chinese J Appl Ecol. 2016;27(12):3944–52. https://doi.org/10.3287/J.1001-9332.201612.015.
Wang X, Wang X, Geng P, Yang Q, Chen K, Liu N, Fan Y, Zhan X, Han X. Effects of different returning method combined with decomposer on decomposition of organic components of straw and soil fertility. Sci Rep. 2021. https://doi.org/10.21203/RS.3.RS-389287/V1.
Wang X, Bai J, Wei T, Feng G, Zhao H, Wei W, Wang M, Zhao Y. Oilseed rape cultivation increases the microbial richness and diversity in soils contaminated with cadmium. J Soils Sediments. 2018;18(7):2451–62. https://doi.org/10.1007/S11368-018-1938-Y/FIGURES/5.
Wang X, Samo N, Zhao C, Wang H, Yang G, Hu Y, Peng Y, Rasul F. Negative and positive impacts of rape straw returning on the roots growth of hybrid rice in the Sichuan Basin area. Agronomy. 2019. https://doi.org/10.3390/AGRONOMY9110690.
Xu ZW, Zhang XY, Yu GR, Sun XM, Wen XF. Review of dual stable isotope technique for nitrate source identification in surface-and groundwater in China. Huanjing Kexue Environ Sci. 2014;35(8):3230–3038. https://doi.org/10.13227/j.hjkx.2014.08.056.
Yang L, Muhammad I, Chi YX, Wang D, Zhou XB. Straw return and nitrogen fertilization to maize regulate soil properties, microbial community, and enzyme activities under a dual cropping system. Front Microbiol. 2022;13:216. https://doi.org/10.3389/FMICB.2022.823963/BIBTEX.
Yang Y, Dou Y, Huang Y, An S. Links between soil fungal diversity and plant and soil properties on the loess plateau. Front Microbiol. 2017. https://doi.org/10.3389/FMICB.2017.02198.
Yu D, Wen Z, Li X, Song X, Wu H, Yang P. Effects of straw return on bacterial communities in a wheat-maize rotation system in the North China Plain. PLoS ONE. 2018;13(6): e0198087. https://doi.org/10.1371/JOURNAL.PONE.0198087.
Yu D, Wen Z, Li X, Song X, Wu H, Yang P. Effects of straw return on bacterial communities in a wheat-maize rotation system in the North China Plain. PLoS ONE. 2018. https://doi.org/10.1371/JOURNAL.PONE.0198087.
Zhang GJ, Yan JW, Zuo WQ, Zhang PP, Zhang WF. Effects of straw return and fertilisation on root growth and nutrient utilisation efficiency of cotton in an arid area. Crop Pasture Sci. 2021;72(7):528–40. https://doi.org/10.1071/CP20259.
Zhang Z, Liu D, Wu M, Xia Y, Zhang F, Fan X. Long-term straw returning improve soil K balance and potassium supplying ability under rice and wheat cultivation. Sci Rep. 2021. https://doi.org/10.1038/s41598-021-01594-8.
Zhao H, Jiang Y, Ning P, Liu J, Zheng W, Tian X, Shi J, Xu M, Liang Z, Shar AG. Effect of different straw return modes on soil bacterial community, enzyme activities and organic carbon fractions. Soil Sci Soc Am J. 2019;83(3):638–48. https://doi.org/10.2136/SSSAJ2018.03.0101.
Zheng L, Wu W, Wei Y, Hu K. Effects of straw return and regional factors on spatio-temporal variability of soil organic matter in a high-yielding area of northern China. Soil and Tillage Res. 2015;145:78–86. https://doi.org/10.1016/J.STILL.2014.08.003.
Acknowledgements
Sanshu Biotechnology Company, Shanghai, China, is acknowledged for the molecular and biotechnology work on this study.
Funding
This research was supported by the National Cotton Industry Technology System Project (CARS-15-12) and Hubei Agricultural Science and Technology Innovation Project (2021-620-000-001-006).
Author information
Authors and Affiliations
Contributions
All authors made equal contribution on study proposal writing, data collection, data analysis and manuscript production. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study did not require ethical approval and consent to participate.
Consent for publication
All the authors have approved this manuscript for publication.
Competing interests
There is no competing interest between the authors or institutions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Wang, Q., Zhang, Y. et al. Influence of decomposition agent application and schedule in wheat straw return practice on soil quality and crop yield. Chem. Biol. Technol. Agric. 10, 8 (2023). https://doi.org/10.1186/s40538-022-00362-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-022-00362-8