Abstract
Background
The negative consequences of industrial agriculture greatly affect human health and the environment. Debating our dietary requirements and challenging the means of food production are necessary. In the first years of transitioning to agroecological production, crop yields normally decrease. Humic acids and beneficial bacteria used as plant growth promoters can be helpful during this stressful time. Metabolite target identification will aid in increasing plant responses to these agents.
Materials
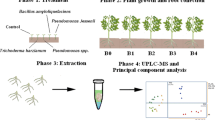
We evaluated the metabolite fingerprints of maize and sugarcane seedlings after 5 days of treatment with like-humic acids isolated from vermicompost coupled with a combined Herbaspirillum seropedicae and Gluconacetobacter diazotrophicus application. The hydromethanolic foliar extracts were submitted for 1H nuclear magnetic resonance analysis, and the data were explored using chemometrics procedures. After the preliminary screening, the extracts were analyzed by gas chromatography coupled to time of flight mass spectrometry to identify metabolite targets.
Results
The biostimulant significantly changed the metabolic fingerprints independent of the plant species. The main proton spectral regions changed by biostimulant use were from 0 to 2.5 ppm and 3.5 to 5 ppm, as revealed by a principal component analysis. The main signals corresponded to amino acid, sugar and organic acid chemical shifts. Aspartic acid was the amino acid present in greatest amounts in both leaf extracts. A significant change occurred in the region normally attributed to (CHn)-protons bound to electron-withdrawing groups, such as carboxyls from mucic, ribonic and saccharic acids derived from sugars and aromatic structures from shikimic acid, 4-hydroxybenzoate and 3,4-dihydroxycinnamic acid. The main organic acids altered by the biostimulant were representatives of the tricarboxylic acid cycle (citric, isocitric, aconitic, malic and fumaric acids). Linoleic and myristic acids, 1-mono palmitin and tocopherol were the major lipid components found at greater levels in the treated leaf extracts. Compounds from the oxidative end products of ascorbic acid metabolism, like threonic, isothreonic and oxalic acids, are putative biomarkers of the biostimulant as are the cyclic polyol identified as quinic acid and trehalose, a disaccharide involved in plant stress responses.
Conclusion
The biostimulant induced significant changes in the metabolite fingerprints of maize and sugarcane seedlings as revealed by nuclear magnetic resonance. Both primary and secondary metabolisms were affected, and 22 putative biomarkers associated with the biostimulant-treated plant phenotype were identified. This agrees with previous work indicating that the stimulation of primary and secondary metabolisms was partially responsible for biostimulant effects on non-leguminous plants. Moreover, these metabolite targets could be used to genetically manipulate metabolic pathways to aid Poaceae breeding programs in increasing biostimulative responses.

Similar content being viewed by others
Introduction
Modern industrial agriculture, having large chemical, nutrient and energy inputs, has contributed to food insecurity, rural poverty and ecosystem degradation in the tropics [1]. Agroecology principles have been successfully applied to overcome these problems [2]; however, in the initial transitional years between systems declining production levels occur. Biological inputs can play strategic roles in aiding plant growth under different stress conditions [3] and in promoting the discussion of technology adaptation [4].
Biostimulants manufactured using like-humic acids (HA) isolated from vermicompost function as vehicles for the field introduction of plant growth-promoting bacteria (PGPB) and have been used with success in different crops [5,6,7,8,9]. Some mechanisms involved in this promotion have been elucidated, especially those involving the increase in the beneficial bacterial population inside the plants, which is promoted by humic matter [10, 11].
An important issue that is usually overlooked in biostimulant use is the origin of the plant materials. These are generally from traditional plant breeding programs, meaning they are produced for chemical input responses. The identification of target metabolites may present a shortcut in the long path to selecting plants that respond better to biostimulants.
The plant metabolome is the entirety of the small molecules present and can be regarded as the ultimate expression of its genotype in response to environmental changes [12]. Metabolomics uses nuclear magnetic resonance (NMR)- or mass spectrometry (MS)-based technology for an unbiased metabolome analysis with a high diagnostic power [13]. Fiehn [12] classified the metabolomics analysis into three main categories: fingerprinting, metabolic profiling and targeted analysis. Metabolite finger printing provides information from the spectra of the metabolites’ total composition, the metabolic profile aims to identify and quantify as many compounds as possible, and the targeted analysis is conducted on a rather limited number of metabolites chosen based on prior knowledge.
Single or a small numbers of metabolites that have the potential to be developed into rapidly accessible biomarkers can be extracted from metabolic fingerprint studies [14]. Small changes in metabolite concentrations may have large biological impacts but are difficult to detect, highlighting the importance of data processing in metabolic fingerprint analyses [15]. In the field of metabolomics, the principal component analysis (PCA) is a popular tool for visualizing datasets and for extracting relevant information when a high level of variance is present [16]. It allows the separation of plants on the basis of treatments and the rapid identification of the metabolites that are responsible for the separation. Meyer and colleagues [13] found a highly significant canonical correlation between biomass accumulation and a specific combination of metabolites. Because of the proven diagnostic power of metabolic fingerprinting and our interest in the metabolic status of growing plants, we determined whether a biostimulant manufactured using HA and PGPB modified the metabolic composition of leaf extracts from Poaceae (maize and sugarcane) seedlings. Thus, the aim of this work was to identify putative metabolite targets involved in plant growth stimulation using gas chromatography coupled to time of flight mass spectrometry (GC/TOF-MS) after a fingerprint analysis using 1H NMR.
Materials and methods
Humic substances
A solution of 0.5-M NaOH was mixed with an earthworm compost (10:1, v/v) under N2 atmospheric conditions. After 12 h, the suspension was centrifuged at 5000×g and the HA were precipitated by adding 6-MHCl until a pH of 1.5 was achieved. After centrifugation (5000×g) for 15 min, the sample was repeatedly washed with water to obtain a negative test result against AgNO3. Subsequently, the sample was dialyzed against deionized water using a 1000-Da cut-off membrane (Thomas Scientific, Swedesboro, NJ, USA) and lyophilized. The HA solution was prepared by solubilizing HA powder in 1 mL of 0.01-M NaOH, followed by adjusting the pH to 6.5 with 0.1-M HCl.
PGPB
Herbaspirillum seropedicae strain HRC54 and Gluconacetobacter diazotrophicus strain PAL 5 were isolated as diazotrophic endophytes from sugarcane [17]. H. seropedicae grown in 5 mL of liquid DYGS medium (glucose, 2.0 g L−1; malic acid, 2.0 g L−1; peptone, 1.5 g L−1; yeast extract, 2.0 g L−1; MgSO47H2O, 0.5 g L−1; l-glutamic acid, 1.5 g L−1; pH 6.0) at 30 °C for 36 h, while G. diazotrophicus was grown in DYGS medium without malic acid. Aliquots (20 µL) of each bacterial species were independently inoculated into 2000-mL flasks of liquid DYGS medium under the same growing conditions for 72 h on a rotatory shaker at 150 rpm. Next, the bacterial biomasses were centrifuged at 2000×g for 10 min, resuspended in sterilized water and adjusted to 109 cells mL−1 using the optical density at 540 nm.
Inoculant
The inoculant was prepared by mixing equal volumes of the suspensions of H. seropedicae and G. diazotrophicus to a final volume of the HA suspension at pH 7.0 to produce a final concentration of 48 mg carbon L−1 and a final bacterial concentration of 2 × 108 cells mL−1.
Treatment of plants
Maize seeds (Zea mays L. Dekalb 815) were surface sterilized by being soaked in 0.5% NaClO for 30 min, rinsed with water and finally, soaked in water for 6 h. Then, the seeds were sown on wet filter paper and germinated in the dark at 28 °C. Four days after germination, six maize seedlings with root lengths of approximately 1.0 cm were transferred into 0.5-L vessels filled with a solution containing 2-mM CaCl2 and the inoculant (48 mg carbon L−1 HA + PGPB 108 cells mL−1). A minimal medium (2 mM CaCl2) was used to avoid any interference from nutrients that may act synergistically with humic matter during plant growth and development. The system was continuously aerated using a low flux pump. The leaves were collected after 5 days of treatment. Maize seedlings were placed in a plant growth cabinet with a photoperiod of 10 h of light and 14 h of darkness, a light intensity of 120 μmol m−2 s−1, and temperatures of 25 °C (night) and 28 °C (day). The control plants were grown only in minimal medium without the inoculant.
Micropropagated sugarcane (var SP 70-1143) were transferred to 5-L pots filled with sand and vermiculite (2:1, v:v). The substrate was previously autoclaved three times. The seedlings were irrigated three times a day with water. At 1 week after transplanting, one-fourth-strength Furlani’s nutrient solution (3527-μM Ca, 2310-μM K, 855-μM Mg, 45-μM P, 587-μM S, 25-μM B, 77-μM Fe, 9.1-μM Mn, 0.63-μM Cu, 0.83-μM Mo, 2.29-μM Zn, 1.74-μM Na and 75-μM EDTA) containing a low concentration of inorganic N (100 μM [NO3− + NH4+]) was applied two times per week. At 45 days after transplanting, plants were treated with 400 mL of inoculant per pot (HAs and PGPB) or with water (control). At 5 days after biostimulant addition, the leaves were harvested.
Leaf extracts
The leaf tissues were obtained from fresh leaves (0.5 g per individual; n = 3 biological replicates), reduced to a fine powder in liquid N2 and then extracted with 20 mL of methanol:H2O (80:20, v/v) for 30 min in an ultrasonic bath at 25 °C. The extracts were filtered at low temperature through a cellulose membrane (0.45-μm), and the solvent was removed at a low temperature.
Metabolic fingerprint analysis
1H NMR spectroscopy was conducted at 25 ± 1 °C using a 400 MHz Avance magnet (Bruker Biospin, Rheinstetten, Germany) equipped with a 5-mm Bruker Broadband Inverse (Bruker Biospin) probe working at the 1H frequency of 400 MHz. Samples were prepared by dissolving 10 mg of exudates in 1 mL of deuterated water (99.8% D2O/H2O, Armar Chemicals). 1H NMR spectra were acquired by applying an on-resonance presaturation of water signal (2 s of presaturation at 54–65 dB for power level attenuation) and setting 16 k data points, four dummy scans, a spectral width of 16 ppm (6410.3 Hz) and 128 scans. Spectra were processed using Mestre-C software (v.4.8.6.0, Cambridgesoft, Cambridge, Massachusetts, USA). In particular, FIDs were transformed by applying a line broadening of 3 Hz.
The 1H NMR spectral dataset was auto-scaled and a PCA performed using the software package Unscrambler X 10.2 (Camo Inc., Oslo, Norway). The dataset consisted of a matrix in which each row represented the spectral average of three leaf extracts of sugarcane or maize. The model validation was carried out using a full cross-validation, and the difference in the variance between the calibration and validation models was less than 5%.
Target analysis (GC/TOF-MS)
For the GC/MS-TOF analysis, three samples of 0.8 mg of leaf extracts were previously derivatized to enhance their thermostability. Carbonyl groups were protected by 10 μL of a solution of 20 mg mL−1 methoxyamine in pyridine at 30 °C for 90 min. Then, 90 μL of MSTFA and 1% TMCS were added for the trimethylsilylation of acidic protons at 37 °C for 30 min. After derivatization, 0.5 μL samples were injected in a randomized sequence into a Gerstel cold-injection system (Gerstel, Muehlheim, Germany) and an Agilent 7890A gas chromatograph (Santa Clara, CA, USA) in splitless mode. The system was controlled by LecoChromaTOF software version 2.32 (St. Joseph, MI, USA). A 30-m long, 0.25-mm i.d. RTX 5Sil-MS column with a 0.25-lm 5% diphenyl/95% dimethyl polysiloxane film and additional 10-m integrated guard column were used (Restek, Bellefonte, PA, USA). The injection temperature was 230 °C, and the interface was set at 280 °C. The helium flow was 1 mL min−1. After a delay of 5 min at 50 °C, the oven temperature was increased at 20 °C min−1 to 330 °C, followed by a 5-min isothermic cool down to 50 °C and an additional 5-min delay. Liners were exchanged automatically every 10 samples. A Leco Pegasus IV time-of-flight mass spectrometer was operated at a transfer line temperature of 280 °C. The ion source was adjusted at 250 °C with a − 70 V electron impact ionization. Mass spectra were acquired at a mass resolving power R = 600 from m/z 85 to 500 at 17 spectra s−1. Quantification was reported as peak height using the unique ion as the default, unless a different quantification ion was manually set in the Bin-Base administration Bellerophon software. Metabolites were unambiguously assigned by the Bin-Base identifier numbers, using retention index and mass spectrum as the two most important identification criteria. All the database entries in Bin-Base were matched against the mass spectral library of 1200 authentic metabolites and the NIST05 commercial library. A quantification report table was produced for all the database entries that were positively detected in more than 50% of the samples.
Results
Metabolite fingerprinting
Spectra of sugarcane and maize leaf extracts are shown in Figs. 1 and 2, respectively. The inoculant containing two endophytic diazotroph bacteria suspended in HA changed the metabolic fingerprints of maize and sugarcane seedlings. The differences between the metabolic fingerprints can be easily visualized using a PCA (Fig. 3). The first two PCs comprised 91% of the variance. They distinguished seedlings by plant species and separated biostimulant treated from control plants. The PC1 captured 63% of the total variance and separated the plant species, while PC2 captured 28% of the total variance and separated the inoculated from control plants. PC1 showed (Fig. 4) that control sugarcane had a low content of protons bond to sp2-hybridized carbons, like aromatic structures, with signals centered at 6.6 ppm and of aliphatic groups with a resonance at 1.07 ppm, which were related to the maize control. In addition, compounds with hydroxyalkyl groups, mainly derived from major carbohydrates, like glucose, glucose-6-phosphate and fructose (strong signals from 3.33 to 3.6 ppm), were prevalent in the control sugarcane extract compared with the control maize extract. PC2 (Fig. 4) distinguished treated from untreated for both sugarcane and maize seedlings. Inoculated sugarcane had high levels of aromatic species, while inoculated maize seedlings had lipid components that allowed the treatments to be distinguished. The metabolic fingerprinting approach using 1H NMR is a powerful technique to compare overall metabolic compositions [18] and was useful in discriminating changes induced by the biostimulant manufactured with HA and PGPB (Figs. 1 and 2). The PCA allowed the quick identification of treatment effects in both maize and sugarcane seedlings (Figs. 3 and 4). In conclusion, the metabolite fingerprints were altered by the biostimulant independent of the plant species (Figs. 1 and 2), and the changes were captured by the PCA (Figs. 3 and 4).
Target analysis
The GC/TOF-MS led to the identification of 356 and 280 compounds present in the leaf extracts of maize and sugarcane, respectively, in inoculated and non-inoculated seedlings. The identifications based on mass fragmentations were unequivocal, and 23 compounds were found in greater concentrations in treated plants than in the controls of both maize and sugarcane seedlings. These compounds are shown in Table 1 and are candidate biomarkers of the biostimulant’s action. The significant increase in concentration was confirmed using an analysis of variance (F test, p < 0.01** and 0.05*). In total, 18 of 23 compounds were from the primary metabolism, including one amino acid (aspartic acid) and six organic acids from the tricarboxylic acid cycle (citric, isocitric, aconitic, malic, maleic and fumaric acids). Linoleic, -mono palmitin and myristic acids were the main lipids found in greater concentrations in both species of inoculated seedlings. The phenylpropanoid pathway was significantly induced, and it was possible to observe an increase in the shikimic acid, 4-hydroxybenzoic acid and 3,4 dihydroxybenzoate levels, with the latter two being precursors of salicylic acid biosynthesis. One intermediate of chlorogenic acid was identified as quinic acid (cyclic polyol) and found in greater concentrations. Organic acids derived from sugar decomposition, such as ribonic acid from ribose and saccharic acid from glucose oxidation, and the sugar alcohol erythritol were present at greater levels. Trehalose, a disaccharide often related to plant stress adaptation, was also present at a greater level. Finally, we found three metabolites, threonic, isothreonic acids and oxalic acids, linked to ascorbic acid metabolism–catabolism. These compounds are involved in redox control and anti-oxidant activities in plant cells. After GC/TOF-MS, the metabolites were unambiguously assigned by the Bin-Base identifier numbers, using their retention indices and mass spectra. The unequivocal identification of ~ 15% and 25% of the compounds present in sugarcane and maize leaf extracts, respectively, was possible.
We explored the differences in metabolite fingerprinting promoted by a biostimulant application based on an 1H NMR analysis and identified metabolic targets that can be used to evaluate maize and sugarcane responses to biostimulants.
Discussion
We discovered 23 metabolic targets that were highly induced by biostimulant inoculations in both maize and sugarcane seedlings (Table 1). We would like to highlight the significantly greater concentrations of these core metabolites in leaf extracts, independent of plant species as being indicative of their usefulness as markers of the positive biostimulation-associated phenotype. The repression or inhibition of cell metabolites, leading to lower concentrations, is equally as, or more, important than high concentrations when discriminating the physiological effects. However, it is much easier to detect one compound by simple GC–MS or HPLC in an extract if this compound is present at a greater concentration. Simplistic solutions to complex problems can lead to ill-advised choices; however, here, we studied the metabolites present in greater concentrations to determine the correlations between their levels and the phenotype/agronomic trait. This strategy has been used previously, including in recent studies of metabolic responses under drought-stress conditions [19].
Among the metabolite targets found in both plants’ extracts, those from the tricarboxylic acid (TCA) cycle and one amino acid indicated that the biostimulant application increased the plants’ metabolic levels. This was reflected in the accumulations of citric, isocitric, aconitic, malic, maleic and fumaric acids (Table 1). The intensification of the TCA flux must be fed by the accumulation of substrates required for enzymatic reaction that will lead to the next step. The main function of the TCA cycle is the generation of ATP, which the cell consumes for energy. The pivotal roles of TCA-related compounds found in greater concentrations include photosynthesis, photorespiration, nitrogen metabolism, reductant transport and the maintenance of photosynthetic redox balance [20]. In addition, the TCA cycle is responsible for the production of various biosynthetic precursors, such as ascorbate, vitamin co-factors, fatty acids and amino acids. We found a number of amino acids at greater concentrations in only one of the plants, such as glycine in maize and proline, phenylalanine, homoserine and glutamine in sugarcane, but only aspartic acid was found in significant amounts compared with the controls in both extracts. Aspartate is the precursor of the essential amino acids lysine, threonine, methionine and isoleucine. It is formed by the transamination of oxaloacetate and may be derived from the TCA cycle [21]. Oxaloacetate is the intermediate compound between malic and citric acids, and the observed accumulations of both concurs with aspartic acid production and oxaloacetate consumption. The use of compounds from the TCA cycle and aspartic acid as markers of biostimulant application is facilitated by these compounds being well described and the availability of kits for their identification by HPLC. Enzymes linked to the TCA cycle were induced by humic substances in maize seedlings [22] as were amino acid, including aspartic acid, synthesis and accumulation [23]. Increased carbohydrate consumption and nitrogen assimilation enzyme activities has been observed in maize treated with an HA plus PGPB biostimulant [6], which was in agreement with the promotion of the primary metabolism observed in this study.
Lipids have essential structural functions in plant cell membranes, are a highly energetic carbon source for cells and can act as cell signal messengers [24]. These compounds are chemically defined by their low-aqueous solubility, and this broad definition includes molecules from primary and secondary plant metabolisms. We identified three fatty acids, linoleic, 2-monopalmitin and myristic, present at greater concentrations in extracts of both treated plant species in comparison with untreated plants. Linoleic acid has a long unsaturated chain (18:2) and is a predominant fatty acid constituent of storage lipids. It has previously been found to preferentially accumulate at high osmotic potentials [25]. 2-Monopalmitin is a glyceride with a fatty acid chain (16:0) covalently bound to a glycerol molecule through an ester linkage, and it can be transported and utilized for energy production or metabolic pathways. Myristic acid (tetradecanoic acid) is another fatty acid (14:0) found at relatively high concentrations in the leaf extracts of treated plants, while tocopherol (vitamin E) is a lipid-soluble molecule that’s biosynthesis is strongly conditioned by the availability of phytyl pyrophosphate, its aromatic biosynthetic precursor [26]. The stimulative effect on the TCA pathway indicates quantitative and qualitative changes in amino acids and lipid accumulation. Future studies can be performed to evaluate these lipid accumulations as markers for biostimulative responses in plants treated with endophytic diazotrophic bacteria and humic substances.
Tocopherol has presented antioxidative properties that protect against the oxygen toxicity of scavenging lipid peroxyl radicals, thereby preventing the lipid peroxidation of membranes [27]. Alpha-tocopherol levels can change in response to environmental cues depending on the magnitude of the stress and species’ sensitivity to stress, and it is generally assumed that increases in tocopherol contribute to plant stress tolerance [28].
Another compound found in greater concentrations in both treated seedlings and often linked to plant stress was trehalose, a disaccharide formed by two molecules of glucose. Trehalose accumulation has been observed in symbiosis and plant–pathogen interactions, as well as during abiotic stress, but its role in plant defense remains unclear [29]. Trehalose is highly soluble but chemically unreactive owing to its non-reducing nature, making it compatible with cellular metabolism even at high concentrations in response to abiotic stress [30]. In addition, trehalose production is a feature of many beneficial microbes, such as rhizobial symbionts [31], and the inoculation of grapevine with a plant growth-promoting rhizobacterium Burkholderia phytofirmans led to the up-regulation of trehalose metabolism and improved chilling-stress tolerance in the plant [32].
Three compounds linked to ascorbate metabolism–catabolism (vitamin C), threonic, isothreonic and oxalic acids, were also observed in greater concentrations in both treated species. The pathways by which ascorbate is catabolized to form oxalic, threonic and isothreonic acids have been previously reported, as well as its roles in many aspects of redox control and anti-oxidant activities in plant cells [33]. Reactive oxygen species (including OH, O−2, H2O2, HO˙2, RO˙, ROO˙ and 1O2) are cytotoxic to plants and induced by various environmental disturbances [34]. Reactive oxygen species are scavenged by various antioxidative defense systems, including hydrophilic and hydrophobic redox buffers, namely ascorbate and tocopherol, respectively. According to Foyer and colleagues [35], tocopherol is an effective scavenger of singlet oxygen species and, in this case, the reduced scavenging form is regenerated by ascorbate. Thus, the greater tocopherol concentration may be linked to the accumulations of ascorbate-degradative metabolites.
Another pivotal compound in secondary plant metabolism found in relatively greater concentrations in both maize and sugarcane was shikimic acid, a precursor of aromatic amino acids, indole compounds and their derivatives and alkaloids. Higher plants possess a mechanism to convert quinic acid to shikimic acid, phenylalanine and tyrosine [36]. Quinic acid, a cyclic polyol, was also found in greater concentrations, which indicates coherence among the metabolite pathways induced by plant inoculations. In addition, 4-hydroxybenzoic acid and 3,4-dihydroxycinnamic acid, two precursors of salicylic acid, which is another compound known to accumulate in response to different stresses, were found at greater concentrations. Salicylic acid is an important plant hormone that regulates many aspects of plant growth and development, as well as resistance to biotic and abiotic stresses [37]. Humic substances can promote phenylpropanoid metabolism, inducing plant accumulations of diverse phenolic compounds [38], and that bacteria used in the biostimulant are able to produce indole derivatives [39]. Thus, it was not surprising to find greater concentrations of shikimic acid and its derivatives in both leaf extracts.
Erythritol is an important nutrient for several α-Proteobacteria, including N2-fixing plant endosymbionts, and is used to feed the pentose phosphate pathway [40]. Here, it was also found at greater concentrations (Table 1). Galactaric acid is also known as mucic acid and is a product of galactose oxidation. Positive correlations between metabolite levels and drought-tolerance traits were identified for galactaric acid and other metabolites (allantoin, gluconic acid, glucose, and a salicylic acid glucopyranoside) in rice genotypes [41]. The concentration of mucic acid also increases in response to heat stress [42] as do other compounds found in this study, like shikimic acid, malonic acid, threonic acid and citric acid, indicating that these metabolites are promising candidate biostimulant markers.
Concluding remarks
We have accumulated field results using a biostimulant manufactured with HA and PGPB in which different crop yields showed increased biomasses and productivity levels in low-fertility soil or under drought-stress conditions [6, 7]. In addition, the efficient use of nutrients, especially nitrogen, was improved after biostimulant applications [7] and the main results and mechanisms of action have been summarized by Olivares and colleagues [11]. Here, we identified metabolites that may be used as markers of biostimulant action in both maize and sugarcane. A further objective is to improve plant responses to the biostimulant. Plant breeding is normally influenced by commercial interests, such as more fertilization, chemical pest and disease control and irrigation [43]. Finally, we recognize that organic (or alternative, biological and ecological) farming and agroecological systems can have lower production rates compared with high industrial intensive agriculture but organic agricultural systems deliver greater ecosystem services and social benefits [44]. Enhancing plant responses to biostimulants can contribute to the ecological intensification of crop production. Transitioning to new farming methods is often complicated in the first years because the plants are adapting to new conditions, such as slower nutrient sources, biological control methods and a different water balance. However, most cultivars used by small holders have been traditionally bred. Here, we reveal compounds that can putatively be used to select plant responses based on metabolite targets and abundance levels using multiple parallel methods, like metabolic fingerprinting, and powerful instrumentation (NMR and GC/TOF-MS). Ideally, the concentration of a metabolite marker will correlate with one or more agronomic traits contributing to the biostimulant response in a wide range of cultivars. We suggest that root traits and nitrogen-use efficiency be linked to the 23 compounds presented in this paper to select plant responses to biostimulants containing HA isolated from vermicompost and PGPB.
Availability of data and materials
Not applicable.
Abbreviations
- 1H NMR:
-
nuclear magnetic resonance of protons
- PCA:
-
principal component analysis
- GC/TOF-MS:
-
gas-chromatography coupled to time of flight mass spectrometry
- HA:
-
like-humic acids isolated from vermicompost
- MSTAF:
-
N-methyl-N-(trimethylsilyl)trifluoroacetamide
- TMCS:
-
trimethylchlorosilane
- PGPB:
-
plant growth-promoting bacteria
- PCs:
-
principal components
- TCA:
-
Krebs tricarboxylic acid cycle
- HPLC:
-
high-performance liquid chromatography
- ROS:
-
reactive oxygen species
- OH· :
-
hydroxyl radical·
- O·− 2 :
-
superoxide radical
- H2O2 :
-
hydrogen peroxide
- HO· 2 :
-
hydroperoxyl radical
- RO· :
-
alkoxy radical
- ROO· :
-
peroxy radical
- 1O2 :
-
singlet oxygen
- RO*:
-
excited carbonyl
References
Sanchez PA, Palm CA, Buol SW. Fertility capability soil classification: a tool to help assess soil quality in the tropics. Geoderma. 2003;14:157–85.
Altieri MA, Funes-Monzote FR, Petersen P. Agroecologically efficient agricultural systems for smallholder farmers: contributions to food sovereignty. Agron Sustain Dev. 2012;32:1–13.
Van Oosten MJ, Pepe O, De Pascale S, Siletti S, Maggio A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric. 2017;4:5.
Gupta AK. Innovations for the poor by the poor. Int J Technol Learn Innov Dev. 2012;5(1/2):28–39.
Baldotto LBE, Baldotto MA, Canellas LP, Bressan-Smith R, Olivares FL. Growth promotion of pineapple ‘Vitória’ by humic acids and Burkholderia spp. during acclimatization. R Bras Ci Solo. 2010;34:1593–600.
Canellas LP, Martínez-Balmori D, Médici LO, Aguiar NO, Campostrini E, Rosa RC, Façanha A, Olivares FL. A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.). Plant Soil. 2013;366:119–32.
Canellas LP, Silva SF, Olk D, Olivares FL. Foliar application of Herbaspirillum seropedicae and humic acid increase maize yields. J Food Agric Environ. 2015;13:146–53.
Olivares FL, Aguiar NO, Rosa RCC, Canellas LP. Substrate biofortification in combination with foliar sprays of plant growth promoting bacteria and humic substances boosts production of organic tomatoes. Sci Hortic. 2015;183:100–8.
Silva SF, Olivares FL, Canellas LP. The biostimulant manufactured using diazotrophic endophytic bacteria and humates is effective to increase sugarcane yield. Chem Biol Technol Agric. 2017;4:24.
Canellas LP, Olivares FL. Physiological responses to humic substances as plant growth promoter. Chem Biol Technol Agric. 2014;1:3.
Olivares FL, Busato JG, Paula AM, Lima LS, Aguiar NO, Canellas LP. Plant growth promoting bacteria and humic substances: crop promotion and mechanisms of action. Chem Biol Technol Agric. 2017;4(1):30.
Fiehn O. Metabolomics—the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–71.
Meyer RC, Steinfath M, Lisec J, Becher M, Witucka-Wall H, Torjek O, Fiehn O, Eckardt A, Willmitzer L, Selbig S, Altmann T. The metabolic signature related to high plant growth rate in Arabidopsis thaliana. PNAS. 2007;1(04):4759–64.
Lindon J, Holmes E, Bollard M, Stanley E, Nicholson J. Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers. 2004;9:1–31.
Steinfath M, Groth D, Lisec J, Selbig J. Metabolite profile analysis: from raw data to regression and classification. Physiol Plant. 2008;132:150–61.
Scholz M, Gatzek S, Sterling A, Fiehn O, Selbig J. Metabolite fingerprinting: detecting biological features by independent component analysis. Bioinformatics. 2004;20:2447–54.
Olivares FL, Baldani VLD, Reis VM, Baldani JI, Döbereiner J. Occurrence of the endophytic diazotrophs Herbaspirillum spp. in roots, stems and leaves predominantly of Gramineae. Biol Fertil Soils. 1996;21:197–200.
Krishnan P, Kruger NJ, Ratcliffe RG. Metabolite fingerprinting and profiling in plants using NMR. J Exp Bot. 2005;56:255–65.
Wedeking R, Maucourt M, Deborde C, Moing A, Gibon Y, Goldbach HE, Wimmer MA. 1H-NMR metabolomic profiling reveals a distinct metabolic recovery response in shoots and roots of temporarily drought-stressed sugar beets. PLoS ONE. 2018;3(5):e0196102.
Araújo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012;35:1–21.
Azevedo RA, Lancien M, Lea PJ. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids. 2006;30:143–62.
Nardi S, Muscolo A, Vaccaro S, Baiano S, Spaccini R, Piccolo A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and Krebs cycle in maize seedlings. Soil Biol Biochem. 2007;39:3138–46.
Vaccaro S, Ertani A, Nebbioso A, Muscolo A, Quaggiotti S, Piccolo A, Nardi S. Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem Biol Technol Agric. 2015;2:5.
Suh MC, Hahne G, Liu JR, Stewart N Jr. Plant lipid biology and biotechnology. Plant Cell Rep. 2015;34:517–8.
Finkelstein R, Somerville C. Abscisic acid or high osmoticum promote accumulation of long-chain fatty acids in developing embryos of Brassica napus. Plant Sci. 1989;61:213–7.
Mène-Saffrané L. Vitamin E biosynthesis and its regulation in plants. Antioxidants. 2018;7(1):2.
Munné-Bosch S, Alegre L. The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci. 2002;21:31–57.
Munné-Bosch S. The role of alpha-tocopherol in plant stress tolerance. J Plant Physiol. 2005;62(7):743–8.
Fernandez O, Bethencourt L, Quero A, Sangwan RS, Clément C. Trehalose and plant stress responses: friend or foe? Trends Plant Sci. 2010;15(7):409–17.
Lunn JE, Delorge I, Figueroa CM, van Dijck P, Stitt M. Trehalose metabolism in plants. Plant J. 2014;79:544–67.
Streeter JG, Gomez ML. Three enzymes for trehalose synthesis in Bradyrhizobium cultured bacteria and in bacteroids from soybean nodules. Appl Environ Microbiol. 2006;72:4250–5.
Fernandez O, Vandesteene L, Feil R, Baillieul F, Lunn JE, Clement C. Trehalose metabolism is activated upon chilling in grapevine and might participate in Burkholderia phytofirmans induced chilling tolerance. Planta. 2012;236:355–69.
Debolt S, Melino V, Ford CM. Ascorbate as a biosynthetic precursor in plants. Ann Bot. 2007;99:3–8.
Kapoor D, Sharma R, Handa N, Kaur H, Rattan A, Yadav P, Gautam V, Kaur R, Bhardwaj R. Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front Environ Sci. 2015;3:13.
Foyer CH, Trebst A, Noctor G. Protective and signaling functions of ascorbate, glutathione and tocopherol in chloroplasts. In: Demmig-Adams B, Adams WW, editors. Advances in photosynthesis and respiration: photoprotection, photoinhibition, gene regulation, and environment. Dordrecht: Springer Science Publishers; 2005. p. 241–68.
Weinstein LH, Porter CA, Laurencot HJ. Role of the shikimic acid pathway in the formation of tryptophan in higher plants: evidence for an alternative pathway in the bean. Nature. 1962;194:205–6.
Dempsey DA, Klessig DF. How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 2017;15:23.
Schiavon M, Pizzeghello D, Muscolo A, Vaccaro S, Francioso O, Nardi S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J Chem Ecol. 2010;36:662–9.
Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31(4):425–48.
Barbiera T, Collard F, Zúñiga-Ripa A, Moriyón I, Godard T, Becker J, Wittmann C, Schaftingen EV, Letesson JJ. Erythritol feeds the pentose phosphate pathway via three new isomerases leading to d-erythrose-4-phosphate in Brucella. PNAS. 2014;111:17815–20.
Degenkolbe T, Do PT, Kopk J, Zuther E, Hincha DK, Köhl KI. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE. 2018;8(5):e63637.
Yu J, Du H, Xu M, Huang B. Metabolic responses to heat stress under elevated atmospheric CO2 concentration in a cool-season grass species. J Am Soc Hortic Sci. 2012;137(4):221–8.
Vidal J. Who should feed the world: real people or faceless multinationals? https://www.theguardian.com/commentisfree/2018/jun/05/feed-the-world-real-people-faceless-multinationals-monsanto-bayer. Accessed 20 Oct 2018.
Reganold JP, Wachter JM. Organic agriculture in the twenty-first century. Nat Plants. 2016;2:15221.
Acknowledgements
Not applicable.
Funding
The stage of NOAC at Genome Center of UC-Davis was possible due CNPq “Science Without Border Program”. Additional support was provided by UENF Pos-Doc fellowship program and FAPERJ, Cientista do Nosso Estado program, INCT for Biological Nitrogen Fixation from National Council for Scientific and Technological Development (CNPq) and FINEP-Pluricana Project.
Author information
Authors and Affiliations
Contributions
NOAC was responsible by the experiments; FLO and LC contribute to experimental idea, discuss the data and reviewed the manuscript. All authors contributed to interpretation of the results and writing of the final paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This manuscript is an original paper and has not been published in other journals. The authors agreed to keep the copyright rule.
Consent for publication
The authors agreed to the publication of the manuscript in this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Canellas, N.O.A., Olivares, F.L. & Canellas, L.P. Metabolite fingerprints of maize and sugarcane seedlings: searching for markers after inoculation with plant growth-promoting bacteria in humic acids. Chem. Biol. Technol. Agric. 6, 14 (2019). https://doi.org/10.1186/s40538-019-0153-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-019-0153-4