Abstract
Background
Under natural conditions, ginger (Zingiber officinale Rosc.) rarely blossom and has seed, which limits new variety breeding of ginger and industry development. In this study, the effects of different photoperiods and light quality on flowering induction in ginger were performed, followed by gene expression analysis of flower buds differentiation under induced treatment using RNA-seq technology.
Results
First, both red light and long light condition (18 h light/6 h dark) could effectively induce differentiation of flower buds in ginger. Second, a total of 3395 differentially expressed genes were identified from several different comparisons, among which nine genes, including CDF1, COP1, GHD7, RAV2-like, CO, FT, SOC1, AP1 and LFY, were identified to be associated with flowering in induced flower buds and natural leaf buds. Aside from four down-regulated genes (CDF1, COP1, GHD7 and RAV2-like), other five genes were all up-regulated expression. These differentially expressed genes were mainly classified into 2604 GO categories, which were further enriched into 120 KEGG metabolic pathways. Third, expression change of flowering-related genes in ginger indicated that the induction may negatively regulated expression of CDF1, COP1, GHD7 and RAV2-like, and subsequently positively regulated expression of CO, FT, SOC1, LFY and AP1, which finally led to ginger flowering. In addition, the RNA-seq results were verified by qRT-PCR analysis of 18 randomly selected genes, which further demonstrated the reliability of transcriptome analysis.
Conclusion
This study revealed the ginger flowering mechanism induced by light treatment and provided abundant gene information, which contribute to the development of hybrid breeding of ginger.
Similar content being viewed by others
Introduction
Ginger (Zingiber officinale Roscoe) is a perennial monocotyledon from the Zingiberaceae family, which is not only an important condiment but also one of the most commonly used Chinese medicines (Miri 2020). Ginger is used worldwide as a flavoring agent in the bread, food, beverage, and bakery. In addition, ginger is commonly used in the pharmaceutical industry as a herbal medicine for treating vomiting, colds, fever and even cancer (Park et al. 2014; Thomson et al. 2014). The flower buds of ginger may developed directly from the rhizome, or it may originated from leaf buds. A pseudo stem could generated and elongated from 3–5 leaves after leaf buds development, and flower buds subsequently emerged at the middle of pseudo stem (Melati et al. 2015). During the growth of flower buds, it gradually changed from slender to round, and formed spikes. Then, flower emerged from each spike, one flower per bract up to three flowers (Melati et al. 2015). However, ginger rarely flowers or sets seed under natural conditions, and it mainly relies on rhizomes for asexual propagation in the cultivation process (Malamug et al. 1991; Sajeev et al. 2011; Melati et al. 2015). Studies have shown the effect of photoperiod and light intensity on ginger flowering (Adaniya et al. 1989; Melati et al. 2015), but floral regulatory genes have not been revealed. Therefore, it is difficult to obtain new cultivars through hybrid breeding, which greatly limits the development of ginger industry.
Environmental factors (such as light and temperature) and endogenous factors (such as plant hormones and age) could regulate flowering time in plants. Light quality and photoperiod control photomorphogenesis, which play an important role in flowering of various plants (Zhang et al. 2022; Sidhu et al. 2021). Plants could sense photoperiod variation of light with different wavelengths through photoreceptor proteins, such as phytochrome or cryptochrome, which would be signals to initiate flower (Weller and Kendrick 2008). For example, long light (16 h/8 h and 24 h/0 h) promoted flowering of Lysimachia mauritiana Lam at low temperature of 5 °C (Im et al. 2020). A significant decrease of flowering time was also observed for Arabidopsis thaliana mutants when constant fluorescent illumination was supplemented with irradiation enriched in red and far red spectrum (Somerville 1990). In photoperiod pathway, CDF1 and GI sense changes of circadian rhythms and transmit signals to CO gene to obtain more CO protein, high CO protein levels then activate the FT gene expression, which in turn promotes the expression of LFY and AP1 genes, and finally induce flowering (Valverde et al. 2004). CDF1 in A. thaliana could cause flowering delay by repressing the transcription of CO and FT genes (Sawa et al. 2007). In addition, COP1 inhibited flowering by degrading CO protein in A. thaliana (Liu et al. 2008).
Transcriptome analysis could accurately obtain transcriptional expression of all genes of studied plant using RNA-seq sequencing, thus provides possibility of important gene mining from obtained data (Sangwan et al. 2013). For example, flowering related genes were identified in transcriptome analysis of strawberry leaves, which reveals flowering regulation mechanism of strawberry under blue light treatment (Ye et al. 2021). Therefore, the identification of genes related to flowering in ginger transcriptome data would also contribute to elucidate the mechanism of induced ginger flowering. In this study, the optimal treatment of different photoperiod and light quality was screened for ginger. Then, an illumina high-throughput sequencing was used to analyze the transcriptional expression during flower bud formation. This study aimed to explore the mechanism for flowering in ginger and provide new insight for future breeding projects.
Materials and methods
Plant material and treatments
The ginger tissue materials involved in experiment were taken from tissue culture seedlings of Zingiber officinale Roscoe cv. Southwest in plant germplasm resource nursery of Chongqing University of Arts and Sciences (Chongqing China, N25° 56′ N, 105° 38′ E). Ginger seedlings were planted in a germplasm resource nursery and grow under green house conditions (temperature: 25 °C, humidity: 60%, light intensity: 200 μEm−2 s−1, photoperiod: 14 h light/10 h dark) to resume growth to three-forked shape stage.
For flowering induction, these ginger plants were grown in a greenhouse treated with the following four photoperiods: T1 (14 h light/10 h dark), T2 (16 h light/8 h dark), T3 (18 h light/6 h dark), and T4 (20 h light/4 h dark). In total, there were 10 pots for each treatment with two plants per pot. Each experimental condition was independently repeated three times. The flowering period of ginger is about 7 months after sowing, which indicates the physiological differentiation of ginger flower buds takes place at 40–60 days after three-forked shape stage (Kumari et al. 2020). Therefore, the number of ginger flower buds in each treatment was counted at 60 days and 70 days after three-forked shape stage to obtain the optimal photoperiod for flowering induction. The formula of flower bud differentiation rate is as follows:
In light quality, ginger plants were cultivated under different colored lights by treated with four different films: red film (R), blue film (B), green film (G) and white film (CK). The photoperiod was set to 18 h light/6 h dark based on previous result. The flower buds were also counted, and the optimal flower-inducing light quality was screened. In total, there were 10 pots per treatment with two plants in each pot, and three replicates were conducted.
Samples collection
Based on previous screened results, ginger tissue culture seedlings were induced to flower with the optimal photoperiod and light quality. Potential flower buds (FI), leaf buds (LI) and leaf buds (LN) under natural conditions (white light and 14 h light/10 h dark) of ginger plants were simultaneously collected at physiological differentiation of ginger flower buds (after 50 days of treatment). These samples were rapidly frozen in liquid nitrogen and stored at – 80 °C. Three independent biological replicates were performed for each treatment.
RNA isolation and library preparation
RNA was extracted using the TRIzol method (Invitrogen, CA, USA) and treated with RNase-free DNase I (Takara, Kusatsu, Japan). RNA was quantified using Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA), then its quality and integrity were assessed using NanoDrop spectrophotometer (Thermo Scientific, DE, USA). Sequencing libraries were constructed using NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA). Finally, the library were sequenced on the Illumina Novaseq 6000 platform by the Beijing Allwegene Technology Company Limited (Beijing, China) and 150 bp paired-end reads were generated.
Transcriptome assembly and functional annotation of transcripts
Raw data (raw reads) with fastq format were processed through in-house perl scripts. To obtain clean data, the reads containing ploy-N and low quality reads were removed from raw data. Then, these clean reads were mapped to reference genome sequence using STAR (Dobin et al. 2012). To obtain unigene library, sequence assembly was performed using Trinity 2.2.0 software (Haas et al. 2013). The transcripts less than 300 bp were discarded and the longest transcript in each cluster was taken as unigene. Unigenes were blasted with non-redundant protein sequences in Non-redundant protein sequence database (NR), gene ontology (GO), Kyoto encyclopedia of genes and genomes (KEGG), search tool for the retrieval of interacting genes/proteins (STRING) databases to obtain annotated information using blastp software (Camacho et al. 2009). E-value distribution was calculated and plotted according to NR library comparison annotation, and the screening criterion was set as E value < 1.0 × e−5.
Identification of differentially expressed genes
Unigenes were mapped to raw reads using Bowtie2 software (Langmead and Salzberg 2012). The expression level of single gene was calculated and normalized using Expectation Maximization in RSEM, and were estimated using the most commonly used FPKM (Li and Dewey 2011). Differentially expressed unigenes (DEGs) with significance in different groups were identified using the edgeR package in R language (Smyth 2010), with threshold value of |log2 (Fold Change)|≥ 1 and q-value ≤ 0.05. GO and KEGG enrichment analysis were performed using hypergeometric distribution in R language (Alexa et al. 2006).
qRT-PCR validation
Eighteen representative DEGs were randomly selected from the comparison groups (FI vs. LN, FI vs. LI and LI vs. LN) for qRT-PCR validation. The actin1 gene was used as internal reference, and all primer sequences were designed using Primer 5.0. Amplification reactions were conducted in a total volume of 20 µl. Cycling parameters were set as follows: 95 °C for 30 s, then 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Gene expression was calculated using 2−∆∆Ct method, and all qRT-PCR reactions were repeated three times.
Results
Effects of photoperiod and light quality on flowering in ginger
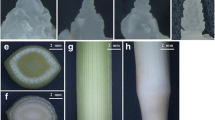
In T2, T3 and T4 photoperiod groups, the flower bud differentiation rates of ginger were 24.62%, 53.33% and 41.67%, respectively. None of flower buds differentiation was observed in T1 photoperiod treatment during whole experiment process. After 70 days, T3 treatment group had the most flower buds number with the most significant increase, from 30.06 on 60 days to 39.21 on 70 days (Fig. 1A, B). These findings indicated that long light condition (18 h/6 h) was beneficial to induced differentiation of ginger flower buds. The light quality results showed that flower bud differentiation rates of ginger under R (red film) treatment were higher than that in control group (white film), with 40.12 and 51.26 flower buds on 60 days and 70 days under R-treated treatment, respectively (Fig. 1C, D). There was no significant difference in flower bud differentiation rate between B (blue film) and G (green film) treatments, but they was significantly lower than that of control group. These results indicated that red light could better trigger flowering in ginger.
The effects of different photoperiod and light quality on ginger flowering. Treatments marked with different letters at a given statistics date are significantly different at P < 0.05.according to Duncan’s test. A The effects of different photoperiod on flower bud numbers; B the effects of different photoperiod on flower bud differentiation; C the effects of different light quality on flower bud numbers; D the effects of different light quality on flower bud differentiation
Statistics of transcriptome sequencing data
RNA-seq was performed for FI (potential flower buds under induced treatment), LI (leaf buds under induced treatment), and LN (leaf buds under natural condition) based on the HiSeq 2500 (Illumina) platform. After downloading raw data, these reads containing adapter and ploy-N and low quality reads were then removed, 59.02 Gb clean data were subsequently obtained. The clean reads of each sample were distributed in range of 40–51 million and more than 90% of reads were mapped in reference sequences (Table 1). The distribution of Q30 bases exceeded 90%, and GC contents ranged from 47.64 to 49.57%. These filtered reads were assembled using Trinity software (Haas et al. 2013), and 33,612 unigenes were obtained with an N50 value of 1123 bp and 40,384,342,800 bp transcriptome data. Functional annotation of proteins was performed using BLASTP based on GO, KEGG, STRING and NR non-redundant protein databases, and a total of 29,985 unigene sequences was annotated (89.21%) (Additional file 1: Table S1).
Differentially expressed genes analysis
To clarify the induction mechanism of ginger flowering, a transcriptome analysis was performed for three samples of FI, LI and LN. Pearson correlation analysis on single-gene expression showed high correlation existed in three biological replicate samples, indicating a high quality and robust sequencing library (Fig. 2A). In comparison of FI and LN, a total of 2230 DEGs were identified (|log2 (Fold Change)|≥ 1 and q-value ≤ 0.05), with 1134 up-regulated genes and 1096 down-regulated genes. In comparison of tissues in LI and LN, a total of 1186 DEGs were identified, with 549 up-regulated genes and 637 down-regulated genes. There were 2221 DEGs identified in comparison between FI and LN tissues, including 1103 up-regulated genes and 1118 down-regulated genes. Besides, there were common 64 DEGs among all three comparisons (Fig. 2B).
Differentially expressed unigenes (DEGs) of gingers among sample comparisons (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition). A Correlation analysis of sample groups; the number in the grid represents the square of Pearson correlation coefficient (R2) and the higher R2 represents the better correlation between samples. B Venn diagrams of DEGs between sample pairs
To verify the accuracy of quantitative and differential expression, 18 DEGs were randomly selected, and primer sequences were designed for real-time quantitative PCR (qRT-PCR). The assays displayed a same trend of gene expression in qRT-PCR and RNA-Seq with a correlation coefficient of 0.85, which indicated that there was a good consistency between RNA-sequencing and qRT-PCR (Additional file 2: Fig. S1).
Annotation and enrichment analysis of DEGs
GO annotation could help us understand the function of identified DEGs. The results revealed that 1699 DEGs identified in FI vs. LI were classified as biological process, cellular component and molecular function in GO database (Additional file 3: Fig. S2). There were 60 terms that were significantly enriched in GO database, which mainly involved with DNA-binding transcription factor activity, terpene synthase activity and transcription regulator activity. In LI vs. LN, 892 DEGs were annotated to the three types, of which 21 terms were significantly enriched in photosystem II oxygen evolving complex, oxidoreductase activity and photosystem I (Additional file 4: Fig. S3). The 1706 DEGs of FI vs. LN were annotated to biological processes, cellular components and molecular functions in GO database (Additional file 5: Fig. S4), of which 59 terms with significantly enriched genes were mainly protein phosphorylation, adenyl ribonucleotide binding and and thylakoid membrane.
The hypergeometric test found that a total of 2230 DEGs were enriched in 120 KEGG pathways, and Figs. 3, 4 and 5 showed the top 19 pathways with the smallest Q values for each group. Among these pathways, only plant hormone signal transduction pathway and starch and sucrose metabolism pathway were found to be associated with flowering (Campos et al 2017; Wahl et al. 2013). In FI vs. LI, 43 DEGs were enriched in plant hormone signal transduction pathway, of which 17 genes were up-regulated and 26 genes were down-regulated. Meanwhile, 18 DEGs were enriched in starch and sucrose metabolism pathway, of which 8 genes were up-regulated and 10 genes were down-regulated (Fig. 3). There were 17 DEGs enriched in plant hormone signal transduction pathway including 12 up-regulated genes and 5 down-regulated genes in LI vs. LN. Eighteen DEGs were enriched in starch and sucrose metabolism pathway with 11 up-regulated genes and 7 down-regulated genes (Fig. 4). In FI vs. LN, 43 DEGs were enriched in plant hormone signal transduction pathway with 29 up-regulated genes and 14 down-regulated genes. Twenty nine DEGs were enriched in starch and sucrose metabolism pathway with 15 up-regulated genes and 14 down-regulated genes (Fig. 5).
KEGG pathway enrichment of DEGs in FI vs. LI. Rich factor refers to the ratio of the number of differentially expressed genes enriched in the pathway to the number of annotation genes. Q value (range 0–1) is P value after correction by multiple hypothesis testing, and the closer the value of Q value is to 0, the more significant the enrichment is. Asterisks indicate significantly enriched pathways. (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition)
KEGG pathway enrichment of DEGs in LI vs. LN. Rich factor refers to the ratio of the number of differentially expressed genes enriched in the pathway to the number of annotation genes. Q value (range 0–1) is P value after correction by multiple hypothesis testing, and the closer the value of Q value is to 0, the more significant the enrichment is. Asterisks indicate significantly enriched pathways. (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition)
KEGG pathway enrichment of DEGs in FI vs. LN. Rich factor refers to the ratio of the number of differentially expressed genes enriched in the pathway to the number of annotation genes. Q value (range 0–1) is P value after correction by multiple hypothesis testing, and the closer the value of Q value is to 0, the more significant the enrichment is. Asterisks indicate significantly enriched pathways. (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition)
Screening of genes related to flowering in ginger
Plants mainly sense and conduct signal transduction through various photoreceptors, and then regulate blossom (Lu et al. 2015). In this study, to find the relevant DEGs that regulate ginger flowering under different light qualities and photoperiods treatment, the transcriptome expression patterns of FI vs. LN were compared. Twenty six homologous genes in ginger were obtained through sequence alignment of ginger transcripts to coding sequence of A. thaliana and Oryza sativa genome (Komeda 2004; Izawa et al 2003). Among them, 10 genes related to flowering regulation were identified with significant differences which included CDF1, COP1, GHD7, RAV, CO, FT, SOC1, AP1_1, AP1_2 and LFY (Fig. 6). In FI vs. LN, four genes located in upsteam of flowering regulation were down-regulated expression while six genes lied in downsteam were up-regulated expression (Fig. 6). The annotation of these DEGs was shown in Additional file 6: Table S2. Compared to the control, fragments per kilobase of transcript per million mapped reads (FPKM) values of CDF1, COP1, GHD7 and RAV in FI were decreased from 38.86, 3.76, 29.12 and 8.75 to 0.69, 0.12, 0.02 and 0.55, respectively. The expression levels of them were significantly decreased by 56.32, 31.33, 1456 and 15.9 fold, respectively. However, the expression of CO, FT and SOC1 were all up-regulated with the FPKM values of 151.31/0.03, 28.34/0.62 and 141.25/20.36 in FI and LN and increased by 5043.67, 45.71 and 6.85 times, respectively (Fig. 6). The AP1 genes at two different sites (AP1_1 and AP1_2) and LFY genes were also up-regulated, with FPKM values of 96.31/13.65 (AP1_1), 41.23/8.33 (AP1_2) and 3.95/0.06 (LFY) in FI and LN (Fig. 6).
The expression profiles of flowering-related DEGs (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition). A A heatmap of flowering-related genes. B The FPKM value of flowering-related genes; the asterisk indicates that unigene is significantly differentially expressed in the comparison and each bar represents one biological replicate; FI, LI, and LN are indicated by red, green, and blue, respectively
Discussion
Alteration in photoperiods and light quality significantly affects flowering process (Baloch et al. 2009; Magar et al. 2018). Plants, such as Moss Rose, Pansy, Snapdragon, Petunia and Annual Verbena, had the minimum time to flower when grow under 17 h photoperiod while it became longer as photoperiod decreased to 8 h (Baloch et al. 2010). In cranberry, the number of flowers under red light was significantly more than that in white and far-red light (Zhou and Singh 2002). In our study, the flower bud differentiation rate of ginger had the highest value under red light and long light (18 h/6 h) treatments compared other groups, which was consistent with previous reports (Baloch et al. 2010; Zhou and Singh 2002). Therefore, further elucidation of flowering mechanism in response to light induction plays an important role in new varieties breeding of ginger. Besides, transcriptome analysis of induced potential flower buds and leaf buds was performed by Illumina high-throughput sequencing technique, and 59.02 Gb clean data were obtained. A total of 33,612 unigenes were obtained with an N50 value of 1123 bp and transcriptome data of 40,384,342,800 bp by assemblying filtered reads with Trinity software. Then, 3395 DEGs were identified from several different comparisons, and qRT-PCR analysis of 18 randomly selected genes also confirmed same expression levels with transcriptome. These results indicated that our transcriptome data are highly reliable and suitable for downstream analysis.
The flowering initiation is an energy-intensive process in plants, and only occurs when energy reserve is sufficient. Trehalose 6-phosphate (T6P), a key energy reserve, is necessary for normal expression of FT gene (Yadav et al. 2014). In A. thaliana, sucrose could promote the expression of TPS gene, which encodes T6P synthase that catalyzes the production of uridine diphosphate glucose (UDPG) reaction using glucose 6-phosphate (G6P) and T6P. Then, UDPG could activate the expression of FT to induce flowering (Wahl et al. 2013). In addition, the mutation of plant vegetative buds into flower buds is caused by the interaction of various hormones, while phytohormones play an important role in flower bud differentiation (Campos et al. 2017). Under certain conditions, plant hormone signals promote or inhibitie the expression of key flowering genes to regulate plant flowering (Conti et al. 2017). In this study, only two KEGG pathways related to flowering were enriched, namely starch and sucrose metabolism pathway and plant hormone signal transduction pathway (Figs. 3, 4 and 5), which indicated that these pathways may be related to light-induced flowering of ginger. Red light and long light treatment could effectively induce differentiation of flower buds in ginger, which also implies that the down-regulated genes involved in theses pathways are possibly negative-regulatory genes related to ginger flowering, while these up-regulated genes play reverse roles.
Referring to the flowering regulation network diagram in A. thaliana and O. sativa, we constructed a hypothetical model for ginger flowering regulation network based on expression patterns of related homologous genes in ginger (Fig. 7). Previous studies have shown that the RAV2-like gene can inhibit flowering by inhibiting the expression of FT and gibberellin production (Luis et al. 2014). In A. thaliana, AtCDF1 inhibits expression of CO and FT by binding to promoter of the two genes, resulting in delayed flowering in photoperiodic pathway (Song et al. 2012; Fornara et al. 2009; Imaizumi et al. 2005). COP1 encodes an ubiquitin E3 ligase, which could promote degradation of CO protein in plants by the ubiquitination process (Liu et al. 2008). In this study, RAV2-like, CFD1, and COP1 gene were also identified and were down-regulated expression in FI when compared with LN (Fig. 6). Therefore, RAV2-like, CDF1, and COP1 may be important negative regulators in photoperiod flowering pathway of ginger. GHD7, a member of CONSTANS-like family, inhibits flowering by silencing Hd3a (homologous gene of FT) in O. sativa under long-day condition (Xue et al. 2009). In our study, GHD7 gene was also identified and the FPKM value decreased by 1456 times in FI vs. LN (Fig. 6). Compared with RAV2-like, CFD1 and COP1, the change of FPKM value in GHD7 is more obvious. Therefore, GHD7 may be another important negative regulator gene in photoperiod flowering pathway of ginger and may play a leading role. In addition, PHYA, PHYB, LHY, ELF3 and other genes located in the upstream of the photoperiod pathway were not differentially expressed (Fig. 6), indicating that the down-regulation of GHD7, COP1, RAV2-like and CDF1 genes may be caused by the comprehensive regulation of the upstream genes of the photoperiod pathway.
The hypothetical model for ginger flowering regulation network was constructed by reference to A. thaliana and O. sativa flowering pathway. Change in expression of photoperiod flowering pathway genes after red and long light (18 h light/6 h dark) treatment. Gene names are given within small boxes whose color indicates the change in gene expression (red: upregulated; green: downregulated; blue: no change). Arrows (promotion) and T-bars (repression) indicate regulation
CO is a key factor that converts light signals into flowering signals, and promotes flowering by activating the expression of FT and SOC1 in A. thaliana (Putterill et al. 1995; Samach et al. 2000). FT and SOC1 are floral integrators, which can integrate various flowering pathway signals and promote the expression of LFY (Jung et al. 2020). LFY play a key role in flower development (Weigel et al. 1992). Mutations in LFY can lead to a shift of flower structure to shoots. For example, tomato LFY mutants resulted in delayed flowering and greater number of leaves (Molinero-Rosales et al. 1999). AP1, another flowering-determining gene, is a MIKC-type MADS-box gene which is located in downstream of LFY. It is positively regulated by LFY, and the coexpression would promote the transformation of stem meristem into floral meristem (Wagner et al. 1999). In this study, CO, FT, SOC1, LFY and two AP1 genes at different loci (AP1_1 and AP1_2) were identified, and were up-regulated expression in both FI vs. LN and FI vs. LI (Fig. 6). Therefore, CO, FT, SOC1, LFY and AP1 genes may positively regulate ginger flowering downstream of the photoperiod pathway. Besides, CDF1, RAV2-like, GHD7 and COP1 may be the key genes for light-induced flowering of ginger. They are regulated by light, then activate expression of CO and FT to result in flowering.
Conclusions
Ginger is an important condiment and medicinal plant, and it is difficult to blossom under natural conditions. However, the flowering related research is important for ginger cross-breeding, which could accelerate the breeding of new varieties. The flowering process is affected by different light conditions and the regulated mechanism of ginger flowering is still unclear. In this study, it was found that 18 h light and red light treatment could effectively induce ginger flowering. In the KEGG pathway, the starch and sucrose metabolism pathway and plant hormone signal transduction pathway may play important roles in ginger flowering. Four down-regulated genes that regulate ginger flowering in the photoperiodic pathway (GHD7, CDF1, COP1 and RAV2-like) were identified, and they may be important negative regulators for light-induced flowering of ginger. In addition, the significant fold change of FPKM value in GHD7 indicated that it may play a dominant role in the photoperiod pathway. In conclusion, this study preliminarily revealed the molecular mechanism of ginger flowering induced by photoperiod and light quality, which provided novel insight into ginger breeding.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FI:
-
Potential flower bud of ginger under induced by photoperiod and light quality
- LI:
-
Induction treatment of ginger leaf bud
- LN:
-
Leaf bud of ginger under natural condition
- NR:
-
Non-redundant protein sequences in NCBI
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- STRING:
-
Search tool for the retrieval of interacting genes/proteins
- DEGs:
-
Differentially expressed unigenes
- qRT-PCR:
-
Real-time quantitative PCR
- FPKM:
-
Fragments per kilobase of transcript per million mapped reads
- T6P:
-
Trehalose 6-phosphate
- UDPG:
-
Uridine diphosphate glucose
- G6P:
-
Glucose 6-phosphate
References
Adaniya S, Shoda M, Fujieda K (1989) Effects of day length on flowering and rhizome swelling in Ginger (Zingiber officinale Roscoe). Jpn Soc Hortic Sci 58:649–656
Alexa A, Rahnenführer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22:1600–1607
Baloch JUD, Khan M, Zubair M, Munir M (2009) Effects of different photoperiods on flowering time of facultative long day ornamental annuals. Int J Agric Biol 11:251–256
Baloch JUD, Khan M, Munir M, Zubair M (2010) Effects of different photoperiods on flowering time of facultative short day ornamental annuals. J Appl Hortic 12:10–15
Camacho C, Coulouris G, Avagyan V, Ning M, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinform 10:421
Campos R, Osorio M, Sanchez B, Us C, Duarte A, Dela P (2017) Plant hormone signaling in flowering: an epigenetic point of view. J Plant Physiol 214:16–27
Conti L (2017) Hormonal control of the floral transition: can one catch them all? Dev Biol 26:1–14
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2012) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21
Fornara F, Panigrahi KC, Gissot L, Sauerbrunn N, Ruhl M, Jarillo JA, Coupland H (2009) Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell 17:75–86
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, MacManes MD, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey CN, Henschel R, LeDuc RD, Friedman N, Regev A (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512
Im NH, Lim SH, Lee HB, An S, Kim K (2020) Growth and flowering responses of Lysimachia mauritiana Lam. to cold treatment and photoperiod. Sci Hortic-Amsterdam 270:109429
Imaizumi T, Schultz T, Harmon F, Ho L, Kay S (2005) FKF1F-BOX protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309:293–297
Izawa T, Takahashi YJ, Yano M (2003) Comparative biology comes into bloom: genomic and genetic comparison of flowering pathways in rice and arabidopsis. Curr Opin Plant Biol 6:113–120. https://doi.org/10.1016/S1369-5266(03)00014-1
Jung H, Jo SH, Jung WY, Park HJ, Lee A, Moon JS, Seong SY, Kim JK, Kim YS, Cho HS (2020) Gibberellin promotes bolting and flowering via the floral integrators RsFT and RsSOC1–1 under marginal vernalization in radish. Plants 9:1–21
Komeda Y (2004) Genetic regulation of time to flower in Arabidopsis thaliana. Annu Rev Plant Biol 55:521–535
Kumari M, Kumar M, Solankey SS (2020) Zingiber officinale Roscoe: ginger. Med Aromat Stimul Plants 12:605–621
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform 12:323
Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yang HQ (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20:292–306
Lu XD, Zhou CM, Xu PB, Luo Q, Lian HL, Yang HQ (2015) Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in Arabidopsis. Mol Plant 8:467–478
Luis MH, Aguilar-Jaramillo AE, Esther MG, Paula SL, Soraya P (2014) RAV genes: reagulation of floral induction and beyond. Ann Bot-Lond 114:1459–1470
Magar YG, Ohyama K, Noguchi A, Amaki W, Furufuji S (2018) Effects of light quality during supplemental lighting on the flowering in an everbearing strawberry. Acta Hortic 1206:279–284
Malamug J, Inden H, Asahira T (1991) Plantlet regeneration and propagation from ginger callus. Sci Hortic 48:89–97
Melati, Palupi ER, Bermawie N (2015) Floral biology of Ginger (Zingiber offcinale Rosc.). Int J Curr Res Biosci Plant Biol 2:1–10
Miri SM (2020) Micropropagation, callus induction and regeneration of ginger (Zingiber officinale Rosc.). Open Agric 5:75–84
Molinero-Rosales N, Jamilena M, Zurita S, Gomez P, Capel J, Lozano R (1999) FALSIFLORA, the tomato orthologue of FLORICAULA and LEAFY, controls flowering time and floral meristem Identity. Plant J 20:685–693
Park GH, Park JH, Song HM, Eo HJ, Kim MK, Lee JW, Lee MH, Cho KH, Lee JR, Cho HJ, Jeong HJ (2014) Anti-cancer activity of Ginger (Zingiber officinale) leaf through the expression of activating transcription factor 3 in human colorectal cancer cells. BMC Complem Altern Med 14:8
Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80:847–857
Sajeev S, Roy AR, Iangrai B, Pattanayak A, Deka BC (2011) Genetic diversity analysis in the traditional and improved ginger (Zingiber officinale Rosc.) clones cultivated in North-East India. Sci Hortic 128:182–188
Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288:1613–1616
Sangwan RS, Tripathi S, Singh J, Narnoliya LK, Sangwan NS (2013) De novo sequencing and assembly of Centella asiatica leaf transcriptome for mapping of structural, functional and regulatory genes with special reference to secondary metabolism. Gene 525:58–76
Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318:261–265
Sidhu V, Bernier-English V, Lamontagne-Drolet M, Gravel V (2021) Effect of light quality and extended photoperiod on flower bud induction during transplant production of day-neutral strawberry cultivars. Can J Plant Sci 102:356–367
Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139
Somerville R (1990) Effect of light quality and vernalization on late-flowering mutants of Arabidopsis thaliana. Plant Physiol 92:770–776
Song YH, Smith RW, To BJ, Millar AJ, Imaizumi T (2012) FKF1 conveys timing information for COSTANS stabilization in photoperiodic flowering. Science 336:1045–1049
Thomson M, Corbin M, Leung L (2014) Effects of ginger for nausea and vomiting in early pregnancy: a meta-analysis. J Am Board Fam Med 27:115
Valverde F, Mouradov A, Soppe W, Ravenscroft D, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303:1003–1006
Wagner D, Sablowski RWM, Meyerowitzt EM (1999) Transcriptional activation of APETALAI by LEAFY. Science 25:52–58
Wahl A, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by Trehalose-6-Phosphate signaling in Arabidopsis thaliana. Science 339:704–707
Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69:843–859
Weller JL, Kendrick RE (2008) Photomorphogenesis and photoperiodism in plants. Photobiology 16:417–463
Xue W, Xing Y, Weng Z, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X (2009) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten HM, Stitt M, Lunn JE (2014) The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 65:1051–1068
Ye Y, Liu Y, Li X, Chen Q, Zhang Y, Luo Y, Liu Z, Wang Y, Lin Y, Zhang Y (2021) Transcriptome profile analysis of strawberry leaves reveals flowering regulation under blue light treatment. Int J Genomics 2021:1–18
Zhang F, Cheng G, Shu X, Wang N, Wang Z (2022) Transcriptome analysis of Lycoris chinensis bulbs reveals flowering in the age-mediated pathway. Biomolecules 12:899
Zhou Y, Singh BR (2002) Red light stimulates flowering and anthocyanin biosynthesis in American cranberry. Plant Growth Regul 38:165–171
Acknowledgements
Not applicable.
Funding
This work was supported by the Grants from National Natural Science Foundation of China (31501273, 82204574), Postdoctoral Science Foundation of China (2021M700623), Scientific Research Fund of Chongqing Municipal Education Commission (No: KJQN202001337), and Social undertakings and livelihood security Project of Science and Technology Committee of Yongchuan District (Ycstc, 2020cc0106, 2021yc-cxfz30018).
Author information
Authors and Affiliations
Contributions
This study was conceived by QD and YJ. The plant material preparations were carried out by KL, GZ and LG. QD, YZ, ZL and MH performed the laboratory experiments and analyses. QD drafted the manuscript. MH and YJ revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing interests or personal relationships that could have appeared to the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Statistics of functional annotation for ginger transcriptome.

Additional file 2: Figure S1.
Validation of DEGs by qRT-PCR. A, validation of DEGs in LI vs. LN. B, validation of DEGs in FI vs. LI. C, validation of DEGs in FI vs. LN. (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition)

Additional file 3: Figure S2.
GO secondary metabolic process of DEGs in FI vs. LI. Asterisks indicate significantly enriched processes. Only 30 terms enriched most significant are shown in this figure. (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition)

Additional file 4: Figure S3.
GO secondary metabolic process of DEGs in LI vs. LN. Asterisks indicate significantly enriched processes. Only 30 terms enriched most significant are shown in this figure. (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition)

Additional file 5: Figure S4.
GO secondary metabolic process of DEGs in FI vs. LN. Asterisks indicate significantly enriched processes. Only 30 terms enriched most significant are shown in this figure. (FI: potential flower bud of ginger under induced by photoperiod and light quality; LI: induction treatment of ginger leaf bud; LN: leaf bud of ginger under natural condition)
Additional file 6: Table S2.
Annotation information of differentially expressed genes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, Q., Zhang, Y., Liu, K. et al. Transcriptome profiles reveal gene regulation of ginger flowering induced by photoperiod and light quality. Bot Stud 64, 12 (2023). https://doi.org/10.1186/s40529-023-00388-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40529-023-00388-7