Abstract
Damage and fracture of archaeological potteries not only jeopardize the long-term preservation but also hinder their exhibition. To repair these pottery sherds effectively, this study introduces a novel inorganic phosphate-based adhesive and evaluates its effectiveness through a series of experiments. To determine the optimal base adhesive, the paper investigates the influence of varying weight ratios of the H2O–H3PO4 system and the Al(OH)3–H3PO4 system on properties including tensile lap-shear strength, microstructure, high-temperature resistance and phase composition. However, the original black color of the traditional CuO–phosphate adhesive limits its application. The innovation of this study lies in the addition of nano-TiO2 to the adhesive, which not only improves the bonding strength but also adjusts the color of the adhesive. This study has defined the optimal formulation (i.e., base adhesive = m[Al(OH)3]:m[H3PO4] = 7:100, filler = 10wt% nano-TiO2), and the final product shows no residual acid in adhesives. Additionally, the fracture surfaces are successfully bonded with a high strength of 3.56 MPa. Various ageing tests including dry-thermal ageing, hygrothermal ageing and UV irradiation ageing are conducted to assess the ageing resistance of the inorganic phosphate-based adhesive. The results indicate strong tolerance of adhesive to high temperature and high humidity environment. Preliminary applications in archaeological pottery restoration suggest that the inorganic phosphate-based adhesive offers considerable promise for repairing shattered pottery.
Graphical Abstract

Similar content being viewed by others
Introduction
Over 10,000 years ago, the advent of pottery heralded the Neolithic Age, swiftly integrating into all aspects of human life. Archaeological pottery excavations provide a vivid snapshot of the political, economic, scientific, technological, cultural and artistic evolution of the times, representing invaluable and irreplaceable resources for historical and cultural heritage worldwide. However, numerous deterioration factors affect the archaeological pottery's structures, leading to surface weathering and fragility. Typically crafted from coarse raw materials and fired at low temperatures (700 ~ 1000 ℃), the pottery exhibits a loose structure with high porosity and variable pore sizes, which substantially diminishes its durability [1]. Consequently, these potteries are less durable and more vulnerable to degradation factors attack including high temperature, underground water and salts [2,3,4,5]. The archaeological pottery was buried underground for an extended period before being excavated. Water, in whatever physical state (solid, liquid or gas), is regarded as the primary deterioration factor causing degradation because of its ability to dissolve salts and other soluble components in pottery matrix [6, 7]. Additionally, external threats like earthquakes, tomb collapses and intentional damage contribute to the fractures of archaeological pottery.
Preserving cultural artifacts through bonding treatments is crucial for extending their lifespan and maintaining their integrity for exhibition. Adhesives have become essential in cultural heritage restoration. The adhesives are classified into three types based on their chemical properties: organic adhesives, inorganic adhesives and organic–inorganic hybrid adhesives. The organic adhesives consist of epoxy resin, acrylic resin, cellulose nitrate, polyvinyl acetate and other polymer compounds [8, 9]. The traditional inorganic adhesives include gypsum, hydraulic lime and silicate adhesives [10]. Furthermore, some organic–inorganic compound adhesives have also been used to bond the broken archeological pottery [11]. In the past few decades, epoxy resin, because of its superior physico-mechanical properties and various cross-linking possibilities, has been a popular choice for conserving stone and pottery cultural heritages [12, 13]. For instance, Wang et al. [14] employed epoxy resin to adhere the shattered terracotta sculptures unearthed from the Hanyang Mausoleum. Lan et al. [15] used a modified epoxy resin to bond the pottery sherds of Qin Terracotta Warriors and Horses. Nevertheless, several drawbacks including high viscosity, brittleness, poor ageing resistance, susceptibility to stress-cracking post-curing and challenges in removing after failure restrict their applications in the conservation and preservation of cultural heritages [16,17,18]. As depicted in Fig. S1 (Supplementary Information), the ageing phenomena, such as bonding strength decrease, browning by light and embrittlement of adhesive, which not only damage the aesthetics of the artifacts but also threaten their long-term preservation. Therefore, the exploration of novel inorganic adhesives for bonding shattered archaeological pottery emerges as a critical research trend.
Inorganic phosphate-based adhesives are highly esteemed for their robust bonding capabilities, exceptional resistance to high temperatures, non-toxic nature, environmental sustainability and their ability to cure at ambient temperatures [19, 20]. These adhesives are versatile, capable of bonding diverse materials such as metals, wood, and glass, and are utilized as matrices in refractory and composite materials. Given these attributes, phosphate-based adhesives are emerging as a formidable candidate for applications in the field of cultural heritage conservation [21]. The phosphate-based adhesive consists of base adhesive, curing agent and filler, among which the base adhesive is generally formulated by enhancing phosphoric acid with a modifier [22]. The curing agents include CuO, AlN, ZnO and MgO [23, 24]. Fillers are incorporated based on specific requirements to enhance the overall performance of adhesives, including modifying curing duration, bonding strength or coloration.
According to the "Principles for the Conservation of Heritage Sites in China" [25], adhesives employed in cultural heritage conservation should possess the features outlined: (1) The ability to solidify at ambient temperature; (2) The bonding strength that is comparable to, or slightly less than, that of the pottery matrix [26]; (3) The color difference value (ΔE*) between adhesive and archaeological pottery should less than 5 [27, 28]. According to the above criteria, CuO was chosen as a curing agent because it enables the adhesive to cure at room temperature. However, the traditional CuO-phosphate adhesive is characteristically black and exhibit inferior bonding strength relative to the pottery matrix. This research modifies the composition and proportions of the base adhesive to amplify bonding strength and reduce curing time. Additionally, we have also explored new fillers to further promote the bonding strength and minimize the color difference between the adhesive and pottery matrix.
This study mainly involves the determination of the optimal formulation, evaluation of ageing resistance and preliminary application effect exhibition of the inorganic phosphate-based adhesive (IPA). The remainder of the paper will be organized as follows: Sect. "Materials and methods" describes the materials and methodology employed to prepare the IPA, characterization methods of the bonding performance and formed final product, as well as the evaluation of ageing resistance. Sect. "Results and discussion" focuses on selecting the base adhesive and filler for bonding pottery sherds, determining the optimal formula for bonding, and presenting the main results from artificial accelerated ageing. Moreover, Sect. "Results and discussion" highlights the restorative effects of the adhesive on two Tang Dynasty handmaiden figurine. Finally, Sect. "Conclusions" lists the principal conclusions drawn from the study and outlines potential avenues for future research.
Materials and methods
Chemicals and reagents
The analytical grade agents Al(OH)3, H3PO4, TiO2 and CuO were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Nano-ZnO and nano-TiO2, with a particle size of 50 nm, were purchased from Aladdin Industrial Corporation (Shanghai, China). Deionized water was used throughout the entire experiment.
Preparation of inorganic phosphate-based adhesive
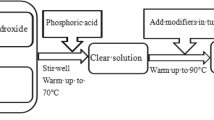
In this study, the phosphate-based adhesive consists of base adhesive, curing agent and filler. In order to explore the optimal ratio of the raw materials, different types of adhesives were prepared with H2O–H3PO4 or Al(OH)3–H3PO4 as base adhesive, CuO as curing agent, nano–ZnO or nano–TiO2 as filler, and their properties were studied and compared.
Effect of the base adhesive
With the purpose of evaluating the impact of different base adhesive systems on the properties of the adhesive, two base adhesive systems were designed in this experiment. The specific preparation methods follow.
Base adhesive 1 H2O and 85 wt% H3PO4 were mixed on a magnetic stirrer at the weight ratios of 1:100, 3:100, 5:100, 7:100 and 9:100 at room temperature (25 ℃), respectively, until a colorless and transparent liquid was obtained. The base adhesive prepared by this method was termed the H2O–H3PO4 system.
Base adhesive 2 Al(OH)3 and 85 wt% H3PO4 were mixed on a magnetic stirrer at the weight ratios of 1:100, 3:100, 5:100, 7:100 and 9:100 at 50 ℃, respectively, until a colorless and transparent liquid was obtained. The base adhesive prepared by this method was termed the Al(OH)3–H3PO4 system. The possible chemical reactions between Al(OH)3 and H3PO4 were shown as follows:
The CuO and base adhesive were mixed and swirled uniformly on the surface of a watch glass according to the weight ratio of 5:3 until the adhesive could form a 5 cm filament. The prepared adhesives were applied to bind the specimens. Additionally, the adhesive was coated on a glass plate and kept in a fume hood at a temperature and relative humidity of 25 °C and 60% for seven days. The obtained adhesive product was used to analyze the microstructure and phase composition.
Effect of the filler
To explore the relationship between the properties of the adhesive and the dosages of filler, the filler (nano-ZnO or nano-TiO2) is added into the adhesive according to the weight ratio of 2.5, 5, 10, 15 and 20 wt%, respectively. On the one hand, the prepared adhesive is coated on the glass plate to record the color and test the microstructure, phase composition and heat resistance after curing. On the other hand, the adhesives are used to bond the standard specimens to test the bonding strength. In addition, the adhesive prepared with the optimal ratio is also applied to bond the fractured terracotta figurines.
Preparation of pottery sample
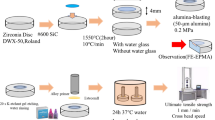
The pottery samples are prepared in the laboratory using local clay. Clay, sand, plant ash and water are mixed in a certain proportion to achieve the requisite plasticity and avoid structural defects in the process of drying. The mixed clay is filled into a metal mold measuring 240 × 100 × 25 mm3. After 48 h, the cubic specimens were taken out of the mold and then dried in natural condition (25 ℃, 60% RH) for a week. The dried samples are placed in a muffle furnace and then burned at a heating rate of 5 °C/min till reaching the desired temperature of 950 °C. After cooling to room temperature, the pottery raw samples are taken out. As shown in Fig. S2, the pottery raw sample was cut into slices with a size of 100 × 25 × 2 mm3 according to the GB/T 7124–2008 regulation [29] to prepare standard specimens for testing the bonding strength.
Characterization method
Bonding strength
The adhesive was applied to the bonding area of the standard specimens (see Fig. S2), and the bonded specimens were fixed and placed on a table. After treatment, the specimens were held for seven days at a temperature of 25 ℃ and relative humidity of 60%. The tensile lap-shear strength was measured using a universal testing machine (SANS–CMT5205, MTS China). The tests were performed at a movement speed of 1 mm/min and the maximum load was recorded upon specimen failure. Each measurement was replicated on five specimens. Finally, the results of the mean value and standard deviation (SD) were recorded. The following equation was used to calculate the tensile lap-shear strength:
where P (N) is the maximum load at failure, A (m2) is the bonding area of the specimen, T (MPa) is the tensile lap-shear strength.
SEM
The morphologies of adhesive products after gold coating were examined with an environmental scanning electron microscope (Quanta 200 FEI), which is operated under a low vacuum at an operating voltage of 20 kV for SEM observations.
TG
The adhesive products were further studied in this experiment to determine the high-temperature resistance. The thermogravimetric-differential thermal analysis (TG–DTA) was conducted using an instrument (TA, SDT Q600) from 25 to 1000 °C at a heating rate of 10 °C/min under nitrogen flow.
XRD
The adhesive product was ground into powder in a mortar and subsequently analyzed using an X-ray diffractometer (XRD) to identify the phases in adhesives. Specifically, the crystalline phases were characterized using a DX-2700 X-ray diffractometer with Cu-Kα radiation using a scintillation detector under the voltage of 30 kV and a current of 40 mA. The XRD data were collected for each sample from 10° to 80°.
pH value
1 g of solid powder was added to 50 mL of deionized water and stirred thoroughly. After static settlement for 1 h, the pH value of the solution was tested using a pH Meter (INESA Scientific Instrument Co., Ltd., PHS-25).
Durability testing methods
In order to evaluate the durability of the IPA, the degradation process was studied and recorded through artificial accelerated ageing in laboratory. Two batch adhesive specimens were prepared for the accelerated ageing experiment. The first batch of specimens was prepared following the method mentioned in Sect. "Effect of the base adhesive". The other batch specimens were prepared following the method mentioned in Sect. "Bonding strength". Considering that temperature, humidity and ultraviolet irradiation are the main factors that contribute to the degradation of adhesive [30], three ageing methods were designed, namely dry-thermal ageing, hygrothermal ageing and UV irradiation ageing, respectively. The specific ageing methods are shown as follows.
-
(1)
Dry-thermal ageing
Three specimens from the first batch and thirty specimens from the second batch, both of the two batch specimens were placed in a ventilated drying oven (Shanghai Blue Leopard Experimental Equipment Co., LTD, BHO-401A) at 105 ℃.
-
(2)
Hygrothermal ageing
Three specimens from the first batch and thirty specimens from the second batch, both of the two batch specimens were stored in a temperature humidity-controlled chamber (Chongqing Yinhe Test Instrument Co., LTD, SDJ6025), with a temperature of 70 °C and relative humidity of 95%.
-
(3)
UV irradiation ageing
Three specimens from the first batch were placed in an ultraviolet ageing chamber, setting the relative humidity is ≤ 85%, the temperature is 34 ± 0.5 ℃, the irradiation intensity is 4.62w/m2, the wavelength range of ultraviolet irradiation is 280 ~ 315 nm for UV irradiation ageing.
After ageing, the first batch of three samples was used for colorimetric measurement, microstructure observation and molecular structure analysis, respectively. For the second batch, five specimens were taken out every seven days for bonding strength test. Since the adhesive cannot be directly exposed to ultraviolet light after bonding the specimens, the bonding strength of the adhesive after UV ageing is not measured.
Colorimetric measurement
The chromatic variations (ΔE*) induced by the degradation of adhesives were determined by a CM-2003d Konica Minolta spectrophotometer. The analyses were carried out according to the European standard UNE–EN 15886:2011 [31]. The results were reported in the CIELab1976 system. The chromatic variation was calculated using the following Equation:
where L1*, a1* and b1* are the lightness and chromaticity values for the specimens before ageing, L2*, a2* and b2* are those for specimens after ageing.
Microscopic observation
Surface morphology observation of the adhesive before and after ageing was carried out by using the three-dimensional ultra-depth of field microscope (Keyence, VHX–5000) at 200 × magnification.
Fourier transform infrared spectrometer (FTIR Spectrometer)
The adhesive specimen was placed in a Fourier transform infrared spectrometer (Bruker Corporation, Tensor 27) and the infrared spectrum of the adhesive before and after ageing was measured by a non-destructive method.
Results and discussion
Selection of the base adhesive
Tensile lap-shear strength analysis
The tensile lap-shear strength is one of the most imperative indicators for evaluating the bonding effectiveness. Figure 1 exhibits the average values and standard deviations of tensile lap-shear strength of the specimens bonding with adhesive prepared using H2O–H3PO4@CuO and Al(OH)3–H3PO4@CuO, respectively. On the one hand, when the base adhesive is H2O–H3PO4 system, the tensile lap-shear strength of the adhesive drops steadily as the water content increases. It declines from the initial 1.244 MPa to 0.638 MPa, resulting in a 48.7% decrease in strength. The strength change is mainly due to the addition of deionized water, which reduces the concentration of phosphoric acid.
On the other hand, when the base adhesive is Al(OH)3–H3PO4 system, the tensile lap-shear strength rises from 1.242 MPa to 1.726 MPa and subsequently drops to 1.558 MPa with the increase of Al(OH)3 content. The adhesive prepared with m[Al(OH)3]:m[H3PO4] = 7:100 depicts the highest bonding strength, reaching a value of 1.726 MPa. However, when m[Al(OH)3]:m[H3PO4] = 9:100, the excessive addition of Al(OH)3 increases the viscosity of the adhesive and improves the temperature required for curing, which results in an inadequate curing reaction and therefore leads to a decrease in bonding strength [32]. Furthermore, the tensile lap-shear strength of the specimen bonded with adhesive prepared using Al(OH)3–H3PO4 system (1.726 MPa) increased by 39.2% compared to the specimen bonded with that of H2O–H3PO4 system (1.049 MPa) at the weight ratio of 7:100, which means that the addition of Al(OH)3 has a positive impact on bonding strength.
SEM analysis
The SEM is used to evaluate the surface morphology of adhesives manufactured based on two base adhesive systems to understand the difference in mechanical strength after curing, and results are shown in Fig. 2. Thereinto, the surface morphologies of the adhesives prepared with the H2O–H3PO4 system as the base adhesive are shown in Fig. 2a, b, c. It can be seen that the adhesive prepared using base adhesive with m[H2O]:m[H3PO4] = 1:100 manifests few holes and integral microstructure, while the adhesive prepared using base adhesive with m[H2O]:m[H3PO4] = 5:100 or 7:100 presents more holes (see arrows in Fig. 2) and more loose structure. Comparing the three images, differences in micrographs among adhesive products of adhesive with varying weight ratios are evident. As observed in Fig. 2a, b, c, the number and volume of holes after curing increases gradually with the improvement of water content in base adhesive, which is attributed to the evaporation of water from the adhesive during the curing process. With the decrease of the active ingredient in the base adhesive, the amount of CuO that did not participate in the reaction increased. The CuO particles display a disordered distribution and structural imperfections, resulting in a decrease in bonding strength.
On the contrary, as shown in Fig. 2d, e, f, when the base adhesive is an Al(OH)3–H3PO4 system, the adhesive product displays a more condensed microstructure and a notable decrease in the number of holes. This compact structure in Fig. 2e, f illustrates that the curing reaction is sufficient enough to provide high strength. With the increase of the amount of Al(OH)3, the content of the aluminum phosphate increased. The participation of aluminum phosphate in the curing reaction is conducive to improving the mechanical strength of the adhesive products, which is consistent with the tensile lap-shear strength tendency of the adhesive in Fig. 1.
TG analysis
As shown in Fig. 3a, the adhesive prepared based on the H2O–H3PO4@CuO contains two evident weight loss stages in the whole process of thermal decomposition. From the results shown in Fig. 3a, it can be seen that the weight of the adhesives continuously decreases but does not change greatly (~ 2%) in the process from ambient temperature heating up to 130 °C, which is mainly due to the evaporation of a small amount of free water [32]. At about 130 °C, the weight loss stage accounts for 5% of the total weight, which originates from the crystallizing water in the adhesive escaping from adhesive products. At around 860 °C, another marked weight loss owes 10% of the total weight, which is ascribed to the decomposition of residuary CuO in adhesive products. CuO undergoes transformation into Cu2O at elevated temperatures [33]. According to the results listed in Table 1, the thermogravimetric rate of the adhesive increases with the improvement of H2O proportion, revealing excess H2O in the adhesive.
Similarly, the adhesive prepared based on the Al(OH)3–H3PO4@CuO (see Fig. 3b) also experiences two significant weight loss stages. Nevertheless, the first stage occurs at 180 °C instead of 130 °C, which may be caused by the Al(H2PO4)3 decomposition. The AlPO4 is formed through a reaction of aluminum dihydrogen phosphate and other phosphates at 180 ℃, the possible reaction equations can be described as follows [34]:
As shown in Fig. 3b, the thermogravimetric rate reduces as the Al(OH)3 content increases, which means that the amount of H2O that can be lost in the adhesive decreases. Therefore, the weight loss rate of the first stage is only about 3%, as listed in Table 1. The second significant weight loss stage appears at 860 ℃ when the weight loss is about 10% (see Table 1). This is because the adhesive changes into a molten state at high temperature and part of the adhesive products decompose.
XRD analysis
The mineralogical compositions and reaction process of adhesives after curing are presented in Fig. 4. The obtained XRD pattern reveals the presence of two phases in adhesive products: First, there are clear peaks of CuO at 2θ = 35.5° and 38.7°. This is because some raw materials do not participate in the curing reaction, so there are residual CuO phases. The other phase is Cu3(PO4)2·3H2O and the rest is amorphous. The bonding effect of H3PO4 and CuO results from a combination of hydrogen bonds and ionic bonds, among which the hydrogen bond energy is rather weak and not the primary component contributing to cohesion. The cohesion of adhesive originates from two factors: (1) Cu3(PO4)2·3H2O forms through a chemical reaction when the CuO is mixed with H3PO4. These needle-shaped and rod-shaped crystals interpenetrate, accumulate and fill the holes of the adhesive during the curing process, and play the function of supporting the adhesive products [35]. (2) The whole curing reaction of adhesives is exothermic. During the curing process, phosphoric acid is dehydrated by heating to form the concentrated phosphoric acid. The horizontal and longitudinal bonding between concentrated phosphoric acid and CuO occurs through "—O—Cu—O—" to create a macromolecular network structure [36]. This reaction process can be concluded in Fig. 4c.
When Al(OH)3 is added to the base adhesive, the XRD pattern of the adhesive product is depicted in Fig. 4b. It can be seen that the adhesive product mostly contains of CuO, Cu3(PO4)2·3H2O, Al(H2PO4)3 and the amorphous phase. The formed copper aluminum phosphate is amorphous and, therefore cannot be detected. The detected Al(H2PO4)3 is the product of the reaction of Al(OH)3 with H3PO4. It has been reported that Al(H2PO4)3 can react with metal oxides to form a strong binding force and the adhesives fill the gaps by chemically reacting with Al(H2PO4)3. Al(H2PO4)3 and other phosphates polymerize to form an amorphous macromolecular structure, which is conducive to the curing of adhesives and improving the bonding strength [37].
Table 2 summarizes the bonding strength, pH value and associated products of adhesives prepared with different base adhesive systems. As observed in Table 2, there is no significant difference in the pH value of adhesives prepared with Al(OH)3–H3PO4 system as the base adhesive. With the increase in the dosage of Al(OH)3, the acidity of the adhesive gradually decreases, and the pH value increases from 6.10 to 6.76. Based on the above analysis, the optimal ratio of the base adhesive is m[Al(OH)3]:m[H3PO4] = 7:100, the bonding strength is up to 1.726 MPa, and the final product is CuO, Cu3(PO4)2·3H2O and Al(H2PO4)3. In addition, the pH value is 6.54, indicating that almost no unreacted acid remains in the final product, so there is no risk of corrosion to the pottery surface.
Selection of filler
On the one hand, the bonding strength value is lower than expected. On the other hand, the color of the adhesive is black, which does not match the archaeological pottery. To further improve the comprehensive properties of the adhesives, this section explored the effect of fillers on color, bonding strength and morphology. Based on the excellent dispersibility and coloring ability of nanoparticles, the nano-ZnO and nano-TiO2 were selected as fillers in this study.
Visible morphology analysis
As can be seen from Fig. 5a, the adhesives containing nano-ZnO as filler are uniformly black in color. The addition of filler did not change the color of the adhesive. When the filler is nano-TiO2, with the weight fraction of the filler gradually increasing, the color change trend of the obtained adhesive is black → dark blue → light blue → gray white → white, as shown in Fig. 5b. The discoloration of the adhesive occurs when nano-TiO2 is introduced because of its white pigment characteristics, high covering power and strong coloring ability. Its chemical properties are stable and almost do not react with the base adhesive. Although nano-ZnO is also a white pigment, its coloring capacity is significantly lower than that of nano-TiO2. Moreover, a part of nano-ZnO reacted with the base adhesive and transferred into Zn2+, therefore losing the coloring ability. As shown in Fig. 5b, the blue shift is found for adhesive after adding nano-TiO2, which may be attributed to the following two reasons: (1) Quantum size effect. As the particle size decreases, the gap between the electron-occupied molecular orbital energy level and the unoccupied molecular orbital energy level increases, and the boundary of the absorption band moves toward the shortwave direction [38]. (2) Small size effect of nanoparticles. Small particle sizes result in a larger surface area. This large surface tension distorts the crystal lattice and destroys the periodic boundary conditions, resulting in a blue shift of the absorption band [39].
Tensile lap-shear strength analysis
The average value and standard deviations of tensile lap-shear strength of adhesives prepared with different fillers are shown in Fig. 6. In general, both fillers have a positive effect on bonding strength, regardless of the concentration of the filler used. However, it is noteworthy that the adhesive containing nano-TiO2 as the filler has resulted in a more significant improvement in tensile lap-shear strength than that of the adhesive with nano-ZnO. Regarding nano-ZnO, the mechanical strength increased from 1.726 MPa to 3.372 MPa, then decreased to 2.900 MPa with the increment of filler concentration. The bonding strength value of the adhesive reaches a maximum (3.372 MPa) when the concentration of nano-ZnO is 15 wt%, which increased by 95.4% compared to the adhesive without filler.
As observed in Fig. 6, the specimens bonded using adhesive with nano-TiO2 undergo a significant increase in tensile lap-shear strength compared to the specimens using adhesive without filler. Specifically, the mechanical strength of the specimens bonded using adhesive with 2.5, 5, 10, 15 and 20 wt% of nano-TiO2 increased by 46.0%, 67.9%, 106.3%, 69.4% and 59.9%, over the specimens using adhesive without filler, which demonstrates that the adhesive is able to cure effectively and form strong cohesion. In general, the adhesive can achieve optimal bonding performance when the amount of nano-TiO2 is 10 wt%. The improvement of bonding strength is mainly attributed to the strong surface superhydrophilicity of nano-TiO2. The Ti–O bond in TiO2 has a high polarity, and the water adsorbed on the surface is dissociated due to polarization, which easily forms hydroxyl groups. The presence of surface hydroxyl groups can strengthen the bonding between TiO2 and other metal oxides when it is used as an adsorbent [40].
SEM analysis
Reasons for the difference in mechanical strength of the adhesive prepared with different fillers can be explained from the microstructure. As shown in Fig. 7(a, b), there are some small pores and holes on the surface of the adhesive product when the filler is nano-ZnO, the unreacted ZnO and CuO particles are encapsulated in the adhesive product. The ZnO has high activity and releases more heat during the curing process. After curing, the adhesive structure is loose, which is not conducive to improving the bonding strength. From Fig. 7c, d, it is observed that nano-TiO2 can be evenly dispersed in the adhesive and fill the original structural defects. Due to the superhydrophilicity of nano-TiO2, the agglomeration phenomenon is reduced, which is conducive to interface bonding [41]. Therefore, the bonding strength of adhesive prepared with nano-TiO2 as filler is higher than that prepared with nano-ZnO as filler.
As observed in Fig. 8, the adhesive product shows a compact and homogeneous microstructure, and the fractured surface is effectively bonded due to the formation of the adhesive product. In this case, no delamination or shrinkage crack is observed at the boundary between adhesive product and pottery matrix, which suggests that the adhesive product is well connected with the pottery matrix. The bonding mechanism between adhesive and the ceramic matrix involves a complicated interaction that includes both mechanical force and intermolecular force. The mechanical force may arise from the pinning effect formed between the adhesive and the rough surface of pottery. The intermolecular force is mainly due to the hydrogen bond between the adhesive (plenty of hydroxyl groups in the final product) and the pottery matrix (rich oxygen-containing groups) [42].
Assessment of the durability of the inorganic phosphate-based adhesive
Tensile lap-shear strength analysis
As observed in Fig. 9, the bonding strength of IPA initially increases and then decreases after both dry-thermal ageing and hygrothermal ageing. After being exposed to a ventilated drying oven at 105 ℃ for 7 days, the tensile lap-shear strength of IPA reaches a maximum value (3.746 MPa), which is 8.6% higher than the initial value. The increased bonding strength may be attributed to high temperature, which promotes further curing of the IPA. After ageing for 42 days, the bonding strength of IPA reduces by 19.0%. When the specimens are placed in the hygrothermal ageing chamber (70 ℃, 95%RH) for 14 days, the bonding strength reaches a maximum of 3.712 MPa, which increases by 7.7% compared to the initial bonding strength. Differently, the appearance of the bonding strength maximum is delayed by about a week compared to the dry-thermal ageing. After ageing for 42 days, the tensile lap-shear strength of IPA decreases by only 9.9% compared with the strength before ageing, indicating that the IPA has good moisture resistance. On the other hand, the decrease of bonding strength induced by dry-thermal ageing is more remarkable than that of hygrothermal ageing, indicating that humidity has little impact on the IPA, and temperature is the main factor affecting the bonding strength.
Color variation analysis
Figure 10 represents the color variation values and surface micrographs of IPA after being exposed to accelerated ageing environments. Under the condition of dry-thermal ageing and hygrothermal ageing, the color variation of IPA gradually increases with increased exposure time. At the first stage (0 ~ 12 days), the ΔE* value increases gradually at a slow rate. When the ageing time is 16 days, the color variation of the IPA reaches the maximum value (ΔE* = 6.56 and 5.82). When exposed to UV irradiation, two different stages can be distinguished in the color variation behavior of the IPA: in the first stage (0 ~ 8 days), the ΔE* value rises gradually at a slow rate. By contrast, in the second stage (8 ~ 16 days), the cured superficial layer exhibits a noticeable color variation. Specifically, the ΔE* value rises from 3.45 to 14.05 at the fastest rate. As observed in Fig. 10c2, c3, the color of the adhesive shows a tendency to yellowing and bluing. This phenomenon can be related to the addition of nano-TiO2. Nano-TiO2 has strong optical activity, and it can excite electrons to form electron holes when absorbing ultraviolet irradiation and cause photochemical alteration in the surrounding medium.
It can be seen from the surface micrographs that the ageing behavior of the adhesive is mainly manifested in the color variation because no delamination or shrinkage crack is observed on the surface of the adhesive after ageing. According to the most widespread classification in cultural heritage conservation [27, 43], the total color variation of an adhesive specimen cannot be detected by a human eye when the ΔE* value is less than 3. The ΔE* values ranging between 3 and 5 imply that the color variation can be perceived by a human eye and therefore exhibits a moderate risk. However, the color change will be considered visible and unacceptable when the ΔE* value is above 5, which means that accelerated ageing has a high risk. Therefore, from the point of view of color variation, the three ageing methods all caused different degrees of discoloration, the significance of the three ageing strategies on IPA decreases in the following order: UV irradiation ageing ˃ dry-thermal ageing > hygrothermal ageing.
FTIR analysis
To investigate eventual chemical changes that occurred during the whole accelerated ageing process, the chemical ageing was monitored by FTIR. The results are summarized in Fig. 11. The FTIR spectra (Fig. 11a) before and after dry-thermal ageing collected have showed remarkable stability, as the molecular composition of the IPA did not change, indicating its exceptional tolerance to high temperatures. This result also may be because the IPA has no detectable characteristic bands, and the molecular changes cannot be detected. As shown in Fig. 11b, the characteristic bands’ position and intensity of IPA are unchanged before and after hygrothermal ageing, indicating that the molecular structures of IPA are not altered under hygrothermal conditions. Similar to the previous dry-thermal ageing and hygrothermal ageing, no notable alterations in FTIR spectra (Fig. 11c) are observed at UV irradiation for up to 16 days, indicating that UV irradiation does not cause the molecular decomposition of adhesive product (Fig. 12).
Preliminary application in the restoration of archaeological pottery
The Sanyuan County Museum, located in Xianyang City, Shaanxi Province, China, has collected more than 80 precious cultural artifacts unearthed from aristocratic tombs in the early Tang Dynasty. These cultural artifacts reflect the social life and artistic development of the Tang Dynasty and have high historical and artistic value. Commissioned by the Sanyuan County Museum, two maidservant figurines need to be restored. To determine the most suitable bonding materials, three commonly used epoxy resins (ER, MER and EP11) and the inorganic phosphate-based adhesive (IPA) were used to bond the standard specimens, the bonding strength results are presented in Table 3.
As observed in Table 3, in terms of the pottery specimen, the tensile lap-shear strength values range from 2.617 MPa to 3.627 MPa, with an RSD value of 16.2%, which indicates the weathering degree of the pottery matrix varies. The bonding strengths of the three organic adhesives, particularly for MER, are markedly higher than that of the PS. According to the principles of cultural heritage conservation [25], it is emphasized that the bonding treatment should avoid the excessive strength effect on the shattered archaeological pottery. While the bonding strength of IPA (3.448 MPa) is 10.4% higher than the PS (3.122 MPa) but slightly lower than the unweathered pottery specimens (about 3.6 MPa), which demonstrates that the IPA is more consistent with the cultural heritage conservation principle. Before bonding, the color difference between the IPA and the two figurines to be bonded is measured, the obtained result shows that the ΔE* values are 3.73 and 4.22, which indicates that the color difference between the adhesive and the pottery matrix could be seen by the human eye but acceptable, so it shows a moderate aesthetic compatibility [43].
Based on the above experimental results, the IPA was initially applied to bond the fractured terracotta figurines. The specific repair steps follow:
-
(1)
The scalpel was used to remove soil or aged adhesives attached to the fracture surface, and then the soft brush was used to sweep the dust. A cotton swab soaked in 75% alcohol was used to clean the surface.
-
(2)
The fracture surface was uniformly covered with the inorganic phosphate-based adhesive. In order to guarantee a strong bonding, the two fracture surfaces were then correctly spliced together and the adhesive was squeezed. It should be noted that the bonding areas need to be fixed firmly to prevent movement and dislocation during the curing process.
-
(3)
The collected terracotta debris was ground into powder using a mortar. After one day of natural curing, the caulking material was prepared by uniformly mixing the adhesive and terracotta powder in a mass ratio of 6:5. We scraped a small amount of caulking material with a bamboo knife and filled it into the gap of the fracture surface to keep it compact.
-
(4)
After the adhesive and caulking material were initially cured, a soft brush was used to sweep off the excessive powder, and the terracotta figurine was placed in an ambient temperature environment for natural curing. Since the restoration process was not carried out in a constant temperature and humidity environment, and the curing cannot be completed within one day, the curing temperature and relative humidity are 20 ~ 30 ℃ and 70 ~ 80%, respectively.
-
(5)
After seven days, the repaired terracotta figurines were shown in Fig. 12b, d, and the results showed a satisfactory bonding effect.
Conclusions
This study introduces a novel inorganic phosphate-based adhesive tailored for the restoration of fractured archaeological pottery, evaluating its effectiveness and durability through a series of tests. Some important conclusions can be summarized as follows:
-
(1) The formulation of the base adhesive is crucial for the adhesive’s efficacy. When the weight ratio of the Al(OH)3–H3PO4 system is maintained at 7:100, the adhesive exhibits optimal comprehensive properties. Under this proportion, the adhesive can be cured at room temperature, the formed Cu3(PO4)2·3H2O, Al(H2PO4)3 and other amorphous phases are considered to be the key contributors to its strength. After curing, the adhesive demonstrates a maximum bonding strength of 1.726 MPa and a dense microstructure with excellent resistance to high temperatures.
-
(2) Integrating nano-TiO2 into the adhesive adjusts its color, reducing the color difference with the pottery matrix. Meanwhile, the superhydrophilicity of nano-TiO2 enhances the connection by increasing the adhesive's affinity for the pottery matrix, thereby improving the bonding strength.
-
(3) The ageing behavior of the IPA predominantly manifests as discoloration and a decline in bonding strength. Despite varied ageing methodologies, the chemical structure of the IPA remains stable, indicating its superior ageing resistance. Comparing the results obtained from the three ageing, it has been found that high temperature and UV irradiation are the primary factors triggering adhesive ageing.
Although the IPA has shown great potential as a bonding material for archaeological pottery, further investigations are needed to optimize its performance. In the subsequent study, we will explore the most suitable reagents that enable the IPA to lose bonding strength in a relatively short time. When better bonding materials are available, the original inorganic phosphate-based adhesive can be simply and quickly removed without causing damage to pottery matrix, thereby enhancing the reversibility of the bonding treatment.
Availability of data and materials
Available upon request by the authors.
References
Mohamed HM. A comparison study of titanium dioxide and zinc oxide nanoparticles for cleaning archaeological pottery. J Nano Res-Sw. 2022;76:61–77.
Ricca M, Cámara B, Fort R, et al. Definition of analytical cleaning procedures for archaeological pottery from underwater environments: the case study of samples from Baia (Naples, South Italy). Mater Design. 2021;197: 109278.
Noghani S, Amiri MC, Emami M. A new approach to the desalination process of archaeological potteries. Mediterr Archaeol Ar. 2018;18(1):255–67.
Elghareb WK. Deterioration and consolidation of some pottery vessels in Tel Ajrud, Suez. Egypt Int J Conserv Sci. 2019;10(3):415–28.
Ibrahim MM, Mohamed WS, Mohamed HM. Evaluation of the efficacy of traditional and nano Paraloid B72 for pottery consolidation. Int J Conserv Sci. 2022;13(1):15–30.
Mohamed Mohamed H, Mohamed WS. Evaluating nano primal AC33 for protection and consolidation processes of archaeological pottery: a comparison study with silica and montmorillonite nanoparticles. Pigm Resin Technol. 2023. https://doi.org/10.1108/PRT-09-2022-0104.
Helmi FM, Hefni YK. Using nanocomposites in the conslidation and protection of sandstone. Int J Conserv Sci. 2016;7(1):29–40.
Gaballah S, El-Nagar M, Abdel-maksoud G, Youssef AM. Presenting shape memory polymers SMP and some reinforcement materials for gaps filling in archaeological bones. Egypt J Chem. 2021;64(7):3605–14.
Ibrahim MM, Mohamed WS, Mohamed H. Comparative and experimental studies for evaluation of Paraloid B-72 in traditional and nano forms for joining of pottery samples. J Nano Res-Sw. 2020;61:61–71.
Ibrahim MM, Mohamed HM. Experimental study to evaluate the efficiency of some gap filling materials of archaeological pottery. Eur J Sci Theol. 2021;17(6):119–28.
Mohamed HM, Mohamed WS. Improving the properties of gap-filling materials for pottery artifacts with nano silica and nano kaolinite polymeric nanocomposites. Pigm Resin Technol. 2023. https://doi.org/10.1108/PRT-03-2023-0024.
Ietto F, Perri F, Miriello D, Ruffolo SA, Laganà A, Le Pera E. Epoxy resin for the slope consolidation intervention on the tropea sandstone cliff (Southern Calabria, Italy). Geoheritage. 2018;10(2):287–300.
Tesser E, Lazzarini L, Bracci S. Investigation on the chemical structure and ageing transformations of the cycloaliphatic epoxy resin EP2101 used as stone consolidant. J Cult Herit. 2018;31:72–82.
Wang H, Song D, Cheng Y, et al. Research of Yangling excavated ceramic relics. Relics and Museolgy. 2009;6:244–50.
Lan D. Research on adhesive materials for restoration of painted pottery figurines excavated from Pit No.1 of the Qin terracotta warriors and horses. Sci Conserv Archaeolo. 2019;31(5):49–59.
Pangallo D, Bucková M, Kraková L, et al. Biodeterioration of epoxy resin: a microbial survey through culture-independent and culture-dependent approaches. Environ Microbiol. 2015;17(2):462–79.
Wang S, Fang S, Zhang B. Exploratory study on ageing process of epoxy bonding material. Sci Conserv Archaeolo. 2017;29(2):15–25.
Alonso-Villar EM, Rivas T, Pozo-Antonio JS. Adhesives applied to granite cultural heritage: effectiveness, harmful effects and reversibility. Constr Build Mater. 2019;223:951–64.
Ma CK, Chen HL, Wang C, Zhang JF, Qi H, Zhou LM. Effects of nano-Aluminum nitride on the performance of an ultrahigh-temperature inorganic phosphate adhesive cured at room temperature. Materials. 2017;10(11):1266.
Sahnoun RD, Bouaziz J. Sintering characteristics of kaolin in the presence of phosphoric acid binder. Ceram Int. 2012;38(1):1–7.
Liu J. Study on preparation and property of phosphate binder [Master]. Harbin: Harbin Enginering University; 2007.
Chen TJ, Wu ZZ, Wang XA, et al. Hierarchical lamellar aluminophosphate materials with porosity as ecofriendly inorganic adhesive for wood-based boards. Acs Sustain Chem Eng. 2018;6(5):6273–80.
Li C, Zhang L, Zhou K, Li Z, Chen Z. Interface microstructure and bonding mechanism of phosphate bonding NiFe2O4 cermet. Chin J Nonferr Met. 2011;21(5):1060–5.
Liu ZX, Sun RN, Mao ZP, Wang PC. Effects of phosphate pretreatment and hot-humid environmental exposure on static strength of adhesive-bonded magnesium AZ31 sheets. Surf Coat Tech. 2012;206(16):3517–25.
ICOMOS. 2015 Principles for the conservation of heritage sites in China.
Rong B, Lan D. Experimental study on adhesive for restoration of Qin terracotta warriors. Relics Museol. 2003;2:71–8.
Rodrigues JD, Grossi A. Indicators and ratings for the compatibility assessment of conservation actions. J Cult Herit. 2007;8(1):32–43.
Realini M, Colombo C, Conti C, et al. Development of neutron imageing quantitative data treatment to assess conservation products in cultural heritage. Anal Bioanal Chem. 2017;409(26):6133–9.
Institute SRPR. 2008 Adhesives—determination of tensile lap-shear strength of rigid-to-rigid bonded assemblies. In. Vol GB/T 7124–2008: Standardization Administration. 8
Delor-Jestin F, Drouin D, Cheval PY, Lacoste J. Thermal and photochemical ageing of epoxy resin—Influence of curing agents. Polym Degrad Stabil. 2006;91:1247–55.
E.C.f. 2011 Standardization, conservation of cultural property—Color measurement of surfaces, in.
Sui LX, Yin CF, Li XC, Chen PA, Zhu BQ. Preparation of a modified phosphate-based adhesive and its hot bonding performance on 316L stainless steel. Ceram Int. 2021;47(11):15585–93.
Chen L, Li L, Li G. Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate. J Alloy Compd. 2008;464:532–6.
Liu G, Wang Z, Jia M, et al. Thermally stable amorphous mesoporous Alumino-phosphates with controllable P/Al ratio: synthesis, characterization, and catalytic performance for selective O-methylation of catechol. J Phys Chem B. 2006;110:16953–60.
Yu L, Lei Y, Yu Q, Zhou Q. Study on adhesive mechanism of inorganic phosphate and copper oxide adhesive. Adhesion. 1998;6:5–11.
Chen Z, Zhang L, Zhou K. Research progress of phosphate inorganic binder for high temperature resistance. Mat Sci Eng Powder Metall. 2009;14(2):74–82.
Xie L. Research on inorganic compatibility adhesive for inorganic cultural heritage [Master]. Xi’an: Shaanxi Normal University; 2018.
Yan X. Grafting modification of nano-SiO2, nano-TiO2 by PMMA and its effect on properties of ink [Master]. Chengdu: Sichuan University; 2009.
Viseu TMR, Almeida B, Stchakovsky M, et al. Optical characterisation of anatase: a comparative study of the bulk crystal and the polycrystalline thin film. Thin Solid Films. 2001;401(1–2):216–24.
Chen Y, Zhang X, Sun J, Lin C. Properties of epoxy resin adhesive modified by nano-TiO2. J Jiangsu Univ. 2013;34(3):335–9.
Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol. 2009;43(12):4227–33.
Guo M, Li G, Cai M, et al. A tough hydrogel adhesive for the repair of archeological pottery. Nano Lett. 2023;23:1371–8.
Pesce C, Moretto LM, Orsega EF, et al. Effectiveness and compatibility of a novel sustainable method for stone consolidation based on di-Ammonium phosphate and calcium-based nanomaterials. Materials. 2019;12:3025.
Acknowledgements
The authors would like to acknowledge the financial support provided by the Ningbo University of Finance & Economics research fund for the master's degree training point under Grant No.1320230933. The authors are also grateful to the Cultural Heritage Bureau of Zhejiang Province for its support of the Conservation Science and Technology Project of Zhejiang Provincial Administration of Cultural Heritage (NO. 2024016).
Funding
This research is supported by the Ningbo University of Finance & Economics research fund for master's degree training point (1320230933), the Conservation Science and Technology Project of Zhejiang Provincial Administration of Cultural Heritage (2024016).
Author information
Authors and Affiliations
Contributions
LX: Investigation, writing, conceptualization, YL and WH: conceptualization and editing, SF: conceptualization, validation and editing, XQC: project administration, writing-reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The consent for the publication of details and images in the manuscript are obtained from all participants.
Competing interests
The authors hold no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, L., Li, Y., Hu, W. et al. A novel inorganic phosphate-based adhesive for bonding archaeological pottery: a preliminary exploration. Herit Sci 12, 164 (2024). https://doi.org/10.1186/s40494-024-01283-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-024-01283-5