Abstract
Removing unwanted materials, such as organic coatings and soil, from the cultural relic surface is a complex and significant task in the field of cultural heritage conservation. Microemulsion-loaded gel can effectively and safely remove those organic coatings and soil. Here, we employed a simple solvent exchange strategy to prepare a microemulsion-loaded polyvinyl alcohol/polyethyleneimine (PVA/PEI) hydrogel. First, PVA and PEI were dissolved into DMSO to form a gel. Then, the gel was immersed into a microemulsion composed of water, ethyl acetate, propylene carbonate, sodium dodecyl sulfate, and 1-pentanol to exchange DMSO. Microemulsion-loaded PVA/PEI hydrogel can be synthesized by completely substituting DMSO. To investigate the microstructure, rheological properties, and mechanical properties of the gel, scanning electron microscopy, a rheometer, and a universal testing machine were used, respectively. Fourier transform infrared (FT-IR) analysis was conducted to explore the synthesis mechanism and confirm the successful loading of microemulsion within the microemulsion-loaded PVA/PEI hydrogel. Furthermore, FT-IR, a depth-of-field microscope, and a glossmeter were utilized to evaluate the cleaning efficiency of the microemulsion-loaded PVA/PEI hydrogel for removing animal glue and soil from the surfaces of cultural relics. Moreover, an X-ray fluorescence spectrometer was used to analyze the element component of the ancient coin. The application results showed that the microemulsion-loaded PVA/PEI hydrogel can effectively remove animal glue from an ancient wall painting surface. Moreover, it is capable of removing soil from an ancient coin surface as well, which helped to confirm the age of the coin. This offers a novel method to prepare microemulsion-loaded hydrogel and demonstrates great potential in the cleaning for cultural heritage.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
A great deal of valuable cultural artefacts and works of art, such as wall paintings, stones, metals, etc., have been produced since the development of human civilization. However, these artifacts are now undergoing a process of aging and are increasingly at risk of being lost to posterity. It is our responsibility to appropriately preserve them for future generations [1,2,3,4,5,6,7,8]. Cleaning unwanted materials such as organic acrylic material, soil, etc., is crucial and challenging work in the field of the conservation of cultural heritage [9,10,11,12,13,14,15,16,17,18,19,20,21]. Initially, conservators employed neat liquids, such as water, alcoholic solution, ketones, etc., to remove organic coatings and soil from the surface of cultural relics [17, 22,23,24]. Nevertheless, neat solvent will dissolve the polymer surface coating, which risks diffusion of the solublised material into the pores of the relic. To tackle this problem, Baglioni et al. developed the so-called nanostructured fluid, which substantially improved the cleaning efficiency, especially for the removal of soil and organic polymer coatings [25,26,27]. The first application of their microemulsions was to clean wax contamination from a wall painting [28, 29]. Since then, microemulsions have received extensive investigations and obtained successful results. To date, various types of microemulsion have been produced: classical microemulsion, Triton X-100-based microemulsion, mineral spirits-based microemulsions, an amine-oxide surfactant-based microemulsion and EAPC (prepared with water, Ethyl Acetate, Propylene Carbonate, sodium dodecyl sulfate and 1-pentanol), where the EAPC has been the most frequently used [13, 16, 30,31,32,33,34,35]. The mentioned microemulsions have been used to remove soil, aged polymers, wax, grime, oils, etc., from wall painting, works on canvas, metal objects and other relics. Nowadays, microemulsions are increasingly employed in the cleaning of artworks [13]. Nevertheless, the surfactants in microemulsion tend to be left on the surface of cultural heritage materials, necessitating a rinsing or clearance step. In addition, most of the oil-in-water microemulsions can be high risk for the cleaning water-sensitive cultural heritage due to their high water content.
To address the limitations of microemulsions, various research groups have confined these water-thin liquid materials into the porous structure of gel to prepare a microemulsion-loaded gel. This strategy enabled microemulsions to be controllably released onto the surface of cultural heritage materials [11, 36,37,38,39]. During the cleaning process, the organic coatings and soil are absorbed to the surface of gel through its micro-pores [13, 40].
In an early attempt to control solvent application, Wolbers et al. proposed a solvent gel method to clean unwanted organic layers from cultural relics’ surface [41]. However, this method involved thickening solvents, risking residues remaining on the surface of the relic during and after cleaning [41]. Subsequently, chemical gels synthesized through crosslinking and precursor polymers were developed to be able to load microemulsions into. For instances, Giorgi et al. created a semi-interpenetrating hydrogel with 2-hydroxyethyl methacrylate and poly (vinylpyrrolidone) as the network framework, with α,α′-Azoisobutyronitrile as the crosslinker [11, 15, 40, 42]. This hydrogel was immersed into EAPC to obtained a microemulsion-loaded hydrogel. While effective in removing acrylic polymers from wall painting surfaces, the synthesis of this hydrogel was complex and time-consuming, taking over one week. Mastrangelo et al. prepared polyvinyl alcohol (PVA) hydrogel loaded with oil-in-water microemulsion, capable of removing hydrophobic grime, [35] where the synthesis process involved freeze–thaw cycle (100–25 ℃). First, PVA was dissolved into water at 100 ºC. Then, for the gel formation, the PVA-water solution was subjected to freeze/thaw cycles at − 23 ºC and 25 ºC, respectively. For this gel too, the whole preparation process was also time-consuming (more than 7 days). Using an elevated temperature (92 ℃), Yipan Yang et al. prepared self-shaping microemulsion gels by incorporating EAPC into a network formed with acrylamide, bacterial cellulose, agarose, N,N′-methylene bisacrylamide, ammonium persulfate, and N,N,N′,N′-tetramethylethylenediamine [43]. This gel has been used to demonstrate effectiveness in cleaning Paraloid B72 (B72) from the surface of a vase. Microemulsion-loaded gels are now increasingly recognized as being a versatile option for the cleaning domain of cultural heritage. However, the development of new methods to synthesize microemulsion-loaded hydrogel remains to be explored.
Solvent exchange method is a simple and efficient way to synthesize hydrogels. For example, Liju Xu et al. [44] employed a solvent exchange route to produce a strong and anti-swelling PVA hydrogel. They first dissolved PVA into dimethyl sulfoxide (DMSO) to form PVA DMSO solution (which is a good solvent for dissolving PVA), which then was poured into a Teflon mold. After being immersed in water (which is a poor solvent for dissolving PVA) for exchanging at room temperature for a 48 h, the PVA hydrogel could be obtained.
For this study, we employed a solvent exchange method to substitute DMSO in the PVA and polyethyleneimine (PEI) gel with microemulsion for the preparation of a microemulsion-loaded hydrogel. In the as-synthesized hydrogel, polyethyleneimine (PEI) was selected due to its good flexibility that may be beneficial for the gel used in the cleaning of cultural heritage [45]. Furthermore, EAPC system that contains water, ethyl acetate (EA), propylene carbonate (PC), and sodium dodecyl sulfate (SDS), and 1-pentanol was the target microemulsion (the resulting gel is referred to as microemulsion-loaded PVA/PEI hydrogel in the subsequent discussion). Fourier transform infrared (FT-IR), scanning electron microscope (SEM), rheometer, universal testing machine, optical microscope etc. were performed to explore the synthesis mechanism, mechanical properties, cleaning effects of the microemulsion-loaded PVA/PEI hydrogel.
Methods
Materials
Polyvinyl alcohol (PVA, alcoholysis degree: 97.5–99 mol%), poly(ethylene imine) (PEI, M.W. 600, 99%), dimethyl sulfoxide (DMSO, analytical reagent), sodium dodecyl sulfate (SDS, gas chromatography), 1-pentanol (gas chromatography), ethyl acetate (analytical reagent), and propylene carbonate (gas chromatography), were purchased from Aladdin Reagent Co., LTD.
Preparation of microemulsion
Microemulsion containing SDS, 1-pentanol, ethyl acetate (EA), and propylene carbonate (PC) was prepared according to the reported method [37]. Briefly, SDS, 1-pentanol, EA, and PC were added into deionized water while continuously stirring. The resulting mixture became transparent, indicating the formation of a microemulsion. The mass percentages of SDS, 1-pentanol, EA, PC, and deionized water are 3.5%, 7%, 8%, 8%, and 73.5%, respectively.
Preparation of microemulsion-loaded PVA/PEI hydrogel
The preparation method follows the reported procedure with modifications [44]. Firstly, PVA was dissolved into 95 g DMSO under 90 ℃ with stirring. Then, PEI was dropwise added. The total mass of PVA and PEI was 5 g and four mass ratios of PVA:PEI (65:35, 70:30, 75:25, and 80:20) were investigated. Once PEI was totally dissolved, the PVA and PEI DMSO solution was poured into a petri dish and stored in a refrigerator at 5 °C for 12 h to form a PVA/PEI DMSO gel. Microemulsion-loaded PVA/PEI hydrogel was obtained after the resulted gel was immersed into microemulsion for 12 h at room temperature. The microemulsion was replaced with fresh microemulsion every 2 h.
Preparation of mockup wall painting
We prepared the mockup wall painting based on previous work [3]. Firstly, we prepared a lime layer by uniformly mixing lime with water at a weight ratio of 0.8. After a half year (to ensure the full carbonation of lime layer), cinnabar mixed with gelatin were brushed to the carbonated lime layer using a brush to obtain mockup wall painting. The mockup was then coated with animal glue by brushing the animal glue solution (~ 5%) onto the surface of the painting. After drying in the lab environment, the mockup wall painting was coated with dirt by brushing soil (collected from the place finding the ancient coin, see section “Laboratory investigations”) aqueous (2%) onto the surface of the painting and left to dry in the lab environment (Fig. 8a).
Characterizations
The FT-IR spectra were acquired using a Bio-Rad FT-IR spectrometer model FTS165 with a resolution of 4 cm−1, 32 scans per spectrum. The spectral range is 4000–400 cm−1. FT-IR was used for explaining the synthesis mechanism of microemulsion-loaded PVA/PEI hydrogel and evaluating the cleaning efficiency of the gel for animal glue and soil from the surface of wall painting and coin. Scanning electron microscope (SEM) images were captured using a Nova 230 instrument (FEI, USA), with the samples coated in a thin layer of gold prior to observation. The parameters were 25 mA of current and 1 min of sputtering time. SEM was carried out to observe the micro structure of microemulsion-loaded PVA/PEI hydrogel. Rheological measurements were performed using a stress-controlled Anton Paar Physica MCR 302 rheometer. Strain sweeps were conducted at a constant angular frequency of 6.28 rad/s, ranging from 0.01% to 100%. Mechanical properties of microemulsion-loaded PVA/PEI hydrogel were tested using universal testing machine (Instron 5942). The sample was loaded at a rate of 1 cm/min. Rheological measurements and universal testing machine were conducted to test the rheological and mechanical properties of microemulsion-loaded PVA/PEI hydrogel. For the rheology or tensile strength tests, three samples were tested in parallel, and the average data was used for plotting. The Optical images were acquired using a VHX-5000 ultra depth-of-field microscope. The glossiness of the sample was tested by glossmeter (3nh, HG268). Depth-of-field microscope and glossmeter were used for evaluating the cleaning efficiency of the gel for animal glue and soil from the surface of wall painting and coin. Three points were tested and the averaged value was taken as glossiness of the sample. Natural evaporations of microemulsion and microemulsion-loaded PVA/PEI hydrogel were determined by weighting them every 10 min using a precision balance. X-ray fluorescence spectrometer (X-MET8000) was used to analyze the element component of the ancient coin.
Results and discussion
Characterization of the microemulsion-loaded PVA/PEI hydrogel
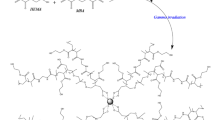
The synthesis process of microemulsion-loaded PVA/PEI hydrogel is illustrated in Fig. 1, involving two steps. Firstly, PVA and PEI were dissolved in DMSO at 90 ℃ with continuous stirring. Once the PVA was totally dissolved, PEI was dropwise added into the PVA solution. After the completion of PEI addition, the mixture of PVA and PEI solution was poured into a petri dish and stored at 5 ℃ for 12 h to form a gel. Secondly, the resulting gel was immersed in the microemulsion for 12 h to obtain a microemulsion-loaded PVA/PEI hydrogel. To ensure fully exchange of DMSO, fresh microemulsion was repeatedly exchanged every 2 h during the immersion process.
The microstructure of microemulsion-loaded PVA/PEI hydrogel with four different weight ratios of PVA:PEI (65:35, 70:30, 75:25, and 80:20) was imaged using SEM (Fig. 2). All the microemulsion-loaded PVA/PEI hydrogels appeared white. Macroscopically, there was no apparent difference observed among these hydrogels (Fig. 2a–d). By freeze drying the four hydrogels with freeze dryer, their structure was explored using microscopy (Fig. 2e–l). It was observed that due to capillary force action, most of the holes had collapsed during the freeze-drying process. The microstructure of the gel was disordered, which is different from the reported PVA or PVA based hydrogels (where the gel microstructure is ordered and organized) [46]. Furthermore, with the increase of PEI proportion (20–35%), the structure of PVA/PEI hydrogel appears even more disordered (Fig. 2e–l). This alteration is likely to be advantageous for the formation of a porous structure within the hydrogel, which facilitates the absorption of the microemulsion.
a-d Pictures of microemulsion-loaded PVA/PEI hydrogel prepared with the weight ratio of PVA:PEI 65:35, 70:30, 75:35, 80:20. e–h Low-magnification SEM images of microemulsion-loaded PVA/PEI hydrogel prepared with the weight ratio of PVA:PEI 65:35, 70:30, 75:35, 80:20. i-l High-magnification SEM images of microemulsion-loaded PVA/PEI hydrogel prepared with the weight ratio of PVA:PEI 65:35, 70:30, 75:35, 80:20
We analyzed the formation mechanism of this disordered structure by collecting FT-IR spectra of PVA, PEI, and PVA/PEI dried gel (Fig. 3a). For PVA, peaks at1100 and 1635 cm−1 are assigned to the vibration of OH and C-O, respectively [46]. The broad peak ranging from 1764 to 1524 cm−1 corresponds to the vibrational absorption of N–H in PEI. Additionally, the peak at 1042 cm−1 is due to the vibration of R2NH of PEI [47]. For PVA/PEI, the intensity of the peak ranging from 1764 cm−1 to 1524 cm−1 shows no obvious decrease, which is different from the reported PVA/PEI hydrogel formed through condensation reactions [47]. The formation of PVA/PEI hydrogel via condensation reaction causes the decline of peak intensity that is ranging 1764 cm−1 from 1524 cm−1. In the current work, it can be deduced that the peak of 1764–1524 cm−1 is the superposition of OH in PVA and R2NH in PEI. This phenomenon is similar to the peak from 1190 to 966 cm−1 in PVA/PEI, which is a combination of C–O of PVA and N–H of PEI. Based on the analysis, it is hypothesized that PVA and PEI chains are arranged in an orderly pattern after they are dissolved into DMSO (Fig. 3b) due to DMSO being a good solvent for them [44]. When the PVA/PEI DMSO gel was immersed into microemulsion to be subjected to solvent exchange, the orderly arranged PVA and PEI chains was disrupted. This is because water, which is a poor solvent for PVA and PEI compared to DMSO, causes the chains to become disordered and tangled. Consequently, the substitution of DMSO with a microemulsion results in the creation of a disordered porous structure within the hydrogel, which absorbs microemulsion and culminates in the formation of the microemulsion-loaded PVA/PEI hydrogel (Fig. 3b).
Rheological and mechanical properties were explored through rheometer and universal testing machine, respectively. The amplitude sweep curves demonstrate that G’ (storage modulus) and G’’ (loss modulus) of the microemulsion-loaded PVA/PEI hydrogels is cross-cover at lower oscillation strain (Fig. 4a). With the increases of PEI content (20–35%), peaks of G’’ of the microemulsion-loaded PVA/PEI hydrogel shift to higher strains (Fig. 4a, arrows), suggesting that the addition of PEI makes the hydrogel compliant. It can be estimated that the addition of PEI reduces the crosslinking of PVA chains via intermolecular hydrogen bonds, thereby decreasing the stiffness of the microemulsion-loaded PVA/PEI hydrogel. Frequency sweeps demonstrated that the storage modulus in the study is higher than their loss storage modulus, and the mechanical properties of the hydrogels decrease with increasing PEI content (Fig. 4b). We also tested the mechanical properties through tensile testing (Fig. 4c). It can be seen that as the PEI content increases, the stress of these hydrogels initially rises and subsequently decreases. Moreover, the strain shows a similar trend (Fig. 4c). Young’s Modulus (calculated from Fig. 4c) of these hydrogels declines with the increase of PEI content, which agrees with the variation tendency noted in the rheological measurements (Fig. 4d). In other words, the microemulsion-loaded hydrogel becomes flexible with increase of PEI content. We propose that the addition of PEI partly hampers the intermolecular crosslinking by forming hydrogen bonds, such as N H (N in PEI with H in PVA). This behavior leads to the formation of disordered porous structure that traps the microemulsion, obtaining microemulsion-loaded PVA/PEI hydrogel. Considering the resilience and the strain (elongation at break) that is benefit to the application of the hydrogel [36], we selected PVA/PEI = 70:30 (Young's modulus and elongation at break) to be the optimal parameter to prepare this microemulsion-loaded PVA/PEI hydrogel.
a Strain dependence, b frequency dependence, c curves of stress–strain of microemulsion-loaded PVA/PEI hydrogel prepared with the weight ratio change of PVA:PEI of 65:35, 70:30, 75:25, and 80:20. d Young’s Modulus and strain of microemulsion-loaded PVA/PEI hydrogel prepared with the weight ratio change of PVA:PEI of 65:35, 70:30, 75:25, and 80:20
The cylindrical PVA/PEI hydrogel (diameter and height: 4 cm) was prepared to determine whether the microemulsion was fully loaded (Fig. 5a). We cut the cylindrical hydrogel each 5 mm (Fig. 5b) and samples were collected at different depths of the hydrogel to perform FT-IR analysis. As for microemulsion alone, a broad peak around 3400 cm−1 is ascribed to the vibration of OH in water and 1-pentanol (Fig. 5c). The peak at 1647 cm−1 is ascribed to the vibration absorption of C = O in EA and PC [48, 49]. The peak at 1425 cm−1 is attributed to vibration of SO42− of SDS [50]. The peak centered at 1022 cm−1 is due to the overlay of C-O vibration in PC and 1-pentanol [51]. The FT-IR spectra of the liquid at different depths of the PVA/PEI hydrogel, including at the surface, 5 mm, 10 mm, 15 mm, and 20 mm, were similar to that of microemulsion. Therefore, we deduced that the microemulsion was fully loaded into the PVA/PEI hydrogel.
a Picture of microemulsion-loaded PVA/PEI hydrogel. Both the diameter and height of the microemulsion-loaded PVA/PEI hydrogel are 4 cm. b Schematic diagram of cylindrical microemulsion-loaded PVA/PEI hydrogel cut every 5 mm from surface to inner. c FT-IR spectra of microemulsion, and liquid collected at surface, 5 mm, 10 mm and 20 mm of PVA/PEI hydrogel. We gently rubbed the gel surface with a cotton swab and squeezed out the liquid to perform the FI-IR
To evaluate the safety of applying microemulsion-loaded PVA/PEI hydrogel for removing unwanted polymer coatings and soil from cultural heritage surfaces, we compared the evaporation rates of the microemulsion and microemulsion-loaded PVA/PEI hydrogel. We plotted the relationship figure of weight loss with time by recording the weight of the sample per 10 min. As shown in Fig. 6, the weight losses of microemulsion and microemulsion-loaded PVA/PEI hydrogel per 10 min are ~ 1% and ~ 3%, respectively, implying that the evaporation of microemulsion is significantly reduced after being loaded into PVA/PEI hydrogel (which is to be expected). This ensures the slow release of the microemulsion onto the surface of cultural heritage material and represents a low risk to the artefact during the removal of polymers and soil.
Removing animal glue and soil from wall painting and ancient coin
Laboratory investigations
Before being applied to cultural heritage materials, we tested the cleaning effect of the microemulsion-loaded PVA/PEI hydrogel using the mockup wall painting. First, we placed microemulsion-loaded PVA/PEI hydrogel on the surface of the mockup wall painting coated with animal glue (Fig. 7a, b)—hereafter called the wall painting mockup for 30 min. It is evident that the reflective intensity of the cleaned area becomes weak (Fig. 7d–g). In addition, the glossiness was reduced to 2.2 GU from 15 GU. The dramatic reduction demonstrates that most of animal glue coated the surface of mockup wall painting is cleaned by the microemulsion loaded PVA/PEI hydrogel. The cleaning effects were also demonstrated by optical microscope. Gradually, the animal glue coated on the surface of the mockup wall painting was removed as time progressed (Fig. 7h–i) and was totally removed thirty minutes later (Fig. 7j and k, no further treatment was carried out with cotton swab or other tools after taking off the microemulsion-loaded hydrogel).
a Mockup wall painting coated with animal glue. b Microemulsion-loaded PVA/PEI hydrogel placed on the surface of the mockup wall painting. c Mockup wall painting after the animal glue is removed. d Partial view of the mockup wall painting coated with animal glue. The microemulsion-loaded PVA/PEI hydrogel placed on the surface of mockup wall painting for e 10 min, f 20 min, g 30 min. h Optical image of the mockup wall painting coated with animal glue. Optical images of the mockup wall painting after microemulsion-loaded PVA/PEI hydrogel being placed its surface for i 10 min, j 20 min, k 30 min
We also examined the cleaning effect of microemulsion-loaded PVA/PEI hydrogel for soil on the mockup wall painting surface (Fig. 8a). The microemulsion-loaded PVA/PEI hydrogel was applied to the soil-covered painting for 5 min (Fig. 8b). It is evident that soil is effectively removed upon the hydrogel's removal (as depicted in Fig. 8c and d), indicating that the hydrogel can efficiently clean soil from the surface of mockup wall painting. The microscope investigation shows that the surface after treatment is consistent with the original painting (Fig. 8e–g). The aforementioned investigations suggest that the microemulsion-loaded PVA/PEI hydrogel can be considered when exploring the removal of animal glue and soil from some heritage surfaces.
a Picture of the mockup wall painting coated with soil. b Placing microemulsion-loaded PVA/PEI hydrogel on the surface of soil coated wall painting. c Picture the mockup wall painting after the removal of the microemulsion-loaded PVA/PEI hydrogel. d Partial view of the cleaned area. Optical images of the e mockup wall painting, f mockup wall painting coated with soil, g mockup wall painting cleaning with microemulsion-loaded PVA/PEI hydrogel
Heritage artefact case study
Ancient murals discovered in the Wu Huifei tomb, designated for the imperial concubine of the Tang Dynasty, have a history of over a thousand years. In 2009, the ancient tomb was robbed. To preserve the murals, conservators decided to transfer them to museum. During the transferring, a strong solution of animal glue (approximately 20%) and flax was applied to the front of murals to safeguard them during excavation. They were moved to the Shaanxi history museum, eventually. Now, to restore the mural and for exhibition, conservators need to remove the animal glue and flax. This necessitated the softening and removal of the animal glue (Fig. 9b). For the microemulsion-loaded PVA/PEI hydrogel was shaped to the desired dimensions and placed on the flax surface (Fig. 9c–e). After 30 min, the hydrogel was removed, facilitating the trimming of flax in the treated area (Fig. 9f). From the macro perspective, the animal glue was no longer observable after being cleaned with the microemulsion-loaded PVA/PEI hydrogel (Fig. 9g). The cleaned area was explored using a light microscope and FT-IR analysis. Prior to the cleaning experiment, a significant amount of reflective animal glue was visible (Fig. 9e). After cleaning, there were no obvious remnants of animal glue observed (Fig. 9h). To detect residue of animal glue after cleaning, we utilized FT-IR, which is a limited instrument for residue detection. Animal glue was collected from the wall painting before cleaning to perform FT-IR. Two distinct peaks at 1630 cm−1 and 1521 cm−1 (Fig. 10), corresponding to the C = O and primary amide vibrations, respectively, demonstrated that the animal glue was somewhat oxidized (aged). After cleaning, the two characteristic peaks of animal glue were no longer detectable with the instrument employed. Three peaks at 1450, 875, and 712 cm−1 were assigned to the vibration absorption of CO32− (Fig. 10), implying that microemulsion-loaded PVA/PEI hydrogel is able to effectively remove animal glue from wall painting surface. Furthermore, this hydrogel had not apparent unwanted effects on the wall painting mockup during the cleaning process.
a Picture of the ancient painting. b Part view of the ancient painting. c Picture of the removal of animal glue coated on the surface of ancient wall painting. d Ancient painting covered with flax that is adhered to the painting surface by animal glue. e Microemulsion-loaded PVA/PEI hydrogel placed on the surface of flax. f The flax was cut with scissor after removing the hydrogel. Optical images of the g flax and h the surface of the wall painting after removing the adhered flax
An ancient coin was found when farming in Shaanxi province of China (Fig. 11a). This ancient coin was found when farming in Shaanxi province of China. The land where the ancient coins was found is loess land. Therefore, the soil covered on the surface of the coin is yellow earth. We carried out XRF to reveal the component of the metal of the coin (Table 1). Result showed that the metal element of the coin is Cu and Fe (Si element should be attributed to component element of yellow earth soil). However, the coin was covered in yellow earth that could not be easily washed off, even after being immersed in water for three days. Based on the historic knowledge, we could only identify two characters on the coin, which are “通” (left) and “寳” (right). The other two significant characters on the top and bottom of the coin were illegible. To estimate the age of the coin without serious damage, we employed a microemulsion-loaded PVA/PEI hydrogel to clean soil from the coin surface. We applied the hydrogel on the top of the coin, exerting a 2 kg weight for 5 min, and then removed the hydrogel (Fig. 11b and c). The soil on the coin surface was partially removed through being absorbed to the hydrogel surface (Fig. 11c). Then, we rinsed off the soil from the hydrogel using the microemulsion and the microemulsion-loaded PVA/PEI hydrogel could be reused. This process was repeated 30 times, resulting in the removal of the majority of the soil (Fig. 11d). After cleaning, we could identify that the  and
and  on the top and bottom, respectively. Therefore, the words on the coin are
on the top and bottom, respectively. Therefore, the words on the coin are  ,
,  ,
,  , and
, and  , which certainly belong to Qing Dynasty and may date back approximately 270 years to the present day. Despite the cleaning process, there is still a small amount of soil remaining on the top surface of the coin, indicating the difficulty of completely removing all the dirt. Prior to cleaning, only the soil was visible (Fig. 11e and f). After cleaning, the metal of the coin was revealed (Fig. 11g and h). However, an amount of soil remained embedded inside the coin, making it challenging to fully remove (Fig. 11g and h, the dotted area).
, which certainly belong to Qing Dynasty and may date back approximately 270 years to the present day. Despite the cleaning process, there is still a small amount of soil remaining on the top surface of the coin, indicating the difficulty of completely removing all the dirt. Prior to cleaning, only the soil was visible (Fig. 11e and f). After cleaning, the metal of the coin was revealed (Fig. 11g and h). However, an amount of soil remained embedded inside the coin, making it challenging to fully remove (Fig. 11g and h, the dotted area).
a Picture of an ancient coin. b Microemulsion-loaded PVA/PEI placed on the surface of the ancient coin. c Picture of the ancient coin and the hydrogel after removed from the surface of the coin. d Picture of the coin after cleaned with the hydrogel for 30 times. Optical images of the e, f ancient coin and g, h coin after cleaned the hydrogel for 30 times
Conclusions
In summary, a solvent exchange method was developed to prepare a microemulsion-loaded PVA/PEI hydrogel which was examined for its effectiveness for removing animal glue and soil from surface of wall paintings and a naturally aged metal coin. The porous structure of the hydrogel absorbs the microemulsion, resulting in a microemulsion-loaded PVA/PEI hydrogel. The evaporation rate of the microemulsion in the PVA/PEI hydrogel reduced approximately three-fold compared to pure microemulsion. The controlled release of the loaded microemulsion helps to ensure the integrity of the cultural heritage surface during the cleaning process. These results demonstrate that the microemulsion-loaded PVA/PEI hydrogel effectively removed animal glue from the surface of wall paintings. Additionally, this hydrogel can help to clean the soil of an ancient coin, assisting us to better identify the era of the coin. This represents a new approach to prepare the microemulsion-loaded hydrogel and contributes to ongoing research into the low-risk removal of organic polymeric coatings and soil from the surfaces of cultural heritage materials and artefacts.
Availability of data and materials
The data in the work is available from the corresponding author with reasonable request.
References
Zhu J, et al. Polydopamine-modified nanolime with high kinetic stability in water for the consolidation of stone relics. ACS Appl Mater Interfaces. 2022;14:13622–30.
Ding J, et al. Quaternary ammonium silane modified nanolime for the consolidation and antifungal of stone relics. Constr Build Mater. 2023;400:132605.
Zhu J, Li X, Zhang Y, Wang J, Wei B. Graphene-enhanced nanomaterials for wall painting protection. Adv Funct Mater. 2018;28:1803872.
Franco-Castillo I, Hierro L, de la Fuente JM, Seral-Ascaso A, Mitchell SG. Perspectives for antimicrobial nanomaterials in cultural heritage conservation. Chem. 2021;7:629–69.
Zhu J, et al. In-situ growth synthesis of nanolime/kaolin nanocomposite for strongly consolidating highly porous dinosaur fossil. Constr Build Mater. 2021;300:124312.
Nevin A, Pouli P, Georgiou S, Fotakis C. Laser conservation of art. Nat Mater. 2007;6:320–2.
Zhu J, et al. Realizing nanolime aqueous dispersion via ionic liquid surface modification to consolidate stone relics. Langmuir. 2023;39:9903–11.
Zhu J, et al. Nano Ca(OH)2: a review on synthesis, properties and applications. J Cult Herit. 2021;50:25–42.
Poggi G, et al. Nanostructured bio-based castor oil organogels for the cleaning of artworks. J Colloid Interface Sci. 2023;638:363–74.
Guaragnone T, et al. Ph-responsive semi-interpenetrated polymer networks of pHEMA/PAA for the capture of copper ions and corrosion removal. ACS Appl Mater Interfaces. 2022;14:7471–85.
Baglioni M, et al. Selective removal of over-paintings from “street art” using an environmentally friendly nanostructured fluid loaded in highly retentive hydrogels. J Colloid Interface Sci. 2021;595:187–201.
Di Napoli B, et al. Gellan gum microgels as effective agents for a rapid cleaning of paper. ACS Appl Poly Mater. 2020;2:2791–801.
Baglioni M, et al. Nonionic surfactants for the cleaning of works of art: insights on acrylic polymer films dewetting and artificial soil removal. ACS Appl Mater Interfaces. 2020;12:26704–16.
Mazzuca C, et al. Cleaning of paper artworks: development of an efficient gel-based material able to remove starch paste. ACS Appl Mater Interfaces. 2014;6:16519–28.
Giorgi R, Baglioni M, Baglioni P. Nanofluids and chemical highly retentive hydrogels for controlled and selective removal of overpaintings and undesired graffiti from street art. Anal Bioanal Chem. 2017;409:3707–12.
Duncan TT, Berrie BH, Weiss RG. Soft, peelable organogels from partially hydrolyzed poly(vinyl acetate) and benzene-1,4-diboronic acid: applications to clean works of art. ACS Appl Mater Interfaces. 2017;9:28069–78.
Prati S, et al. Cleaning oil paintings: NMR relaxometry and SPME to evaluate the effects of green solvents and innovative green gels. New J Chem. 2019;43:8229–38.
Mastrangelo R, et al. Twin-chain polymer hydrogels based on poly(vinyl alcohol) as new advanced tool for the cleaning of modern and contemporary art. Proc Natl Acad Sci USA. 2020;117:7011–20.
Andrea C, David C, Piero B. Advanced methodologies for the cleaning of works of art. Sci China Tech Sci. 2023;66:2162–82.
Weththimuni ML, Girella A, Ferretti M, Sacchi D, Licchelli M. Nanostructured emulsions as smart cleaning materials for removing aged polymer coatings from stone substrates. Sustainability. 2023,15, 8117.
Zhu J, et al. Effectively removing animal glue coated on the surface of ancient mural via dissolution of PVA hydrogel induced by thermal treatment. J Cult Herit. 2022;55:179–84.
Mazzuca C, et al. Innovative chemical gels meet enzymes: a smart combination for cleaning paper artworks. J Colloid Interface Sci. 2017;502:153–64.
Sansonetti A, et al. A review in using agar gels for cleaning art surfaces. J Cut Heritage. 2020;44:285–96.
Baglioni P, Baglioni M, Bonelli N, Chelazzi D, Giorgi R. Nanotechnologies and nanomaterials for diagnostic, conservation and restoration of cultural heritage. Amsterdam: EIsevier; 2019. p. 171–204.
Baglioni M, Alterini M, Chelazzi D, Giorgi R, Baglioni P. Removing polymeric coatings with nanostructured fluids: Influence of substrate, nature of the film, and application methodology. Front Mater. 2019;6:311.
Rosciardi V, Chelazzi D, Baglioni P. “Green” biocomposite poly(vinyl alcohol)/starch cryogels as new advanced tools for the cleaning of artifacts. J Coll Interface Sci. 2022;613:697–708.
Alcalá S, et al. The use of nanostructured fluids for the removal of polymer coatings from a Nuxalk monumental carving—exploring the cleaning mechanism. J Cult Herit. 2022;55:18–29.
Chelazzi D, Giorgi R, Baglioni P. Microemulsions, micelles, and functional gels: how colloids and soft matter preserve works of art. Angew Chem Int Ed Engl. 2017;57:2–10.
Chelazzi D, Bordes R, Giorgi R, Holmberg K, Baglioni P. The use of surfactants in the cleaning of works of art. Curr Opin Coll Interface Sci. 2020;45:108–23.
Baglioni P, Chelazzi D, Giorgi R, Poggi G. Colloid and materials science for the conservation of cultural heritage: cleaning, consolidation, and deacidification. Langmuir. 2013;29:5110–22.
Baglioni M, et al. Complex fluids confined into semi-interpenetrated chemical hydrogels for the cleaning of classic art: a rheological and SAXS study. ACS Appl Mater Interfaces. 2018;10:19162–72.
Ormsby B, et al. Mineral spirits-based microemulsions: a novel cleaning system for painted surfaces. J Am Inst Conser. 2016;55:12–31.
Baglioni M, et al. An amine-oxide surfactant-based microemulsion for the cleaning of works of art. J Coll Interface Sci. 2015;440:204–10.
Baglioni M, Sekine FH, Ogura T, Chen SH, Baglioni P. Nanostructured fluids for polymeric coatings removal: surfactants affect the polymer glass transition temperature. J Coll Interface Sci. 2022;606:124–34.
Mastrangelo R, Montis C, Bonelli N, Tempesti P, Baglioni P. Surface cleaning of artworks: structure and dynamics of nanostructured fluids confined in polymeric hydrogel networks. Phys Chem Chem Phys. 2017;19:23762–72.
Bonelli N, Poggi G, Chelazzi D, Giorgi R, Baglioni P. Poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for the cleaning of art. J Coll Interface Sci. 2019;536:339–48.
Carretti E, et al. New frontiers in materials science for art conservation: Responsive gels and beyond. Acc Chem Res. 2010;43:751–60.
Al-Emam E, et al. Removal of a past varnish treatment from a 19th-century belgian wall painting by means of a solvent-loaded double network hydrogel. Polymers. 2021;13:2651.
Baglioni P, Chelazzi D, Giorgi R. Nanorestart: nanomaterials for the restoration of works of art. Herit Sci. 2021;9:1–5.
Bonelli N, Montis C, Mirabile A, Berti D, Baglioni P. Restoration of paper artworks with microemulsions confined in hydrogels for safe and efficient removal of adhesive tapes. Proc Natl Acad Sci USA. 2018;115:5932–7.
Baglioni P, Chelazzi D. Nanoscience for the conservation of works of art. London: Royal Society of Chemistry; 2013. p. 382–3.
Domingues JAL, et al. Innovative hydrogels based on semi-interpenetrating P(HEMA)/PVP networks for the cleaning of water-sensitive cultural heritage artifacts. Langmuir. 2013;29:2746–55.
Yang Y, et al. Self-shaping microemulsion gels for cultural relic cleaning. Langmuir. 2021;37:11474–83.
Xu L, et al. A solvent-exchange strategy to regulate noncovalent interactions for strong and antiswelling hydrogels. Adv Mater. 2020;32:2004579.
Chen Z, Lv Z, Sun Y, Chi Z, Qing G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J Mater Chem B. 2020;8:2951–73.
Shao L, et al. PVA/polyethyleneimine-functionalized graphene composites with optimized properties. Mater Design. 2016;99:235–42.
Li Q, Wu C, Zhang B. Hybrid hydrogels based on polyvinyl alcohol, branched polyethylenimine, polydopamine, and phosphonium-based ionic liquid for effective synergetic antibacterial applications. Colloid Surface A. 2022;648:129277.
Wang W, Zhang G, Xie L, Wang Z, Deng G. Fast assay of ethyl acetate content of double-base oblate spherical propellant after balling process by near-infrared spectroscope. Infrared Phy Techn. 2021;112:103579.
Ahmad NH, Isa MIN. Characterization of un-plasticized and propylene carbonate plasticized carboxymethyl cellulose doped ammonium chloride solid biopolymer electrolytes. Carbohyd Polym. 2016;137:426–32.
Saad P, Flach CR, Walters RM, Mendelsohn R. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int J Cosmetic Sci. 2011;34:36–43.
Ikezawa Y, Ariga T. In situ FTIR spectra at the cu electrode/propylene carbonate solution interface. Electrochim Acta. 2007;52:2710–5.
Acknowledgements
Not applicable.
Funding
This research is supported by the National Natural Science Foundation of China (52372035, 52002304, 52072228), the Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20190806155605415), the Key Research and Development Program of Shaanxi Province (Program No. 2024SF-YBXM-676, 2020KWZ-018, 2020ZDLGY10-03), the Key Research and Development Program from Xianyang (2021ZDYF-GY-0024), Open Project of Gansu Province Dunhuang Cultural Relics Protection Research Center (GDW2021ZD02), the Fundamental Research Funds for the Central Universities supported by Northwestern Polytechnical University (23GH02026).
Author information
Authors and Affiliations
Contributions
Jinmeng Zhu and Xuanhua Li conceived the idea and designed the experiment. Jinmeng Zhu and Xuanhua Li wrote the manuscript. Jiapeng Wang, Jia Wang and Jinghan Ding performed the experiments. All the authors edited and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, J., Wang, J., Wang, J. et al. Preparing a microemulsion-loaded hydrogel for cleaning wall paintings and coins. Herit Sci 12, 149 (2024). https://doi.org/10.1186/s40494-024-01269-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-024-01269-3