Abstract
The gelatine emulsions used in historical photographs can shrink and become brittle under alternating dry–wet environmental conditions, which would result in curling and fracture of the gelatin paper photographs, thereby degrading their quality and threatening the long-term inheritance of such cultural heritage. To improve the stability and flexibility of gelatine films under dry–wet cycling, glycerol triglycidyl ether (GPE) was employed as a synergistic crosslinking and plasticising agent. The plasticising effect of GPE on the dimensional stability and flexibility of gelatine films in alternating dry–wet environments was firstly studied. Gelatine films with different contents of GPE were prepared and their dimensional changes during dry–wet cycling were investigated. The results indicate that GPE greatly enhances the dimensional stability of the films during cycling. By analysing the swelling behaviour, molecular structure, mechanical strength, fracture cross-sections, and other properties of the gelatine–GPE films, it was confirmed that the addition of GPE greatly reduces the moisture absorption and swelling of gelatine and improves its moisture stability. Furthermore, benefiting from GPE as a crosslinking agent, the mechanical strength and flexibility of the gelatine films were both enhanced. In this study, the modification of gelatin film by GPE provides experimental evidence for the subsequent research on the application of restoration and conservation for the gelatin paper photographs.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Gelatine is an inexpensive protein with excellent film-forming properties, which is widely used in coatings, medicine, food, photography, and other fields [1,2,3,4,5]. Because of its brittleness and sensitivity to hygrothermal environments, the application of gelatine is greatly limited [6, 7]. The brittleness and moisture/heat stability of gelatine can be controlled by a crosslinking reaction between the functional groups of gelatine and some suitable crosslinking agents. The crosslinking of gelatine with glycerol epoxy resin to form a hyperbranched polymer was shown to reduce its brittleness and increased its water resistance [8]. Other studies used glycerol triglycidyl ether to modify collagen fibre scaffold materials and pigskin biological dressings, which increased the hydrophilicity of the protein dressing [9, 10]. The aggregation behaviour of gelatine in aqueous solutions affects the grafting density of glycidyl in the gelatine [11]. When the amount of added ethylene glycol diglycidyl ether reaches a critical concentration, the swelling rate of gelatine remains constant, and the amino functional groups are completely consumed [12]. A study of amino conversion in the grafting reaction of allyl glycidyl ether using the van Sleek method proposed that the grafting of epoxy compounds and gelatine mainly occurs via the free NH2 group of gelatine [13]. These findings show that the chemical crosslinking between epoxy resin and gelatine can control the brittleness, hydrophobicity, mechanical properties, and stability of gelatine films.
Polyethylene glycol, glycerine, sorbitol, and other polyol small-molecule plasticisers can be inserted into the gelatine polymer chain to form a hydrogen-bond network, thereby increasing the fluidity and flexibility of gelatine molecules, which contributes to plasticising the gelatine film [14,15,16]. The epoxy group in polyol glycidyl ether opens the epoxy ring and reacts with the amino group in the gelatine molecule to generate a hydroxyl group, forming structures similar to the above polyol structures. Early research showed that the aggregation of protein molecules of an emulsified gelatine film under cyclic dry–wet conditions resulted in the macroscale shrinkage of the film, which was identified as the main cause of the embrittlement, curling, and fracture of gelatin paper photographs [17]. As shown in Fig. 1, the structure of the gelatin paper photographs is composed of a paper base and a barium, emulsion and gelatin protective layer, and the main film-forming materials of the last three layers are gelatin molecules. Therefore, ensuring the stability and flexibility of gelatine films in a hot and humid environment is an effective way to prevent the degradation of gelatin paper photographs. In addition to its role as a crosslinking agent, polyol glycidyl ether would be a plasticiser, which would further help protect gelatin paper photographs. To date, there has been no report about the plasticising effect of polyol glycidyl ether on gelatine films.
Against such a background, this study employed glycerol triglycidyl ether (GPE) as a representative plasticiser to investigate its effect on the dimensional stability and flexibility of photographic gelatine films during dry–wet environments. The size change and shrinkage of GPE–gelatine composite films were investigated under cyclic dry–wet environmental conditions and the effect of GPE on reducing the shrinkage of gelatine films was analysed macroscopically. The plasticising and flexing effects of GPE on gelatine films were further studied by analysing the swelling, molecular structure, mechanical strength, and fracture cross-sections of the composite films. Research results provide theoretical support for further research on the prevention and treatment of gelatin paper photographs to overcome embrittlement, curling, and fracture.
Experimental Section
Sample preparation
An appropriate amount of gelatine powder (photographic grade, Aladdin Chemical Reagent Co., Ltd.) was weighed and soaked in distilled water. After complete swelling, the gelatine was heated at 65 °C for 40 min under stirring to produce a transparent and uniform solution with a concentration of 5wt%. Then, a pipette gun was used to add an appropriate amount of GPE (biological grade, Aladdin Chemical Reagent Co., Ltd.) to prepare gelatine–GPE solutions with concentrations of 0wt% (control), 0.5wt%, 1wt%, 1.5wt%, and 2wt%. After adding GPE, the mixed solution was heated at 65 °C with stirring for 90 min, then transferred to a culture dish and tape-casting was used to form a composite film, which was naturally dried at room temperature. The preparation process of blank and composite films is controlled at the same condition, thereby the thickness values of the blank and composite films are almost same (about 80 μm) after drying.
Characterisation methods
To study dimensional stability, the prepared pure gelatine control film and gelatine films containing various fractions of GPE were cycled many times between dry and wet environments until the dimensions of the sample became stable. The high-humidity environment had a relative humidity (RH) of 85% and temperature (T) of 23 °C, while the dry environment had a RH of 15% and T of 50 °C. The sample was placed 24 h at each condition, and 48 h for one cycle. The effects of different fractions of GPE on the shrinkage of the gelatine films were analysed.
To study film swelling, all film samples were cut into 3.0 cm × 7.0 cm pieces and placed in a desiccator containing silica gel for 24 h. The samples were weighed to obtain W1 and then immersed in a container with deionised water. After 5 min, full swelling was achieved and the wet film from removed from the container, wiped with filter paper to remove excess water, and weighed to obtain W2. The degree of swelling was calculated from the water uptake indicated by the weight change after immersion, as shown in Eq. (1).
To study surface structure, the molecular structure of the gelatine films with different fractions of GPE was characterised by Fourier transform infrared spectroscopy (FTIR; Perkin Elmer Spectrum 2, Vertex70, Bruker, Karlsruhe, Germany) in total reflection mode at room temperature and ambient humidity. The measurements were performed over a wavenumber range of 500–4000 cm−1 with a step size of 4 cm−1. To reduce the influence of CO2 and water vapor on the infrared spectra, the background was deducted from all spectra. The crystal structures of the GPE–gelatine films were analysed using X-ray diffraction (XRD; Smart Lab, Rigaku Corporation, Japan) using a voltage of 45 kV, current of 200 mA, scan speed of 5°/min, and 2θ scan range of 2–50°.
To study mechanical properties, the various films were cut into 12 cm × 1.5 cm rectangles and their film thicknesses were measured. A universal material testing machine (RGT-10, Xi'an Mingke Testing Equipment Co., Ltd.) was used to measure the mechanical properties of the films at room temperature. Tensile tests to obtain the tensile strength, Young's modulus, and elongation at break were performed at a speed of 10 mm/min.
To study fracture cross-sections, the films were broken in half by an external folding force, and the fractured samples were attached to aluminium sample holders with conductive tape. The sample cross-sections were sprayed with gold for 80 s with an ion-sputtering device (SCD005, Baltek, Liechtenstein, Germany) and the surface morphology of the samples was observed by scanning electron microscopy (SEM; Hitachi, TM3030).
Results and discussion
Dimensional stability
The expansion and contraction of the control film and GPE-plasticised films varied during environmental cycles (Fig. 2) and the final shrinkage is shown in Fig. 3. During cycling, all samples experienced expansion (swelling by the uptake of moisture) and contraction (drying shrinkage), and irreversible shrinkage generally occurred in the length by the end of cycling. The dimensions of the control film fluctuated greatly during cycling, and the final shrinkage rate was 5.54% after 17 cycles. However, the magnitude of the changes in expansion/shrinkage of the composite gelatine–GPE films decreased during cycling, and the final shrinkage decreased to 1.68% with increasing 2.0wt% of GPE. These experimental results show that the addition of GPE as a plasticiser reduces the dimensional changes that the gelatine film experiences during environmental cycling to some extent. This indicates that GPE could provide a solution for the stable preservation of the gelatine emulsion in crimped historical photographs.
Film swelling
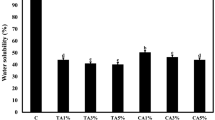
Figure 4 shows that the swelling rate of the film decreases significantly with the addition of GPE. The maximum swelling rate of the pure gelatine control film is ~ 707%, while that of the gelatine film containing 2.0wt% GPE is ~ 197%. It is interpreted that GPE belongs to the class of amphiphilic small molecules, which contains hydrophilic epoxy groups and hydrophobic alkyl chains. The hydrophobic chains reduce the water absorption of hydrophilic compounds, thereby reducing their swelling capacity. Although the water absorption of the gelatine film was lower with the addition of GPE, the ductility of the film after water absorption and swelling increases with increasing GPE concentration (Fig. 5), which may be due to reduced intermolecular forces in the gelatine macromolecular system after the addition of GPE. The results discussed so far indicate that GPE acts as a plasticiser in the gelatine system.
Structural properties
To characterise the chemical interactions between GPE and gelatine molecules, FTIR spectra of the films were analysed (Fig. 6a). The characteristic FTIR spectrum of gelatine includes a stretching vibration peak of the carbonyl group in the amide I band of the gelatine molecule at 1650 cm−1. Peaks related to C–N stretching vibrations and N–H in-plane deformation vibration of the amide II band of the gelatine molecule are observed at 1525 cm−1. In addition, the peak at 1238 cm−1 is the C–N stretching vibration peak of the amide III band of the gelatine molecule [18]. Figure 6a shows that the intensities of the absorption peaks related to the amide I, amide II, and amide III bands in the gelatine molecule do not change with increasing GPE fraction. In addition, compared with the pure gelatine control film, the C–O–C stretching vibration of the gelatine–GPE composite films at 1010 cm−1 gradually increases with increasing GPE fraction; this is related to the ether bonds in the GPE molecule. However, the cross-linking reaction between the epoxy group of GPE and the side chain amino group of gelatine was not detected, which may be related to the small amounts of GPE used in this study. The FTIR results prove that physical intermolecular interactions occur between the GPE and gelatine molecules. GPE acts as a plasticiser in the gelatine system but does not change the chemical structure or properties of gelatine film itself.
To further investigate the effect of GPE on the crystal structure of gelatine molecules, we obtained the XRD patterns of the composite films. Figure 6b shows that the control film has two major characteristic diffraction peaks at 2θ positions of 7.5° and 21.6°. The diffraction peak at 7.5° is the triple helix characteristic peak of gelatine [19, 20], while the peak at 21.6° is the amorphous characteristic peak of gelatine [21]. With the addition of GPE, the intensity of the triple helix characteristic peak decreased significantly, while that of the amorphous characteristic peak decreased only slightly. It is speculated that this structural change is due to the electrostatic adsorption and hydrogen bonding between GPE small molecules and hydroxyl or amino groups on the side chain of gelatine molecules. This hinders the orderly arrangement of the triple helix structure of gelatine, thus reducing the brittleness (increasing the flexibility) of the composite films.
Mechanical properties
To study the effect of GPE on the mechanical properties of gelatine films, the tensile strength, Young's modulus, and elongation-at-break of the various samples were tested, and the results are summarised in Table 1. These data show that the tensile strength and elongation-at-break of the plasticised film increase significantly with increasing GPE fraction, increasing by 80.91% and 135.77%, respectively. Furthermore, the Young's modulus decreases with increasing GPE content, with a final decrease of 33.95% for the film with 2.0% GPE. It is inferred from these results that the increased tensile strength may be due to the partial chemical crosslinking between GPE and gelatine molecules. In addition, the plasticising effect of GPE in gelatine resulted in an increase in the elongation-at-break and a decrease in Young's modulus of the composite films compared to the control film. The high tensile strength and excellent flexibility of gelatine-based emulsion films are very important for their long-term stability and application. The strength and flexibility of the gelatine film were modified synergistically by adding GPE.
Fracture cross-sections
To further verify the toughening effect of GPE on gelatine films, the micro-morphology of the film fracture cross-sections was observed. Figure 7 shows the cross-sectional morphology of the gelatine control and GPE-plasticised gelatine films after dry–wet cycling. The fracture profile of the pure gelatine film after cycling showed regular curved features (Fig. 7a), indicating brittle fracture behaviour [22]. In contrast, the cross-section of a GPE-plasticised gelatine film (1wt% GPE, Fig. 7b) is rough with a wire-drawing phenomenon. Therefore, we believe that the addition of GPE converts the fracture mode of the gelatine film from brittle to ductile fracture. This indicates that the introduction of GPE plasticiser can avoid the brittle fracture of gelatine films during the long-term preservation of cultural gelatin paper photographs.
Conclusion
Based on the previous research about the disease causes of curling and fracture in the gelatin paper photograph relics, this work focusses on studying the GPE physical modified gelatin films, which is the preliminary experiment and demonstration of the practical application of real photographic objects. We propose a new strategy for increasing the stability of the gelatine film during humidity and temperature changes in the surrounding environment by modifying the gelatine film with GPE molecules, which provides synergistic crosslinking and plasticising functions. The following conclusions are drawn from the experimental findings.
First, the GPE can greatly minimise the shrinkage of gelatine films during environmental changes, thereby improving the dimensional stability of the film. Second, GPE greatly reduces the hygroscopicity and swelling behaviour of gelatine, thereby increasing its moisture stability. Finally, GPE increases the strength and flexibility of the gelatine film, which is favourable for the application of gelatine emulsions. In summary, GPE is a suitable plasticiser for modifying the physical and chemical properties of gelatine films. The initial findings imply that the addition of GPE to gelatine could be a useful method for controlling the brittle fracture and curling of historical photographs. This study provides theoretical support for the application of GPE in the actual restoration and protection of gelatin paper photograph relics, and the application research work is in progress.
Availability of data and materials
All data are available on request.
Abbreviations
- GPE:
-
Glycerol triglycidyl ether
- RH:
-
Relative humidity
- T:
-
Temperature
- FTIR:
-
Fourier transform infrared spectroscopy
- XRD:
-
X-ray diffraction
- SEM:
-
Scanning electron microscopy
References
Mosleh Y, de Zeeuw W, Nijemesland M, Bijleveld JC, van Duin P, Poulis JA. The structure-property correlations in dry gelatin adhesive films. Adv Eng Mater. 2020;23:2000716.
Abdelhedi O, Salem A, Nasri R, Nasri M, Jridi M. Food applications of bioactive marine gelatin films. Curr Opin Food Sci. 2022;43:206–15.
Kosel J, Ropret P. Overview of fungal isolates on heritage collections of photographic materials and their biological potency Science Direct. J Cult Herit. 2021;48:277–91.
Gimat A, Michelin A, Belhadj O, Pellizzi E, Massiani P, Rouchon V. Paper sizing with gelatine: from the macro-to the nano-scale. Cellulose. 2021;28:2419–32.
Alavijeh RZ, Shokrollahi P, Barzin J. A thermally and water activated shape memory gelatin physical hydrogel, with a gel point above the physiological temperature, for biomedical applications. J Mater Chem B. 2017;5:2302–14.
Yue L, Li J, Zhu WY, Xu J, Li TD. Fabrication, and characterization of polydimethylsiloxane/glycidol-grafted gelatin film. J Coat Technol Res. 2017;14:225–32.
Yakimets I, Wellner N, Smith AC, Wilson RH, Farhat I, Mitchell J. Mechanical properties with respect to water content of gelatin films in glassy state. Polymer. 2005;46:12577–85.
Wang XC, Zhu JB, Liu XH, Zhang HJ, Zhu X. Novel gelatin-based eco-friendly adhesive with a hyperbranched. Ind Eng Chem Res. 2020;59:5500–11.
Dan NH, Dan WH, Ding J. Hydrophilicity of acellular porcine dermal matrix cross-linked with glycerol glycidyl ether. Funct Mater. 2011;42:803–6 (In Chinese).
Ju HY, Wang KY, Su DQ. Study on the properties of collagen fiber scaffold modified by glycerol glycidyl ether. China Leather. 2008;37:24–8 (In Chinese).
Xu J, Li TD, Tang XL, Qiao CD, Jiang QW. Effect of aggregation behavior of gelatin in aqueous solution on the grafting density of gelatin modified with glycidol. Colloid Surface B. 2012;95:201–7.
Vargas G, Acevedo JL, Lopez J, Romero J. Study of cross-linking of gelatin by ethylene glycol diglycidyl ether. Mater Lett. 2008;62:3656–8.
Li TD, Tang XL, Yang XD, Guo H, Cui YZ, Xu J. Studies on the reaction of allyl glycidyl Ether with gelatin by van slyke method. Asian J Chem. 2013;25:858–60.
Cao N, Yang XM, Fu YH. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocolloid. 2009;23:729–35.
Suderman N, Sarbon NM. Optimization of chicken skin gelatin film production with different glycerol concentrations by response surface methodology (RSM) approach. J Food Sci Technol. 2020;57:463–72.
Murrieta-Martínez C, Soto-Valdez H, Pacheco-Aguilar R, Torres-Arreola W, Rodríguez-Felix F, Ramírez-Wong B, Santacruz-Ortega H, Santos-Sauceda I, Olibarría-Rodríguez G, Márquez-Ríos E. Effect of different polyalcohols as plasticizers on the functional properties of squid protein film (Dosidicus Gigas). Coatings. 2019;9:77.
Liu JJ, Li YH, Hu DD, Chao XL, Zhou YJ, Wang JL. An essential role of gelatin on the formation process of curling in long historical photos. Polymers. 2021;13:3894.
Manshor NM, Rezali MI, Jai J, Yahya A. Effect of plasticizers on physicochemical and mechanical properties of chitosan-gelatin films. IOP Conf Series Mater Sci Eng. 2018;358: 012040.
Pereda M, Ponce AG, Marcovich NE, Ruseckaite RA, Martucci JF. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocolloid. 2011;25:51372–81.
Awasthi SK, Bajpai SK, Utiye AS, Mishra B. Gelatin/poly(aniline) composite films: synthesis and characterization. J Macromol Sci A. 2016;53:301–10.
Badii F, Macnaughtan W, Mitchell JR, Farhat IA. The effect of drying temperature on physical properties of thin gelatin films. Dry Technol. 2013;32:30–8.
Shabbir A, Huang Q, Chen Q, Colby Ralph H, Alvarez NJ, Hassager O. Brittle fracture in associative polymers: the case of ionomer melts. Soft Matter. 2016;12:7606–12.
Acknowledgements
The authors would like to thank the conservators in the Second Historical Archives of China for their contributions.
Funding
This research is supported by the National Natural Science Foundation of China (22202129), and the Fundamental Research Funds for the Central Universities (G2022KY05101).
Author information
Authors and Affiliations
Contributions
JL and RC conceived the project. JL performed the experiments and wrote the original manuscript. JY, YL and DH assisted in sample testing and data analysis. RC provided support and guidance for this study, and revised the original manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Yang, J., Li, Y. et al. Effect of glycerol triglycidyl ether on the dimensional stability and flexibility of photographic gelatine films. Herit Sci 11, 49 (2023). https://doi.org/10.1186/s40494-023-00892-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-023-00892-w