Abstract
The Antonine Wall was commissioned by the Roman Emperor Antoninus Pius around 142 CE and stretches for c. 60 km across the central belt of Scotland, marking the Empire’s most north-western frontier. This vanguard research reports on the materials referred to by Antiquarian sources as having been applied during the sixteenth century for the redecoration of an iconic Distance Sculpture that was once embedded into the mural barrier. Portable non-invasive technologies, including pXRF and in-situ microphotography were deployed. These techniques were further supplemented by micro-sampling for SEM/EDS, FTIR–ATR and microscopy of embedded cross-sections. The validity of applying these complementary techniques has been confirmed. They provide a comprehensive account of the polychromy present, including pigments that could have been applied during the Roman period and others that were only available from the fifteenth or sixteenth Centuries. The work has confirmed stratigraphic sequencing of the pigments which will, in due course, permit the digital reconstruction of how this Classical relief sculpture would have been adorned during the Renaissance.
Similar content being viewed by others
Introducing an Antonine wall Roman distance sculpture (Hunterian Museum number: GLAHM.F1)
The Antonine Wall was commissioned by the Roman Emperor Antoninus Pius around 142 CE and stretches for c. 60 km across the central belt of Scotland. Constructed of turf, the Wall marked the Roman Empire’s most north-western frontier [1,2,3]. A total of 21 sandstone relief sculptures have been recovered from south of the mural barrier [4]. These are inscribed with abbreviated Latin text recording measured sections of the frontier constructed by three Legions assigned to the task (Legio II Augusta, Legio VI Victrix and Legio XX Valeria Victrix).
These Distance Sculptures are unique inscribed reliefs [5, 6] that were originally adorned in vibrant polychromy to reinforce decorative details and iconographic scenes [7]. One is thought to originate from east of Auchendavy fort [8] or the central sector of the Wall between Auchendavy and Twechar [9], hence its common nomenclature as the ‘Auchendavy’ sculpture (Fig. 1), but its provenance is unrecorded. This sculpture has a rich and diverse history [10], having been installed at various times into prominent positions at Dunnottar Castle, Aberdeenshire, by the Earls Marischal in the sixteenth Century. It remained visible there in 1642 [11,12,13,14,15,16] prior to being moved to the Marishal Museum in Aberdeen and its eventual donation by George Keith, the Tenth Earl Marischal [from 1712–78], to the Hunterian Museum in 1761 [9] where it was assigned museum number GLAHM.F1 [Roman Inscriptions of Britain [RIB] No. 2173] [17].
Carved from buff sandstone that was probably quarried from the vicinity of the Wall, the sculpture comprises a central inscription panel framed with triple ribbed border, swirling ivy tendrils above and below and flanked on either side by elongated peltae depicting plumage of open-beaked griffins mounted with central rosettes. Two crampholes dovetailed at the top confirm it was originally embedded into a frame [18], probably also constructed of stone.
The inscription reads:
IMP CAESARI T AELIO HADRIANO ANTONINO AVG PIO P P VEXILLATIO LEG XX VAL VIC F PER MIL P III
(for the Emperor Caesar Titus Aelius Hadrianus Antoninus Augustus Pius, Father of his Country, a detachment of the Twentieth Valerian and Victorious Legion built this over a distance of 3000 units of measure).
A well-known Antiquarian [11] and Ambassador to Denmark for Queen Anne [16], George, the Fifth Earl Marischal (from 1581–1623) travelled extensively during a Renaissance period that prompted the rediscovery of Classical philosophy, literature and art [19]. This doubtless exposed him to Classical architecture and art so he would have been acutely aware of this inscription’s cultural significance and motivated to erect it in a prestigious position at his ancestral stronghold on a majestic promontory off the north-east coast of Scotland.
Macdonald hypothesised the Distance Sculptures were likely to have originally been “brightly, if crudely, coloured… [though] no vestige of anything of the sort is visible on them now” [5]. Although he briefly refers to the gilding on inscribed letters not originating from this particular sculpture’s creation in the second century, he does not draw out the vibrant polychromy that once adorned this relief and does not refer to it in his second edition [8]. This is despite Camden’s [11] explicit mention of the sculpture being gilded under the direction of the Fifth Earl and Horsley [14] reporting the presence ‘now’ of black paint, suggesting a potential later episode of repainting some features by the early eighteenth century. Anderson [15] makes clear this painting was not the work of university staff upon its gifting to the University of Glasgow, while Gibb [20] confirms the paint was “very properly washed off” before traces were once more revealed during cleaning in 1976 [9].
These tantalising traces of polychromy permit a detailed exploration of at least one episode in the sculpture’s itinerary. To identify, for the first time, the pigments used in past conservation treatments as well as their sequence and chronology of application we have undertaken multi-technique analyses, including in-situ non-invasive technologies supplemented by micro-sampling.
Methods
Non-invasive technologies, including portable X-ray Fluorescence (pXRF) and in situ microphotography were deployed for detailed surface examination and to analyse elemental and mineral compositions of surface pigments on each sculpted feature to determine whether historical accounts referencing their sixteenth Century application are verifiable. These techniques are supplemented by micro-sampling to provide invaluable information on chemical composition of the pigments and binders using Scanning Electron Microscopy/Energy Dispersive X-ray Spectroscopy (SEM/EDS) and Fourier Transform Infrared spectroscopy with Attenuated Total Reflectance (FTIR–ATR) as well as, critically, microscopy of cross-sections to identify stratigraphic layers and determine whether later layers overlie and preserve original pigments applied in the Second Century by Roman artisans.
Portable X-Ray Flourescence (pXRF)
The pXRF instrument used was a Niton XL3t 900 SHE GOLDD Alloy Analyser, with a 50 kV Ag X-ray tube, 80 MHz real time digital signal processing and two processors for computation and data storage respectively; analyses were undertaken in the ‘mining’ calibration with resolution of c.165 eV at 35 keV which has been found most suitable for analysis of pigments. Analysis time was 80 s (with 30 and 30 s on the filters for light and low energy spectral lines respectively and 20 s on the filter for high energy spectral lines) and the area of analysis was 3 mm2. Several of the thirty-six elements that the instrument can in principle detect in this mode were present below the limit of detection (LoD), and light elements with fluorescent peaks at low energies were poorly resolved at low concentrations.
A total of twenty-seven analysis spots were captured and composition tables comprising the full datasets are contained in Additional files 1 and 2: Appendix SI grouped according to sculpted features and the elements related to each feature are discussed in-text. Elemental concentrations are expressed in parts per million (ppm). Some elements, including Al, K, Ti, Cr, Zn, Rb, Sr and Zr have been excluded from the broader discussion on analysis as naturally occurring in the sandstone as confirmed with six background spots located on the sides and rear of the sculpture where pigments were not expected to have been applied, though it is possible that some of these may be present as trace elements of pigments. Some surface patination was visible in areas resulting from post-depositional processes, including episodic cleaning, weathering or atmospheric pollution. The remaining 18 elements provided a level of quantification at various spots in concentrations sufficiently above background levels to confidently infer the presence of pigments.
Microsamples
Microsamples were collected from 12 areas by scraping with a scalpel and sealing them in labelled glass vials (Additional file 3: Appendix SII). They were studied under a Leica M80 microscope with incident LED light and images were captured using integrated digital camera as well as a Leica Wild M420 fitted with LM digital SLR adapter connected to a Canon EOS. These were supplemented by in-situ images captured on a Dinolite Edge Digital Microscope for comparison. Some samples were then embedded in Technovit® 2000 LC, a fast light-curing methacrylate based resin, and hardened by UV light in the Technotray CU light curing device (Heraeus Kulzer GmbH, Wehrheim, Germany) then ground with a Beuhler Metaserve grinder before hand-polishing with Micro-Mesh polishing cloths.
Fourier Transform Infrared spectroscopy with Attenuated Total Reflectance (FTIR–ATR)
FTIR–ATR was carried out on microsamples, these were separate to the samples used for SEM/EDS and were not embedded in resin. The FTIR–ATR used was a Perkin Elmer Spectrum One FTIR–ATR Spectrometer with Spectrum software version 5.0.1 and fitted with a Universal ATR Sampling Accessory. The ATR crystal used was a diamond/thallium-bromoiodide (C/KRS-5) with a penetration depth up to 2 µm (FTIR–ATR is primarily a surface technique). 16 accumulations were used at a resolution of 8 cm−1. Unless otherwise stated samples were placed on the ATR with the surface level facing downwards.
Scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS)
A small number of embedded samples were selected for exploratory SEM/EDS analysis and a more comprehensive programme of work will commence soon to build on these preliminary results. Most of the samples were coated in gold for maximum conductivity, two containing visible gold gilding were carbon-coated. The samples were then secured with copper tape to fix samples in place and assist electron conduction. Sample characterisation was performed at ISAAC, University of Glasgow, and backscatter electron images obtained with Carl Zeiss EVO scanning electron microscope (SEM) at high vacuum conditions with an accelerating voltage of 15 kV. Mineral identification was performed with Oxford Instruments Aztec integrated EBSD/EDS system.
Mapping the monument’s pigments
Initial pXRF analysis of this monumental inscription in 2013 [4] hinted at Sixteenth Century application of the visible pigments given their elemental composition, particularly the gilded frame and very high lead content on almost all painted features consistent with that period [21] as opposed to haematite or goethite [22] browns that were more common in the Roman artists’ palette. That said, lead-based pigments were also commonly used by Roman artists so, given the intriguing trajectory of this sculpture and the absence of any similar studies of polychromy on sandstone statuary, it served as a unique platform to test the applicability of a comprehensive suite of non-destructive analytical techniques further supported by targeted invasive analyses for comparison and to establish a chronological framework for the pigment application.
All the painted areas displayed a cracked, resinous, waxy and degraded surface with visible pigments surviving only in some areas, largely due to episodic cleaning over the centuries. Systematic survey of the sculptural features provided the undernoted results.
Ivy tendril framing the top and bottom of the inscribed panel
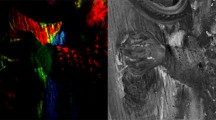
The carved ivy tendrils framing the top and bottom of the inscription panel retain visible light brown pigment (Fig. 2A) overlying a clearly visible light pink layer (Fig. 2B). Microphotography and samples under microscope and in cross-section (Fig. 2B, C) confirm this pattern, with a clear definition between a base of heterogeneous pinkish pigment interspersed with numerous white inclusions of various size and occasional red inclusions. This is overlaid with an orangey-red with occasional white inclusions, followed by a slightly darker red then brown top layer.
Deep, narrow, sculpted grooves made this feature challenging to target for pXRF and in situ microphotography, but elements detected on one analysis spot confirmed peaks of Fe and Cl at higher than background levels as well as traces of As and Pb. The high Cl may be explained by reported cleaning episodes or, perhaps, related to the use of a size [23] since EDS mapping detects this in the base layers of other features, discussed below. The Fe could indicate red ochre mixed with a lead-based pigment, white lead or lead sulphate, as confirmed with the white inclusions overlain with layers of red lead and possibly realgar, though lead and arsenic are linearly correlated with no evident addition of an As-rich material. Further analysis, including EDS mapping and others, will be carried out in a future programme of work to provide molecular information to clarify this. There is some debate surrounding the mixing of arsenic sulfide and lead-based pigments as the former can be unstable in alkaline conditions causing an adverse reaction with the copper and lead in lead-oxides [24]. But the presence of these mixtures applied with no evidence of negative impact on medieval wall paintings across Europe [25, 26], makes it perfectly plausible for arsenic and lead-based pigments to have been used together here, but in layers rather than mixing on the palette as confirmed in cross-section.
The SEM image (Fig. 2D) confirms this stratigraphy more clearly and FTIR–ATR spectrum indicates the presence of an organic material (Fig. 2E). From the shape and position of the bands it is most likely to be a resin. The bands at 2926 and 2853 cm−1 are associated with the aliphatic hydrocarbon chains but are less sharp than those longer chains found in oils and waxes. The carbonyl absorbance at around 1700 cm−1 further confirms this as this is associated with the carbonyl acid. The spectrum is shown beside a shellac standard spectrum to highlight the likelihood of the presence of a resin. It is not possible to determine if the resin is shellac or a tree resin from its spectra. To determine this gas chromatography mass spectrometry would be required.
From this spectrum (Fig. 2F) the presence of inorganic materials can also be deduced. The spectrum shows the likelihood of the presence of lead sulphate and quartz due to the strong wide absorbance at 1040 cm−1. The presence of a shoulder around 1060–1070 cm−1 and sharp shoulders either side of it at 1173 and 971 cm−1; bands at around 1400 and 1626 cm−1 and the sharp bands at 698 and 594 cm−1are indicative of lead sulphate.
The presence of lead sulphate as a ground layer is reported as rare and is usually thought to be due to degradation of other pigments and a comprehensive review has found it to be present in a number of works crossing the prehistoric to Medieval periods [27]. It was identified in a study of the Room of the Beds in the Royal Bath of Comares of the Alhambra monument in the Iberian peninsula redecorated during the Renaissance period and the intentional presence of lead sulphate cannot be fully disregarded [28]. From the analysis reported here, it is not possible to state that its presence was intentional or unintentional, nor whether it derives from a degraded lead white in a sulphate-rich gypsum substrate [27] originating from an original Roman layer. The absence of evidence for green or blue pigments on a feature depicting foliage is intriguing.
Griffin peltae
The zoomorphic shapes of griffins flanking the central inscription panel retain visible dark greyish/black pigment, predominantly in the grooves where past cleaning episodes failed to reach. Microsamples were taken from a groove in the plumage and eye pupil for analysis.
Griffin plumage
Some slight cracking is visible on the surface of the plumage sample (Fig. 3A, B and D), but less so than other pigmented areas and in cross-section (Fig. 3C) it appears very resinous with sporadic black and white inclusions overlying a distinct orange-red layer. The pXRF results reveal a low peak of Pb with traces of As which might be suggestive of a realgar or realgar mix base layer along with a resinous pigment mixed with some black and red lead could explain the thin red base layer, while the Cl peak could derive from soap during episodic cleaning. This could be clarified through SEM/EDS mapping. Trace levels of P were picked up by pXRF; however, phosphate was not detected with FTIR–ATR. This discounts the presence of Ivory or Bone black in the surface layer which provided a deep warmer black than other carbon-based black pigments [29, 30]. Taken together with the C evident on black inclusions in this layer of the griffin eye (below), then, this is most likely a carbon-based black.
The FTIR–ATR of this sample shows the presence of lead sulphate and a resinous material which explains the visibly resinous appearance of this sample, and a hint of proteinaceous material, probably a binder. As with the ivy tendril, above, the presence of an organic material has been detected. Given the position and shape of the prominent absorbance bands 2920, 2852 and 1710 cm−1 this is likely to be a resin (Fig. 3E).
Again, the spectrum (Fig. 3F) shows the likelihood of the presence of lead sulphate and quartz due to the strong wide absorbance at 1040 cm−1. The presence of a shoulder around 1060–1070 cm−1 and also sharp shoulders either side of it at 1173 and 971 cm−1; bands at around 1400 and 1626 cm−1 and the sharp bands at 698 and 594 cm−1are indicative of lead sulphate.
Griffin eye
Close inspection of the griffin eye (Fig. 4A) reveals white painted directly over the resinous layer with black inclusions that covers the griffin plumage. This defines eye whites (sclera) that were then topped with a surface layer of shiny black pigment forming a circular pupil in the centre. Visible traces of white pigment are also extant on the crest of the right griffin’s head plumage, suggesting all four griffin-heads were very likely crowned with white. This stratigraphy is confirmed in cross-section (Fig. 4B) of the microsample (Fig. 4C) and in situ (Fig. 4D) with crisp and very clearly defined layers of a thin orange-red base covered by the resinous layer then an additional thick band of white with a final black surface layer comprised of heterogeneous black angular inclusions. Ultra Violet (UV) light microscopy showed the white to be comprised of heterogeneous white and cream inclusions (no image included).
The pXRF results detect much higher levels of Pb than the groove sample, confirming the presence of a lead-based pigment to define the eye whites. Intriguingly, high levels of Sn and traces of Cu are also present at this feature indicating the possible presence of a copper and tin-based pigment, perhaps to create a shiny metallic surface for the pupil or eye white but this cannot be confirmed here. The absorbance bands 2920, 2852 and 1710 cm−1 are most likely to be associated with an organic resin as noted in the ivy tendril and griffin plumage. The spectrum is shown in comparison to shellac, although it should be made clear that from the FTIR–ATR it is not possible to determine the specific resin (Fig. 5G).
Inscription panel frame—grey/black exterior. A Detailed image; B LM image showing clear stratigraphic sequence of pigments in cross section; C Microsamples; D In situ microphotograph; E (i–ix) EDS Mapping (scale on images); F FTIR–ATR spectrum of organic resin; G FTIR–ATR spectra of quartz and lead sulphate
In this sample the presence of calcium carbonate is clearly seen in the large broad band at 1392 and the bands at 872 and 710 cm−1 (Fig. 5H).
High levels of Cl (Fig. 4viii) are also detected here with pXRF and confirmed by EDS mapping in the base layer where Na (Fig. 4ix) is detected in the same context which may indicate a size.
SEM imaging (Fig. 4E) confirms the heterogeneous character of inclusions in each layer. EDS mapping (Fig. 4i–ix) detects C in the top layer (Fig. 4ii), confirming a carbon-based black pigment. EDS further validates the presence of a lead pigment with a strong signal for Pb (Fig. 4iv) in both the white band immediately below the black as well as in the base layer interspersed by a calcium-rich layer (Fig. 4v) with carbon black inclusions similar to the griffin groove sample. This appears to confirm the presence of a lead pigment depicting the griffin’s eye white and a thin base layer of red lead, possibly mixed with realgar which would explain the pXRF detection of As. Sn (Fig. 4iii) and S (Fig. 4vii) are also present in the white layer, the latter may suggest a lead sulphate (PbSO4) or sulphate products from degradation. Sn is present uniquely in this white layer, distinguishing it chemically, and visibly, from the red lead base. A feasible deduction would be that some lead tin yellow may have been mixed in with this to achieve a desired colour for the eye white. The Cu detected by pXRF suggests a copper-based pigment mixed into one of the layers in this feature, most likely the surface or eye white. Ca is restricted to the layer immediately underlying the lead sulphate, identifying the location of the calcium carbonate sandwiched between the red lead possibly mixed with a realgar base layer and the white above.
Griffin rosette
No significant pXRF results were detected in the rosette centre. Conversely, the pXRF results for the petal of the central rosette contain only trace levels of Pb with high levels of Fe, S and Mg, indicating this decorative feature was depicted in a different pigment, possibly red ochre. That said, these elements could derive from soil particles and/or sulphation and the Pb traces may indicate an original lead pigment. No microsamples or in situ microphotography images were taken of this feature since no pigment traces were visible.
Inscription panel frame
The inscription panel is set within a triple-ribbed carved frame that retains visible traces of a mid-brown pigment overlain with gold gilding (Fig. 6) and flanked by informal borders in grey/black pigment around the exterior (Fig. 5) and very shiny black pigment in the interior (Fig. 6A, top left). Each of these painted features are dealt with in detail below.
Inscription panel gilded frame. A Detailed image; B LM image showing complex pigment and resinous layers in cross section; C In situ microphotograph; D UV Cross section; E Microsample; F SEM image; G (i–xi) EDS Mapping (scale on images); H FTIR–ATR spectrum of organic resin; I FTIR–ATR spectra of quartz and lead sulphate
Exterior frame
The inscription panel frame is bordered around the exterior by a grey/black pigment (Fig. 5A). In cross-section (Fig. 5B) this comprises three distinctive layers: a very bright orange-red sandwiched between a black surface and a whitish/pink base with large crystalline inclusions. Visual inspection of the cross-section, microsample (Fig. 5C) and in situ (Fig. 5D) appears to confirm the central orange-red underlying the black surface to be the same pigment as on the exterior of the griffins (Additional file 3: Appendix II, pXRF13), confirming a layer of red was painted across the entire area up to the carved frame then overlaid with a band of black on the exterior of the carved frame, perhaps to highlight the gilded area, and in some places this has extended onto the high carved area of the frame. Again, pXRF results confirm a Pb-based pigment with traces of As. One sample from this area (17.4) records high levels of P, Fe, Au which suggest this reading was taken from an area contaminated by an underlying Fe-rich bole with glue applied to the gilded frame (see below).
SEM imaging (Fig. 5E) shows the heterogeneous character of these layers and EDS mapping (Fig. 5Ei–ix) combined with targeted spot analyses confirm Pb (Fig. 5iii) dominates the base layer with frequent Ca (Fig. 5v) and Si (Fig. 5vii) inclusions, suggesting the mixing of lead sulphate and red lead. The presence of As and Pb detected by pXRF and the vibrancy of the red layer as well as EDS mapping of O (Fig. 5iv) strongly suggests the mixing of red lead and realgar also evidenced on other features.
The absorbance bands 2920, 2852 and 1710 cm−1 are most likely to be associated with an organic resin as noted in in the ivy tendril, griffin plumage and griffin eye are also seen here (Fig. 5F).
The spectrum (Fig. 5G) shows the likelihood of the presence of lead sulphate and quartz due to the strong wide absorbance at 1040 cm−1. The presence of a shoulder around 1060–1070 cm−1 and sharp shoulders either side of it at 1173 and 971 cm−1; bands at around 1400 and 1626 cm−1 and the sharp bands at 698 and 594 cm−1are indicative of lead sulphate. Si–O-Si bands at around 1060, 780 and 450 cm−1 and correspond to quartz.
Taken together, cross section and SEM images suggest the black surface pigment is likely carbon-based, similar to the griffin eye pupil. Traces of P in one sample could point to a bone/ivory black, but that analysis spot is anomalous with the other samples from this feature and may be a contaminant from the gilded frame which contains similar elements. The FTIR–ATR results do not detect P-containing compounds which renders the possibility of bone/ivory black unlikely. EDS spectra on samples from this surface layer detect Pb (Fig. 5iii) and traces of Na, Mg (Fig. 5ix), Cl (Fig. 5viii) and Al (Fig. 5vi) and Ca (Fig. 5v) in the base layer which, combined with the Pb here, suggest the mixing of red lead and/or lead sulphate and calcium carbonate as calcium was detected by the EDS.
Gilded frame
The carved frame is finished with a layer of gold gild (Fig. 6A) over mid-brown that is visible in microsample (Fig. 6E) and in situ (Fig. 6C). In cross-section (Fig. 6B) at least 9 stratigraphic layers of pigments are discernible, including a white base layer, then a yellowish layer with abundant crystalline inclusions that may constitute seepage or contamination from the ground white followed by an orange-red layer characteristically similar to that evident on other features then three layers of a resinous orange with occasional black inclusions interspersed by light resinous brownish layer then a final very thin surface of gold gild.
In common with the gilded letters, discussed below, pXRF results from this area show the broadest range of elements from any of the sculpted features, including Ba, As, Au, Pb, W, Fe, P and Cl. One spot contained additional traces of Bi, Cu, Ni, Co, Mn, Cl and Mg with lower than ground level of Si and Ca (the latter common to almost all pXRF results), which correlates to the readings for the inscription background, below, suggesting cross contamination from the panel pigment in at least some parts of the frame.
The organic bands and shape point to the presence of a resin. Again, from the FTIR–ATR (Fig. 6H) it is not possible to identify the specific resin. Despite the presence of gold on the sample there was no indication of proteinaceous glue however gas chromatography with mass spectrometry would perhaps have indicated its presence or otherwise.
As in previous samples, the presence of lead sulphate, which may be a degradation product of lead white, and quartz can be seen in the gilded frame sample (Fig. 6I).
Since W is detected at significantly high levels only where gilding visibly survives, this element is likely connected to the gold gilding. Bismuth has been identified under metal leaf in a study of the use of powdered bismuth in Late Gothic painting and sculpture polychromy [31]. This is a complex sample that perhaps indicates more graphically than all others the extensive expertise of the artist deploying these pigments and surface treatments. In cross section, it is evident that every layer was carefully and skilfully applied then left to dry before application of the next, this is most clear in the UV image (Fig. 6D) which shows no seepage between levels.
All layers are clearly discernible in the SEM image (Fig. 6F) which depicts many large inclusions in the base layer of glassy structure. EDS mapping (Fig. 6Gi–xi) confirms Ca (Fig. 6xi) mixed with Pb (Fig. 6iv) in the base layer, suggesting the presence of a lead sulphate which may be a degradation product of lead white likely mixed with calcium carbonate detected in FTIR–ATR. Some mixing of orpiment may explain the yellowish hue just above here, though the EDS mapping did not stretch to mapping As in this sample to corroborate the source of the As detected with pXRF. This yellow hue might derive from yellow ochre in a size layer underlying the bole [23]. A compilation of yellow ochre, linseed oil, varnish and minium (red lead) are recorded as a mordant for matte gilding during the seventeenth century [32] and complex recipes for gilding preparatory layers of orpiment with other arsenic sulphides are known from fifteenth–sixteenth century German sources, including orpiment, chalk vermillion and hematite [33] or orpiment, red lead and gum [34].
Occasional traces of Fe are present in the base layer and also in the orange-red layers above, most prominently on the penultimate layer, the latter could then be a bole, comprised predominantly of clay with naturally occurring iron oxides to which red pigments are added since metal leaf is generally not burnished over oil or resin-based layers which can tear delicate gilding due to their sticky texture [35]. In northern Europe during the thirteenth century a smoothed white ground of chalk or gypsum mixed with animal-skin glue was commonly overlain with a carefully prepared poliment (polisher) which was moistened with water before gilding [35]. Bole was used as poliment throughout Europe from the mid-thirteenth century, particularly from the fifteenth century, and the red colour resulted in a warm tone of the gold gilding applied above [36]. White mordant comprised of lead white mixed with other additions is also known from German-speaking countries during the fifteenth–eighteenth centuries, confirmed in a fifteenth century manuscript Cod. Pal. Germ. 558 [37], but this is currently only known to have been applied below white metal leaf [23]. This tradition of layering later became common during the seventeenth Century when Rembrandt and other artists built up multiple layers commencing with orange-red ochre in oil as a primer interspersed with thin resinous layers which effectively sealed in those below, preserving the vibrancy of their colour [38]. There is no evidence for glaze layers above gilded surfaces common to paintings from this period, but the concentration of Cl (Fig. 6vii) and Na (Fig. 6viii) in the base layer is strongly suggestive of a size [23]. Further research is necessary to validate this hypothesis.
Informal black border inside panel frame
The black informal border depicting the interior of the carved gilded frame (see Fig. 6A, top left) has been applied directly over the smalt covering the inscription panel. This pigment differs markedly from all others on the sculpture. The substance is highly shiny and the extraction of microsamples was challenging due to its sticky pitch-like character. As and Pb detected here by pXRF likely derive from the layers underlying the black (see inscription background, below) which is probably carbon based and therefore not detectable with pXRF. Natural wax is the only material detected by FTIR–ATR on this sample (Additional file 4: Appendix SIII with detailed band absorbance information).
Inscription background
Close inspection reveals a heavily cracked and degraded surface layer across the inscription panel (Fig. 7A) that has survived fragmentarily, perhaps accelerated through successive cleaning episodes [9]. The colour appears greyish-brown and the surface is less shiny than other surface features. This layer appears to have been applied to the entire internal panel in advance of the application of pigments and gilding on the inscribed letters, see below. Critically, a small splash of barely visible, but very vibrant, blue is evident in the bottom right section of the panel (Fig. 7D) which demands more detailed interrogation and a separate microsample of this blue was taken for FTIR–ATR analysis (Fig. 7E). Stratigraphically, this blue splash blends into the top-most layer of pigment here and microscopic in-situ inspection of various points across the panel using a DinoLite microscope (Fig. 7C) confirms a pigment with heterogeneous matrix dominated by brownish crystalline grains interspersed by small flecks of blue and red.
Inscription panel background. A Detailed image; B LM image showing clear stratigraphic sequence of pigments in cross section; C In situ microphotograph; D In situ microphotograph of blue splash; E Microsample; F SEM Image (scale on images); G FTIR–ATR spectra of smalt and beeswax; H FTIR–ATR spectra of quartz, lead sulphate and smalt
As with the other pigments present, pXRF confirms Pb is the dominant element with peaks of As, Cu, Ni and Co also detected along with a low reading of Ca, in common with other analysis spots, and traces of Bi. The presence of these elements combined with the tantalising traces of visible blue identify the presence of smalt [39]. Commonly used from the late fifteenth—early eighteenth Centuries, smalt is a vibrant blue derived from a potash silicate glass coloured with cobalt and often substituted for more expensive pigments [40]. Due to its siccative [41] and refractive properties which could result in the migration of cobalt ions and leaching of potassium from potash glass flux used in its manufacture the colour was unstable and could discolour to brownish grey [40,41,42,43,44]. This could explain the grey-brown colour of the inscription panel and cracked surface from shrinkage in a dry museum environment [39].
In cross-section (Fig. 7B) three distinct layers are defined, including a blueish/green surface pigment, a brownish central layer constructed of heterogenous inclusions and a pinkish base layer with large inclusions. The SEM image confirms this stratigraphy more clearly. These suggest a surface layer of smalt overlying a possible red lead and/or orpiment/realgar.
Two micro samples were taken from the panel background. A sample was taken from the visible blue area (Fig. 7E) and here the presence of smalt can clearly be seen due to the characteristic broad peak at 1002 cm −1 (Fig. 7G). In previous samples it was difficult to determine its presence due to interferences from quartz which also contains the Si–O–Si grouping. These species partly undergo condensation reactions creating more bridging Si–O–Si. As a result, in the FTIR–ATR spectra the Si–O–Si stretching appears to become more intense and to shift to higher wavenumber [39]. Beeswax is also detected in this sample due to the strong aliphatic absorbance between in the area 2956–2848; around 1710; 1642 and 720 cm−1.
Calcium carbonate was not detected, though this was a deliberately surface sample which did not encompass lower layers). Beeswax has been recorded in the seventeenth century mixed with smalt to create a high gloss surface to the panel to imitate the more expensive lapis lazuli [45]. The FTIR–ATR of the second sample (Fig. 7H) showed its composition to be very much like that of the ivy tendril and griffin plumage samples. Here it is difficult to determine the presence of smalt as lead sulphate and quartz are both present. The spectrum shows the likelihood of the presence of lead sulphate and quartz due to the strong wide absorbance at 1040 cm−1. The presence of a shoulder around 1060–1070 cm−1 and also sharp shoulders either side of it at 1173 and 971 cm−1; bands at around 1400 and 1626 cm−1 and the sharp bands at 698 and 594 cm−1 are indicative of lead sulphate.
Inscribed letters
The letters are painted with a reddish pigment with waxy appearance then overlain with gold gilding (Fig. 8A, D and E). As with all other painted areas, a high Pb reading is present in the pXRF results along with high Au, Fe, Mn, P and Cl with lower levels of Si in areas with visible gilding. Au is not detected in the analysis spots where gilding is not visible and Ba, Bi and P are lower than the gilded areas with Ca and Pb detected at lower levels than the background sandstone. In common with the panel background and frame, peaks of As, Cu, Ni and Co are detectable along with a low reading of Ca. Together, this suggests a layer of smalt was applied to the entire panel and frame (or at least some parts of the frame) including the inscribed letters, before the letters were overlaid with an iron-rich pigment, possibly ochre.
In this sample the presence of smalt and a natural beeswax are clearly seen. Beeswax detected in this sample due to the strong aliphatic absorbance between in the area 2956–2848; around 1710; 1642 and 720 cm−1. The presence of smalt can clearly be seen due to the characteristic broad peak at 1002 cm−1.
As with the gilded frame, a yellow-ish hue is definable in the base layer with abundant glassy inclusions in cross section (Fig. 8B and C). This is followed by a thick blue layer of smalt, a brown/red layer, an orangey-red layer with multiple inclusions of various size and colour, including dark red, golden yellow, orangey-red and black, then a gilded surface. SEM imaging (Fig. 8F) confirms this stratigraphy and EDS mapping (Fig. 8Gi–viii) clearly detects C (Fig. 8ii) throughout the sample, with As (Fig. 8iii) identified in all but the base layer, confirming the presence of orpiment or realgar. The yellowish layer immediately above the blue is most likely orpiment with realgar in the reddish layer and Fe (Fig. 8v) and Pb (Fig. 8iv) above confirming a penultimate bole layer consisting of iron oxides mixed with realgar and red lead immediately below the surface gilding. Pb is also dominant in the base layer along with Ca (Fig. 8vii) which suggests a preparatory layer of lead sulphate and calcium carbonate possibly mixed with some red lead given the EDS mapping of O here.
‘Pockmark’ indentations
The readings from circular gouges referred to by Keppie [9] as ‘pockmarks’ in the stone closely mirror those of the ground sandstone along with extremely lowered readings of calcium, chlorine and sulphur with elevated magnesium in one reading. This, combined with their obliterating of underlying features, including parts of the upper panel frame and letters, as well as the absence of any evidence for pigments, confirm this damage must have occurred after the final episode of painting, perhaps during a siege of Dunnottar by Cromwell in 1651–2 [10].
Results
The results are reported as found with little or no speculation. These have established the elemental composition of surface treatments defined by pXRF (Table 1), drawn out a palette of the pigments present (Table 2) and identified complexities in their mixtures, stratigraphic layering and application (Table 3) that have hitherto been unexplored for repainted Classical statuary [10], with the exception of a marble relief from Bursa, Turkey, repainted centuries later in the nineteenth century [46]. The palette comprises white, reds, black, blue, yellow and gold which, perhaps unsurprisingly, reflects contemporary tastes for colours applied to architectural features, statuary [47] and framed paintings [36, 48] combined with the antiquarian penchant for the collection and display of Classical sculpture [49, 50]. This has facilitated an authentic digital reconstruction of the sculpture from the Renaissance period in full polychromy for publication in a future article [10].
Conclusion
This vanguard research has successfully deployed a suite of analytical techniques to fingerprint surface treatments applied to a unique Classical relief sculpture repainted during the Scottish Renaissance. Given the innovative context of this work, comparative research is limited, but we have effectively stripped back multiple layers to ascertain, with confidence, the stratigraphic sequencing of pigment application and, critically, the timeframe for this episode in the sculpture’s trajectory. This validates accounts of antiquarian writers who attribute the visible polychromy to the 16th C under the direction of George Keith, the Fifth Earl Marischal.
The combination of mixing in pigments with siccative properties with other pigments to maximise the impact of each painted feature and allow for the rapid drying of each layer before the application of subsequent layers [38, 47] align with contemporary practice of a highly skilled artist who doubtless was commissioned to undertake the painting. A resin has been clearly detected in the letters, hints of this are also present in other samples. Further detailed analysis would be required to fully identify the type of resin present.
Yet, there remains much to be revealed about this unique monument that invites us to delve deeper and peel back additional layers, including the complexities and diversity evident in base layers of some features that may be associated with original pigments applied in Antiquity. Aside from the smalt and lead sulphate detected on various features and the lead tin yellow present in the griffin eye, the other pigments identified could equally date from the Roman era. Indeed, the lead sulphate detected in six out of nine samples could very well originate from a degraded lead white in a sulphate-rich gypsum substrate [27] originating from a Roman application. This and other aspects, including the potential presence of metal soaps, will be fully explored through a comprehensive programme of SEM–EDS of samples from all features and other analysis using cutting-edge materials science techniques.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LM:
-
Light microscopy
- FTIR–ATR:
-
Fourier transform infrared spectroscopy with attenuated total reflection
- pXRF:
-
Portable X-ray fluorescence
- SEM/EDS:
-
Scanning electron microscopy/ energy-dispersive X-ray spectroscopy
- UV:
-
Ultra-violet
References
Hanson WS, Maxwell GS. Rome’s north west frontier: the Antonine Wall. Edinburgh: Edinburgh University Press; 1983.
Breeze DJ. The Antonine Wall. Edinburgh: Birlinn; 2015.
Robertson AS. The Antonine wall. In: Keppie LJF, editor. A handbook to Scotland’s Roman frontier. 6th ed. Glasgow: Glasgow Archaeological Society; 2015.
Campbell L. Reading the writing on the wall: discovering new dimensions to the Antonine wall distance stones. J Anc Hist Archaeol. 2020. https://doi.org/10.14795/j.v7i2.513.
Macdonald G. The Roman Wall in Scotland. Glasgow: James Maclehose and Sons; 1911.
Ferris IM. Enemies of Rome: barbarians through Roman eyes. Stroud: Sutton Publishing; 2000.
Campbell L. Polychromy on the Antonine wall distance sculptures: non-destructive identification of pigments on Roman Reliefs. Britannia. 2020;51:175–201. https://doi.org/10.1017/S0068113X20000124.
Macdonald G. The Roman wall in Scotland. 2nd ed. Oxford: Clarendon Press; 1934.
Keppie LJF. Roman inscribed and sculptured stones in the Hunterian Museum, University of Glasgow. London: Society for the Promotion of Roman Studies; 1998.
Campbell L. In prep. Tracking Trajectories: Projecting Epigraphic Propaganda from Antoninus Pius to the Earls Marischal.
Camden W. Britannia, sive florentissimorum regnorum Angliae, Scotiae, Hiberniae, et insularum adiacentium. Chorographica descriptio: nunc postremo recognita, plurimis locis magna accessione, et chartis chorographicis illustrata. London; 1607.
Gibson E. Camden’s britannia: newly translated into english: with large additions and improvements. London: F. Collins; 1965.
Gordon A. Itinerarium Septentrionale. London: Abe books; 1726.
Horsley J. Britannia Romana or the Roman antiquities of Britain. London: John Osborn and Thomas Longman; 1732.
Anderson J. Of the Roman Wall between the Forth and Clyde and of some Discoveries which have lately been made upon it. Glasgow: Andersonian Library University of Strathclyde; 1771.
Macfarlane W. Geographical Collections (Scottish Historical Society volume 53), vol. 3. Edinburgh: National library of Scotland; 1908.
Collingwood RG, Wright RP. The Roman inscriptions of Britain, vol. 1. Oxford: Clarendon Press; 1965.
Keppie LJF. The distance slabs from the Antonine Wall: some problems. Scottish Archaeological Forum. 1976;7:57–66.
Chaney E. The evolution of the grand Tour: Anglo-Italian cultural relations since the renaissance. London and New York: Routledge; 1998.
Gibb A. New measurement of the Vallum of Antoninus Pius. North Notes Queries. 1902;63:17–26.
Fortunato G, Ritter A, Fabian D. Old Masters’ lead white pigments: investigations of paintings from the 16th–17th century using high precision lead isotope abundance ratio. Analyst. 2005;130:898–906.
Dooryhée E, Anne M, Bardies I, Hodeau JL, Martinetto P, Rondot S, Salomon J, Vaughan GBM, Walter P. Non-destructive synchrotron X-Ray diffraction mapping of a Roman painting. Appl Phys. 2005;81:663–7.
Richter M. Art technological source research and scientific examination: a comparative study on the technique of coloured glazes applied to metal leaf. In: Kühlenthal EE, Richter M, editors. Lüsterfassungen des Barock und Rokoko. Coloured Glazes on Metal Leaf from the Baroque and Rococo. München: Anton Siegl Fachbuchhandlung; 2013. p. 39–84.
Howard M. Pigments of english medieval wall painting. London: Archetype; 2003.
Fitzhugh EW. Red lead and minimum. In: Feller RL, editor. Artists pigments: a handbook of their history and characteristics, vol. 1. Cambridge: Cambridge University Press; 1986. p. 109–39.
Rodwell W. Westminster part i: The art, architecture and archaeology of the royal Abbey. London: Routledge; 2020.
Gliozzo E, Ionescu C. Pigments—lead-based whites, reds, yellows and oranges and their alteration phases. Archaeol Anthropol Sci. 2021;14(1):17.
Arjonilla P, Ayora-Canada MJ, Rubio Domene R, Correa Gomez E, de la Torre-Lopez M, Domingues-Vidal A. Romantic restorations in the Alhambra monument: spectroscopic characterization of decorative plasterwork in the Royal Baths of Comares. J Raman Spectrosc. 2019;50(2):184–92.
Winter J, Fitzhugh EW. Pigments based on carbon. In: Berrie BH, editor. Artists’ pigments: a handbook of their history and characteristics, vol. 4. New York: Oxford University Press; 2007. p. 1–37.
Apted MR. Painting in Scotland from the 14th to the 17th centuries, with particular reference to painted domestic decoration, 1550–1650. Unpublished Doctoral dissertation, University of Edinburgh; 1964.
Čechák T, Trojek T, Sefcu R, Chlumska S, Restikova A, Kotrly M, Turkova I. The use of powdered bismuth in Late Gothic painting and sculpture polychromy. J Cult Herit. 2015;16(5):747–52.
Félibien A. Des principes de l’architecture, de la sculpture, de la peinture, et des autres arts qui en dependent. Avec un dictionnaire des termes propres à chacun des ces arts. Paris; 1676 :281–91.
Bartl A, Krekel C, Lautenschlager M, Oltrogge D. Der, “Liber illuministarum” aus Kloster Tegernsee, Edition, Übersetzung and Kommentar der kunsttechnologischem Rezepte. Stuttgart: Franz Steiner; 2005.
Seidensticker P. Mittelniederdeutsche Mal- und Färberrezepte aus der Niedersächsischen Staats- und Universitätsbibliothek Göttingen. In: Kramer W, Scheuermann W, Stellmacher D, editors. Gedenkschrift für Heinrich Wesche. Neumünster: Karl Wachholtz; 1979. p. 287–304.
Nadolny J. All that’s burnished isn’t bole. Reflections on medieval water gilding, part 1: early medieval to 1300. In: Plahter U, Nadolny J, Kollandsrud K, editors. Medieval painting in Northern Europe: techniques, analysis, art history studies in commemoration of the 70th birthday of Unn Plahter. London: Archetype Publications; 2006. p. 148–62.
Powell C, Allen Z. Italian Renaissance frames at the V&A. London: Elsevier; 2010.
Oltrogge D. Datenbank mittelalterlicher und frühneuzeitlicher kunsttechnologischer Rezepte in handschriftlicher Überlieferung. Cologne: Fachhochschule Köln, Institut für Restaurierungs- und Konservierungswissenschaften; 2006.
Plesters J. Cross-sections and chemical analysis of paint samples. Stud Conserv. 1956;2(3):110–57. https://doi.org/10.2307/1505000.
Robinet L, Spring M, Pagés-Camagna S. Vibrational spectroscopy correlated with elemental analysis for the investigation of smalt pigment and its alteration in paintings. Anal Methods. 2013;5(18):4628–38.
Boon JJ, Keune K, van der Weerd J, Geldof M, van de Boer Asperen JRJ. Imaging microspectroscopic, secondary ion mass spectrometric and electron microscopic studies on discoloured and partially discoloured smalt in cross-sections of 16th century paintings. Chimia Int J Chem. 2001;55(11):952–60.
Spring M. New insights into the materials of fifteenth-and sixteenth-century Netherlandish paintings in the National Gallery. Herit Sci. 2017;5:40.
Easthaugh N, Walsh V, Chaplin T, Siddall R. Pigment compendium: a dictionary and optical microscopy of historical pigments. London and New York: Routledge; 2013.
Kugler V, Spring M, Hudson J. Quantitative SEM-EDX Analysis of Smalt Pigment Under Variable Pressure Conditions. Proc Microsc Microanal. 2013;19(Suppl 2):1428–9.
van Loon A, Noble P, de Man D, Alfeld M, Callewaert T, van der Snickt G, Janssens K, Dik J. The role of smalt in complex pigment mixtures in Rembrandt’s Homer 1663: combining MA-XRF imaging, microanalysis, paint reconstructions and OCT. Herit Sci. 2020;90:8. https://doi.org/10.1186/s40494-020-00429-5.
Kunckel J. Ars vitraria experimentalis oder vollkommene Glasmacher-Kunst. Frankfurt; 1679:366–67.
Abbe M, Verri G. The “Bursa Relief”: a Non-Invasive Analytical Investigation of an Exceptionally Painted Roman Marble Portrait. In: Liverani P, Pallecchi P, Paolucci F, Bracci S, Giachi G, editors. Polychromy in Ancient Sculpture and Architecture. Livorno: Sillabe; 2018. p. 167–82.
Stone I. The Thomas Sutton Memorial in Charterhouse Chapel. 2019 https://ianstone.london/2019/03/18/the-thomas-sutton-memorial-in-charterhouse-chapel// Accessed 20 April 2022.
Simon J. The Art of the Picture Frame: Artists, Patrons and the Framing of Portraits in Britain. London: National Portrait Gallery; 1996.
Scott J. The pleasures of antiquity. New Haven: Yale University Press; 2004.
Bristow IC. Interior house-painting colours and technology, 1615–1840. New Haven: Yale University Press; 1996.
Acknowledgements
Sincere thanks are due to colleagues at the University of Glasgow for their support and access to specialist equipment, including Professor Christina Young and Dr Mark Richter (Technical Art History), Dr Richard Jones (Archaeology) and to the Collections Management Team (Hunterian Museum) for permitting access to the sculpture. Thanks also to Dr Liene Spruzeniece of ISAAC for the SEM/EDS analysis service.
Funding
Grant funding was most gratefully received from Historic Environment Scotland (Grant Number HEAP2470491033), the University of Glasgow’s Lord Kelvin Adam Smith Leadership Fellowship, the Scottish Renaissance Society Museum and Galleries Research Award and the Archaeology Incentivisation Fund at the University of Glasgow.
Author information
Authors and Affiliations
Contributions
The concept for this research derived from LC. LC undertook exploratory pXRF and the historical/archaeological research relating to the sculpture and samples. LC extracted and embedded the samples for cross section analysis and for SEM/EDS analysis and undertook microscopy and microphotography of the samples. LC and MS jointly undertook the pXRF and FTIR–ATR data capture and analysis of the pXRF and SEM/EDS results. MS undertook FTIR–ATR analysis. All authors read and approved the final manuscript.
Authors’ information
Dr Louisa Campbell is Lord Kelvin Adam Smith Leadership Fellow in Archaeology at the University of Glasgow and leads on the Paints and Pigments in the Past (PPIP) research project.
Dr Margaret Smith is an Affiliate Researcher at the Kelvin Centre for Conservation and Cultural Heritage Research, University of Glasgow.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix SI.
pXRF Analysis Spots.
Additional file 2: Appendix SI.
pXRF results.
Additional file 3: Appendix SII.
Microsample Locations of GLAHM.F1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Campbell, L., Smith, M. Multi-technique analysis of pigments on sandstone sculptures: Renaissance re-painting of a Roman relief. Herit Sci 10, 156 (2022). https://doi.org/10.1186/s40494-022-00790-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-022-00790-7