Abstract
The Foyemiaowan-Xindiantai cemetery, with more than 10,000 tombs, is located in north-west Gansu Province. The cemetery was active during the Sixteen-State Period of the Western Jin Dynasty (265–439 CE). As the largest ancient tomb group in the Dunhuang region, its cultural core is still mainly based on the cultural tradition of the Central Plains. Metal objects were texturally and chemically analyzed by optical microscopy (OM), scanning electron microscopy coupled with energy-dispersive spectrometry (SEM–EDS), and Raman spectroscopy. The analysis of 36 copper-based objects showed that the main alloying elements were tin and lead, while three objects were brass, with copper and zinc as the main elements. These three pieces of brass are the earliest brass objects found in Gansu Province. The brass contains numerous sulfide inclusions, indicating that the raw materials used to make these objects contained a large amount of residual sphalerite instead of being “pure” calamine. They were then hot forged directly from the original smelted brass, without having been remelted. Brass may have reached China during the Wei Jin Southern and Northern Dynasties (220–589 CE), but it was not widely used in China before the Ming Dynasty (1368–1644 CE). According to the statistics and relevant studies of brass unearthed before the Ming Dynasty in China, although the distribution of brass objects spans a considerable period, it concentrates in three areas from the early to the late stage, showing phased development characteristics. This includes accidental smelting in the Central Plains and Haidai areas, followed by external introduction in the northwest, and the emergence of local production in northeast China. Most brass objects contain lead and tin, which requires further exploration.
Similar content being viewed by others
Introduction
Brass, the alloy of copper and zinc, has historically similar uses as bronze, the copper-tin alloy. However, its production technology and history differ significantly from bronze, due to the particular properties of zinc as the main alloying element. The smelting of metallic zinc requires special condensing and collecting devices to capture the highly volatile metal, even though it is relatively easily reduced from its ores. Therefore, its regular production as a metallic element occurred only in the very early 2nd millennium CE in India [1], and the mid-2nd millennium CE in China [2, 3] much later than other commonly used metals in ancient times, such as copper, tin, lead and iron. Before it was possible to smelt metallic zinc in order to alloy it with copper to make brass, there were other methods of making brass, such as smelting natural copper-zinc composite ore, or the smelting of artificially mixed zinc ore and copper ore. Of particular significance was the cementation processes, where zinc oxide is reduced to metallic zinc vapor in a closed vessel in the presence of metallic copper, which absorbs the zinc to form brass [4]. Previous research focused mainly on the production technology of early brass, based on data from ancient documents, simulation experiments, and the study of excavated brass objects and brass production remains.
Early brass making
The ores of copper and zinc often have different geological formation backgrounds, but can also occur closely combined and related, which is worthy of attention [5]. Some scholars who have discussed the smelting process of prehistoric brass argue that it could have been smelted from mixed oxidic copper-zinc ore in a solid-state reduction process to make the brass [6]. Such a natural copper-zinc alloy is thought to have been the material referred to as oreichalkos or ‘mountain copper’ in Iron Age Greek sources, and later as aurichalcum, or ‘golden copper’, described by classical authors of the very late 1st millennium BCE as no longer being made [7,8,9].
With regard to the early brass production in the Near East before 1000 BCE, it is suggested that brass was recognized and selected as a special alloy for pins and needles, among others. The analysis of excavated samples found at Tepe Yahya and Nuzi shows that brass with 14 to 19 wt% zinc was used to make artifacts already before the middle of the second millennium BCE, and that the use of these brasses was the result of the intentional choices of early metal craftsmen [5, 10, 11]. The conscious smelting of brass through cementation at a large scale is thought to have emerged around the first century BCE, to mint Roman brass coins in Asia Minor. Brass soon became popular in other fields in the region, especially in metal ornaments [8]. Evidence such as crucibles used to produce and work brass were found in several places across the Roman empire [12,13,14,15,16], mostly from the very first few centuries CE. After a gap of about half a millennium, brass-making by cementation re-emerged in Central Europe in the late first millennium CE [9, 17]]. During the Islamic period, brass is the dominant copper alloy across the Middle East [18], but so far, no archaeological production evidence for its production outside Europe is known.
In the process of cementation, copper metal and zinc ore are heated together with charcoal in a closed crucible to make brass. Depending on the process temperature, this can be a solid-state process or result in a pool of liquid brass at the bottom of the crucible, which is then cast into ingots for further processing. Zinc ores used mainly include smithsonite, calamine, potentially sphalerite, and artificial zinc oxide. Sphalerite is the most widely distributed mineral of zinc ore, but unsuitable for cementation. It is generally believed that the use of sphalerite for brass making occurs after roasting and desulfurization to produce zinc oxide, and then brass is smelted through the cementation process [8, 19].
Simulation experiments are also a way to explore brass smelting technology. Scholars have carried out simulation experiments on the cementation process [20, 21], the “dianhua method (点化法)” [22], the combined smelting and melting method [23,24,25], and the simulation experiments of calamine melting with pure copper [26]. Most zinc ores used in these experiments were calamine, smithsonite and complex copper-zinc ores, but not sphalerite. However, it is worth exploring whether sphalerite was used for smelting brass in ancient China.
Early brass in China
The earliest brass objects in China were unearthed at Jiangzhai near Xi’an, Shaanxi Province, and are thought to have been made from naturally-mixed copper and zinc ores, using a low-temperature smelting process with solid-state reduction which would have minimized zinc evaporation [27]. Early brass objects have also been unearthed at Beiliu (Shaanxi Province), Sanlihe (Shandong Province), and Zhoujiazhuang (Shanxi Province), which all show characteristics of brass made from mixed ore, typical of the early stage of brass smelting. From southwest Asia’s prehistoric brass and bronze studies, brass may have been taken as tin-bronze by local consumers ignorant of or ambivalent about the very different mechanical properties of these two alloys, and the linguistic separation of these two metals in the 1st millennium BCE.
Some scholars believe that in the second-third centuries CE, cementation brass smelting technology of the Roman Empire spread to Persia, and brass objects may have reached China during the Wei Kingdom and the Jin Dynasty to the Southern and Northern dynasties (220–589 CE) [28, 29]. Brass also spread to Northwest China through the exchange of objects [30, 31]. The sites with brass objects excavated on the Silk Road also include the Xiaohe Han Jin cemetery in Xinjiang (third century CE), Yingpandi in Xinjiang (fourth century CE), and the Tulan Tubo cemetery in Qinghai (ninth century CE). According to the documentary records, “toushi” (鍮石), thought to be brass, was first introduced into China from Persia through the Silk Road. After the Tang Dynasty, it mainly came from India, Kashmir, and other Asian countries. In the history of the Song Dynasty Records of Food and Goods, known as songshishihuozhi《宋史·食货志》, Brass is called “toushi”, and brassware is called “toushi qi”. In short, “toushi” reached China mainly through inland Asia. The specific time of its introduction is not quite clear, but the “toushi” crafts were favoured by the dignitaries in the Han and Tang dynasties [32].
More recent research suggests that it is the accidental combination of Hellenistic influence and the acceptance of Buddhism in Gandara in the Indus Valley in the early centuries of the common era that led to the traditional phenomenon of casting Buddhist statues in brass rather than bronze. This tradition, together with Buddhism itself, may then have crossed the western Himalayas into China, passing through oases and temples in the Taklimakan Desert, and then entered central China along the Hexi Corridor in the early to mid-1st millennium CE [33].
The conscious production of brass in China began no later than in the Five Dynasties (907–960 CE) and at the very latest in the Northern Song Dynasty (960–1127 CE). In the Jin Dynasty (1115–1234 CE), brass products made by local smelting appeared in China [34]. According to chujiashenpindan method《储家神品丹法》in the Five Dynasties (907–960 CE), which was recorded in the rihuazidiangenfa (末日华子点庚法) of the Song Dynasty, calamine was used for smelting brass. According to the waidanbencao《外丹本草》materia medica, the recipe for brass making consisted of “three kilos of copper, and one kilo of calamine, which after casting gives one and a half kilos of toushi”. Thus, brass is made of solid copper mixed with solid calamine and heated in a crucible to produce liquid brass. These records show that from the Five Dynasties to the Northern Song Dynasty (907–1127 CE), calamine and pure copper were used to consciously smelt brass [35]. Based on literature research and analysis of objects, the Chinese used metallic zinc to make brass coins from at least 1621 CE, consistent with the evidence for metallic zinc distillation in China at that time [2, 36].
Before the successful smelting of metallic zinc, there were two main processes of brass smelting in ancient China, namely, the “melting process” and the “solid reduction process”. “Melting process” refers to the process in which the product goes through a liquid state. “Solid-state reduction process” refers to the process that does not experience the melting state, that is, the product always maintains a solid-state during the formation process. Some scholars have carried out simulation experiments on brass obtained by cementation using melting and solid-state reduction processes, respectively, and then conducted micro-XRF synchrotron radiation analysis. The distribution of elements in the brass obtained by the two methods is quite different. Whether brass is obtained by the cementation process or co-smelting calamine and malachite, the distribution of zinc in brass samples prepared by the melting process is uniform, with the lead particles gathering on grain boundaries in a point distribution. In the simulation experiment, the distribution of zinc and lead in bulk brass obtained by the solid-state reduction method is uneven, and the zinc and lead content in different regions are also significantly different. An irregular zinc and lead distribution in the excavated brass sheet strongly suggests that this very early object was made by a solid-state reduction process [24].
Archaeological site
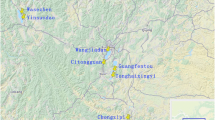
The Foyemioawan-Xindiantai tomb group has been excavated repeatedly since the 1940s. According to the currently known data, most of the tombs can be traced back to date from the Sixteen-State Period of the Western Jin Dynasty (265–439 CE) to the Tang Dynasty (618–907 CE), covering an area of approximately one million square meters (Fig. 1). From 2015 to 2016, Gansu Provincial Institute of Cultural Relics and Archaeology excavated 180 tombs in 8 sections within the protected area of the Foyemioawan-Xindiantai cemetery in Dunhuang, most of which were from the Wuliang Period of the Western Jin Dynasty (265–439 CE), while a few tombs were from the Cao-Wei (220–265 CE) and Sui (581–618 CE) and Tang (618–907 CE) dynasties. A large number of pottery, a small number of copper and iron objects, and a large number of tomb murals were unearthed in the cemetery. Compared with other ancient populations, the genetic diversity of the Foyemiaowan population is dominated by Tibetan and Han Chinese ancestry. However, the presence of Northeast and Southeast Asian as well as Western Asian populations is quite high, with the 2nd highest score for mitochondrial haplogroup diversity out of 30 studied cemeteries from the wider region, indicating a complex population influx consistent with the geographic position of the site in the Hexi Corridor [37].This excavation enriched the archaeological data of this period in the Hexi region, and is of great significance to further explore the history, culture, and funeral customs of the Hexi region. The earliest brass excavated from the Foyemiaowan-Xindiantai cemetery is from the Cao-Wei Period, which makes it the earliest brass object found in the Hexi Corridor area. This paper mainly explores the production technology of brass from the Foyemiaowan-Xindiantai cemetery in Dunhuang, and further discusses the origin and spread of brass in ancient China by combining research on brass before the Ming Dynasty (1368–1644 CE).
Materials and methods
Thirty-six copper-based objects were selected for analysis, including 18 pieces of personal adornment such as hairpins, belt hooks, or other decorations, 16 coins, and 1 each knife and figurine. Among the eight hairpins, three were identified as brass, which are the earliest brass objects in Gansu Province of China (Fig. 2), with finds numbers IM9:48, IIIM35:33, and IIIM50:21. Tomb IM9, rich in a variety of funeral objects, belonged to three people who were buried together, in which three ceramic vessels were unearthed with a description of the age, as “zhengshi seven years (246 CE)”, “zhengyuan two years (255 CE)”, “ganlu three years (258 CE)”. The interval between the deaths of the three tomb owners is 12 years, placing the tomb in the late Cao Wei period. The burial time of M35 can be traced back to the late Western Jin Dynasty to the early Qianliang Dynasty (265–363 CE), while the burial date of M50:21 can be dated back to the middle and late Western Jin Dynasty (265–316 CE) [38].
The corroded status of the finds made non-invasive surface analysis insufficient; instead, a single sample was removed from each selected object as a compromise between preserving the integrity of the cultural relics, and obtaining scientifically sound analytical data. An effort was made to include both near-surface and core material in the selected cut, to test for heterogeneity in the primary metal composition prior to corrosion. To observe the metallographic structure, the samples were etched with 3% hydrochloric acid and ferric chloride in ethanol, and then the microstructure of the samples was photographed with a Keyence VHX-6000 Superfield three-dimensional microscope.
After re-polishing, a Vega 3 XMU scanning electron microscope with a Bruker Nano 610 m X-ray spectrometer was used to observe the morphology and determine the composition of the samples. The accelerating voltage was 20 kV to optimize the excitation of transition metals, and the working distance was 15 mm. The counting time was set to 60 s, resulting in total count rates of around 3 kcps. To test for potential heterogeneity of the samples, ten areas from different parts of each sample were analyzed. The selected area size was a compromise between the availability of sound metal areas within the available samples, and the need to cover larger areas to reduce the effect of analytical bias from scattered inclusions, primarily lead metal. Considering the advanced state of corrosion of many of the samples, where possible we selected well-preserved metallic areas; however, most areas included variouthe Chinese used metallic zinc to make brass coins from at least s degrees of corrosion, from incipient grain boundary corrosion to more fully oxidized areas. In most samples, the main alloying elements, tin and zinc, were found to be present at sufficiently low concentrations to prevent the formation of intermetallic phases, and thus ensure sample homogeneity at the scale of the analyzed area.
The Bruker Nano 610 software was used to convert cps data for each element into weight percent, based on a factory-installed multi-element calibration and established ZAF correction factors. The quality of the data acquisition and quantification procedure was tested by analyzing a certified brass sample from the CHARM set under the same settings and procedure as the unknown samples [39, 40]. The data is reported in Table 1, showing a very good agreement between published values and those determined by our instrument for elements above c 1 wt% absolute concentrations. For lower concentrations, the relative error increases above 10%, with particularly high values for lead, bismuth and antimony. Overall, the CHARM test results indicate a high degree of accuracy for the data in this study for elements present at concentrations above c 1 wt%, and reasonable accuracy for lower concentrations.
In addition to SEM–EDS spot analyses, Raman spectroscopy was used to identify inclusions with a Scientific LabRAM XploRA PLUS (France HORIBA). Raman samples are directly tested with embedded and polished samples. After calibrating the instrument with a silicon wafer, the sample was placed under a 5 × objective lens for observation. After finding the point to be measured, the 532 nm laser was used for detection under a 50 × objective lens. The laser energy on the sample surface is about 0.5–2.5 mw, and the integration time used was 60 s.
Results
The majority of objects were made of leaded bronze (Table 2 and Fig. 3), two objects (one hairpin and one decoration) are made from lead, one belt hook is predominantly iron, and one coin almost pure copper. More than half of the samples were severely corroded, particularly the coins, where 13 of the 16 samples have more than 3 wt% combined oxygen and chlorine. In contrast, only 6 of the 17 other objects are corroded, including the two lead objects, the copper figurine, and the iron-rich belt hook. The less corroded objects have between 4.5 and 10.4 wt% tin (average 7.3 wt%) and from 1 to 17.1 wt% lead, with an average of 6.1 wt%. In contrast, the three uncorroded coins have much lower tin and lead contents, averaging just over 3 wt% for each. Even the corroded coins have only on average about 5 wt% Sn, an element that is typically enriched in corroded bronzes. Thus, the copper alloy used for the coins is significantly lower in tin than that used for the production of personal ornaments. The lead content seems to vary more strongly in the coins, although the corrosion status of most coins makes it difficult to assess their original lead content.
The projection of the metal compositions into the relevant ternary system (Fig. 3) shows that the melting temperatures of the original alloys would have been between 950 and 1050 °C, with two of the three coins plotting at even higher temperatures, due to their low tin content. The corrosion leads in several samples to an apparent enrichment of lead and/or tin, due to the selective removal of copper. This phenomenon is well-known from ancient copperwork [41], and highlights the need for careful selection and critical evaluation of analytical data from such objects. Further discussion of these objects is beyond the remit of this paper, which is focused on the early brass objects.
The brass objects
Two of the three brass hairpins are well preserved in their cross section (Fig. 4a, b), enabling a comprehensive metallographic and compositional analysis. In contrast, the third (DFX09) is severely corroded, with only small areas of sound metal preserved (Fig. 4c). The results of the inspection of the microstructure of the three brass objects are summarized in Table 3. DFX13 has α equiaxed crystals and twin crystals (Fig. 5a), with relatively small grains and elongated inclusions, which is the result of hot forging. Before etching, DFX16 shows isometric crystal boundaries and some twin preferential corrosion (Fig. 5b), and there are a small number of local slip lines. After etching, in DFX16 there are α equiaxed crystals and twin crystals visible, and the grain size is uneven, with an obvious stratification. The middle region sees larger twins, close to a compact surface structure with smaller grains (Fig. 5c). Overall, we assess that also the hairpin DFX16 is made by hot forging (Fig. 5d). From the back scatter images and the multiple area analyses at different parts of the cross sections of the two non-corroded samples by SEM–EDS, it can be seen that lead and zinc are evenly distributed. This indicates that the brass was liquid and homogenized during the formation process (Fig. 4 and Table 3). According to the metallographic analysis, DFX09 is severely corroded; however, the remaining metallographic features indicate that it also was hot forged (Table 4).
Metallographic photograph of brass, etched with 3% hydrochloric acid and ferric chloride in ethanol. a Metallographic photograph of DFX13 (hot forging structure); b Metallographic photo of DFX16 (not etched); the equiaxed crystal boundaries are preferentially corroded; c Metallographic photograph of DFX16; d Metallographic photograph of DFX16
There are a large number of Cu2S and ZnS inclusions in the three samples (Fig. 6). Raman spectra show that some inclusions are sphalerite and FeO(OH)·nH2O, the latter probably corroded iron metal (Fig. 7). ZnS generally coexists with lead particles, while Cu2S generally exists alone. In addition, there was an iron and arsenic enriched phase among the inclusions, and no PbS inclusion was found. Details of the inclusion compositions are shown in (Additional file 1: Table S1).
The composition of the three brass objects is shown in Table 3, reporting the average values of multiple small area analyses. For the two well-preserved samples, 10 individual analyses were done, while for DFX09 only three sound metal areas were found. The average zinc contents of DFX13 and DFX16 are 15.0 wt% and 22.3 wt%, respectively, based on ten individual analyses. Confirming the microscopic observation, the data from the individual analyses is closely clustered, confirming the compositional homogeneity across the entire section with no surface enrichment or gradient towards the core area. Tin and lead were present in the low percent range, while sulfur was detected at even lower levels, with some of the individual area analyses in DFX13 and DFX16 containing up to 0.2 wt% and 0.4 wt%, respectively. DFX16 contains 1.5 wt% iron. Based on the three analyses of less-corroded regions, DFX09 has the same zinc content as DFX13, but lower tin and no lead detectable by SEM–EDS. In the fully corroded region, the zinc content is only about one third of the original content, reflecting the common phenomenon of dezincification of brass during corrosion.
Apart from the very high zinc content, the low tin and lead contents differentiate the brass objects from the other decorative items from the cemetery, but resemble the concentrations found in the uncorroded coins.
Discussion
The brass products unearthed from Foyemiaowan-Xindiantai cemetery
The three brass objects stand out from the other 33 bronzes, which do not contain any zinc above the detection limit of the instrument used, estimated to be around 0.1 to 0.2 wt%. Conversely, the tin and lead contents of the brass objects are much lower than in the bronze decorative objects, even though they are still detectable by SEM–EDS. Thus, the two groups of decorative copper-based objects fall into two separate compositional groups that do not seem related to each other. Overall, the brass objects account for a relatively small proportion of the detected metal objects, indicating that at the time of their burial in the late 3rd to mid-fourth century CE, brass has not been used on a large scale. The three brass hairpins unearthed in the cemetery came from different tombs, but are compositionally similar enough in principle to consider them together. One of the key properties of ancient metals for both consumers and craftsmen is their color, and the associated value perception. The most well-known example of this is gold, easily recognized by its clarity and strength of color. Of the common copper alloys, high-tin bronzes and brasses with more than about 20 wt% Zn are closest in appearance to gold. Thus, of the copper alloy objects analysed here, the three brass hairpins would have had the noble color of gold, while the bronze objects would have been easily distinguished by their dull and more brown appearance [42]. This indicates that the three brass hairpins were deliberately made from this alloy and recognized as a different, probably more valued alloy—either for their semblance of gold, or just as an unusual and exotic alloy.
The zinc contents of DFX13 and DFX16 are 15 wt% and 22.3 wt%, respectively; even the partly corroded object DFX09 still has 14.8 wt% Zn, falling to 5.8 wt% Zn in the fully corroded part. These levels of zinc are typical for objects made from intentionally made cementation brass; however, some very early objects, most likely smelted from naturally mixed ores, can also reach up to 20 wt% Zn in the alloy [10, 11] Judging from their microstructures, all three objects were hot forged. The working of the metal is also evident from the deformation and broad alignment of some of the inclusions, as a result of the ‘flow’ of the metal under the hammer. Some inclusions contain approximately 30% sulfur and 60% zinc, with the balance to 100 wt% being mostly copper (see OSM Table). Pure zinc sulfide has 67.1 wt% Zn and 32.9 wt% S, confirming that the inclusions here are close to the stoichiometric composition of ZnS. with some substitution of copper for zinc. The presence of zinc sulfide inclusions also indicates that the three pieces are newly manufactured brass, because if the alloy is re-melted, the sulfur content will be increasingly oxidized and finally disappear [43]. Therefore, the three brass objects analyzed were hot forged directly from freshly smelted brass, without evidence for repeated remelting. In addition to the zinc sulfide inclusions, there are iron arsenic-rich inclusions and copper sulfide inclusions up to 20 µm in diameter. Since both iron and arsenic are known to accompany sphalerite ore, their occurrence in the brass is not a surprise. In fact, arsenic is similarly volatile as zinc, and would have concentrated in the brass during the cementation process. The iron could also have come from the copper, and combined with arsenic during the solidification of the brass.
Overall, the chemical and textural observations on the three brass objects studied here are consistent with observations on early brass from West and Central Asia, where binary brass is mostly hammered, while cast brass has mostly added tin and lead [44].
Raw materials and smelting technology
The brass objects contain a large amount of sulfide inclusions. Does it imply that sphalerite was used as the raw material for brass? In theory, there are 2 ways to obtain brass from sphalerite. One is to first roast it into oxide and then use this to obtain brass. The other is that copper replaces zinc in the sulfide, and the zinc goes into copper to form brass. The mixed smelting activity of malachite and zinc sulfide ore has been simulated experimentally. When the two are mixed, ZnS and CuS can be obtained, and CuS will be oxidized into Cu2S so that a large number of Cu2S inclusions are distributed in the products obtained (CuO + ZnS → CuS + ZnO; CuS + Cu → Cu2S). However, the content of zinc in the metal alloy obtained from malachite and sphalerite is very low, indicating that brass objects cannot be obtained by this method [45]. In fact, no one seriously suggests that sphalerite was once used as the main ore in the ancient manufacture of brass. Instead, it is likely that the ore used to make brass contained a large amount of residual sphalerite, rather than being “pure” calamine. Therefore, although it is theoretically correct to assume that the result of calamine used for cementation is sulfur-free brass, the actual ore formation often produces mixed ore, which cannot be separated mechanically before the ancient process is used.
Alternatively, the sulfur content could have come from the raw copper used for the brass cementation. In fact, the sulfur content of most of the analyzed bronze objects from the cemetery is higher than the sulfur content of the brass samples (Tables 2, 3). During the melting stage, sulfur would dissolve in the liquid metal; during solidification, it would concentrate in the residual liquid until it exceeds the solubility threshold and precipitates as discrete particles. According to the relevant Ellingham-type diagram for heavy metals and sulfur, copper, zinc and iron have similar affinity for sulfur, while lead is less likely to bond with it [46,47,48]. This is consistent with the prevalence of copper and zinc sulfides, and the absence of lead sulfides reported above. This passing of the sulfur through the liquid state in the alloy makes it impossible to determine the source of it from either of the two raw materials; in fact, both could have contributed to the final sulfur content. However, it is possible to posit, due to the easy oxidation of sulfur during remelting, that the existence of these sulfide inclusions indicates that these objects are very close to primary products and did not undergo repeated recycling operations.
As discussed above and based on extensive research on European and western Asian brass, brass can be obtained by three different technologies: co-smelting of (naturally) mixed ores, cementation of copper with zinc oxide, either natural calamine or artificial tutty, and alloying of metallic copper and zinc. In the West, the three processes follow a broad chronological sequence, with natural alloys dominating in prehistoric brasses, cementation brass appearing from around the BCE/CE divide, and alloying emerging only after the invention of zinc distillation in the early to mid-2nd millennium CE.
The chemical composition of the resulting brass is a further indicator of the process used. Natural brass is generally assumed to be relatively low in zinc, rarely exceeding 15 wt%. It also may contain a number of other elements, such as arsenic, antimony, nickel and lead, depending on the composition of the naturally complex ore. In contrast, cementation brass is often thought to contain higher zinc concentrations, reaching up to about 30 wt%. The exact upper limit has been hotly disputed and explored through a series of experimental studies, but few if any archaeological brass objects of such high zinc content are known to have been made by cementation. Finally, alloying metallic zinc and copper enables the production of a wide range of compositions, including those with more than 30 wt% Zn. These general rules are further complicated by the fact that when brass is melted for casting, zinc is sequentially lost from the alloy due to the volatility of zinc at the high temperature, and the easy oxidation of zinc [21, 49]. Thus, the zinc content in brass decreases a little with each re-melting, particularly when not covered with charcoal or a protective slag.
Considering the relatively high levels of zinc in the three brass objects and their date in the early first millennium CE, it is likely that they were made by cementation. The presence of numerous sulfide inclusions indicates that they were close to their original composition, with little if any re-melting history. The cross section microscopy and even composition across the entire section indicates that the pins were not made by direct cementation in the solid state, where one would expect a gradient with higher zinc content near the surface and lower zinc towards the core. Instead, we assume that the fresh alloy was fully molten and cast into shape before the pins were finished by hot forging. This assessment, of course, assumes that the broad general observations regarding the different brass-making technologies as sketched above hold true also for Chinese material; at this stage of research in Chinese brass making, this is difficult to ascertain with confidence.
It is uncertain whether the brass products in Foyemiaowan-Xindiantai are locally smelted or imported from the West. It is worth noting that the main cultural core of Foyemiaowan-Xindiantai is still dominated by the cultural tradition of the Central Plains. However, a unified norm had not been formed in the combination and evolution of the burial system and funerary objects. The burial pattern inherited the local tradition, and there was no element from Western Regions that could be found among the excavated relics. If these three brass hairpins were indeed introduced from the West, they should have been accompanied by the trade and exchanges of other ‘Western’ artefacts. However, we cannot find western regions’ cultural indicators among the many funerary objects. Therefore, the possibility of local smelting cannot be ruled out.
Brass objects unearthed in China before the ming dynasty (before the fourteenth century CE)
According to current knowledge, fewer than 50 brass objects dating to before the Ming Dynasty have so far been excavated in China, excluding bronze Buddha statues; their geographical distribution is shown in Fig. 8. Compared with bronze, copper and zinc alloys had never become the dominant alloying element until the Ming Dynasty, when brass was used at a large scale in coins.
Distribution of brass objects dated before the Ming Dynasty. 1 Jiangzhai site, Shaanxi (4700–4000 BCE). 2 Beiliu site, Shaanxi (3900–3000 BCE). 3 Sanlihe site, Shandong (2300–1800 BCE). 4 Zhoujiazhuang site, Shanxi (2500–2100 BCE). 5 Shandong Changdao (1046–771 BCE). 6 Shirenzigou site, Xinjiang (400–200 BCE). 7 Xinjiang Han Jin Xiaohe site (third century CE). 8 Dunhuang Foyemiaowan Xindiantai tombs, Gansu (third century CE). 9 Yingpan cemetery, Xinjiang (fourth century CE). 10 Qilangshan cemetery, Chayouzhong banner, Inner Mongolia (fourth century CE). 11 Zhouyuan site and sites around its tombs, Shaanxi (seventh century CE). 12 Tibetan tombs in Dulan, Qinghai (ninth century CE). 13 Liao Zuling, Liaoning (tenth century CE). 14 Taizicheng Ancient City Site, Hebei (12th-13th Century CE). 15 Yuan Shangdu, Inner Mongolia (Neimongol zizhiqu) (thirteenth century CE)
There are 6 prehistoric brass objects known from the Central Plains and Haidai region. From the Han Dynasty to the Western Jin Dynasty, the number increased to 10, mainly distributed in northwest China. After the arrival of the Liao Dynasty, brass products gradually entered the stage of large-scale use, mainly distributed in the northeast (see Fig. 8, sites numbers 10–15). Although the number of analyzed brass objects from before the Ming Dynasty is small considering the long time-period covered, it showed a continuous and concentrated area of three periods. Prehistoric brass objects from the Yangshao and Longshan periods were mainly discovered in the Central Plains and Haidai areas. The brass in this period was mostly considered to be obtained by accident through smelting naturally mixed ore. From the historical period to the Liao Dynasty, brass was mainly found in Northwest China (Gansu, Xinjiang, and Qinghai). Brass in this period is mostly believed to have spread from the west to China through the Silk Road. From the Liao Dynasty to the Ming Dynasty, brass objects are mainly found in Northeast China, and locally made brass products appeared, such as Tai zicheng Ancient City Site brass in the Liao Dynasty. Most of these objects were worked while hot, judging from metallographic studies. In terms of the types of objects, tools include hairpins and awls. Decorations include rings, earrings, bracelets, etc., while weapons only include arrowheads.
The composition of brass objects from the Yangshao and Longshan periods include a nickel-brass with a nickel content of 3.8 wt% and zinc content of 14.0 wt% at the Zhoujiazhuang site in Jiang County, and two objects with an even higher zinc content of 20.2 wt% and 32 wt%, respectively. One of the characteristics of these very early brass products is that there are more impurities in them. These early brass objects, including the brass pieces and brass tubes in Jiangzhai and those unearthed at the Sanlihe site, Beiliu site and at the Zhoujiazhuang site, all show the characteristics of brass smelted from naturally mixed ores, and represent the earliest stage of brass smelting.
After the fourth century CE, the zinc content of brass objects excavated in China was between 10 and 33 wt%, with an average of 19 wt% Zn. The tested samples also contained impurities such as tin, lead, and iron, which could have come from the copper metal used for cementation brass making. The introduction of lead in brass could in part also be due to the symbiotic occurrence of lead and zinc in most ores, leading to brass often containing lead. From the ternary phase diagram of brass in the early periods (Fig. 9), it can be seen that tin only contributes a small amount to the brass composition; only in a few pieces, tin is detected in levels above a few wt%. The metallogenic background of tin and zinc is quite different, and their respective ores will not normally occur with each other. Therefore, there are two main possibilities for the introduction of tin, namely adding tin intentionally to the brass alloy, or introducing zinc by cementation or alloying into a pre-existing copper-tin alloy.
The distillation of metallic zinc was introduced in China during the Ming Dynasty, and enabled the production of brass by alloying the two metallic elements, zinc and copper. However, cementation may well have continued, and it is not always easy to distinguish between brass made by cementation and by alloying metallic zinc and copper, particularly for objects with c 20 to 35 wt% Zn. Cowell and co-workers concluded from a large number of analyses that the content of the trace element cadmium in copper coins can be an indication for the process used. According to their work, cadmium in coins issued before the first year of the Tianqi era (1621 to 1627 CE) was extremely low, and often difficult to detect. However, the content of cadmium in copper coins increased significantly after the first year of Tianqi [50]. Similarly, Zhou Weirong's experimental analysis found that from Jiaqing to Wanli, the content of cadmium in copper coins was very low (Cd < 0.0001 wt%), while that in copper coins after the Tianqi era (1621 to 1627 CE) was relatively high (Cd 0.001–0.0038%). Since zinc and cadmium are congeneric elements, zinc ores are often accompanied by a small amount of paragenetic cadmium ores. In cementation brass making using calamine ore, cadmium is easy to volatilize and difficult to retain in the alloy due to its low boiling point. However, in brass made of elemental zinc obtained by distillation, there is a condensation process and cadmium is easily retained in the zinc metal, and then enters the brass alloy. According to the literature, in 1621 CE, China began to use brass made with metallic zinc to cast coins. The laboratory data cited above is consistent with the literature. Therefore, cadmium in brass can be used to distinguish brass obtained from ores by cementation and brass made with metallic zinc [36]. However, zinc ore with little or no cadmium will produce zinc metal with the same characteristic, making it then difficult to distinguish the brass made from this metallic zinc from cementation brass. Vice versa, calamine ore rich in cadmium may result in elevated Cd levels in cementation brass, rendering the cadmium level of brass only an indicative element for the production process.
The Ming Dynasty brass coins obtained from ore smelting and metallic zinc, respectively, as identified by their cadmium content, are shown separately in the Zn-Sn scatter diagram, together with the brasses before the Ming Dynasty (Fig. 10) [36]. The zinc content of brass coins made with metallic zinc is on average higher than those made by cementation, and even the lowest is higher than 24 wt%. They still contain a certain amount of tin, but mostly less than 2 wt%, which means that most of the tin may have been introduced as an impurity in the added copper. The cementation brass coins fall into 2 groups according to their tin content. One is the kind that has low or no tin content, typically less than 1.5 wt% Sn; this group has rather high zinc content, of 25 to 35 wt%, similar to the brass coins made with metallic zinc. In the other group, the content of tin in these coins was relatively high, often more than 4 wt%, and reaching as high as 8 wt% Sn; their zinc content is around 10 to 20 wt%, in line with typical cementation brass compositions. Since the alloy production for coins was strictly controlled, it is likely that the tin in these brass coins might have been intentionally added.
It is remarkable that there is more or less tin in brass from all periods. It can be assumed that in addition to pure copper, a copper-tin alloy or even intentionally added tin may have been used in brass smelting. The solubility of tin in copper decreases with the increasing of zinc content, and even a small amount of tin will improve its strength and hardness. However, when tin is added in excess of the reduced solubility, a brittle tin-rich intermetallic compound will form. The tin content of modern cast brass is therefore kept low, typically well below 1 wt%; otherwise, the machinability of the alloy will decline. In the early Chinese brasses, the content of tin is generally less than about 2 wt% (Fig. 10), as can be expected from the use of either fresh copper metal containing hardly any tin, or from recycled low-tin bronze or ‘dirty’ copper with just a very few wt% tin. Remarkable is the assemblage of Ming Dynasty brass coins with around 4 to 9 wt% tin and relatively low zinc contents of 10 to 20 wt% Zn (Fig. 10, central part), suggesting the use of tin bronze for cementation brass making, or the addition of tin to the alloy, at a time when most brass coins were made using metallic copper and zinc. These have rarely more than 1 or 2 wt% Sn, and range from 25 to more than 35 wt% Zn. The relationship between tin and brass is worthy of further exploration in the future, particularly for those early brass objects with tin in the low weight percent range, from 1 to c 5 wt%.
Conclusions
The three brass objects from Foyemiaowan are the earliest brass products so far found in the Hexi Corridor. Zinc was not found in the other copper alloys in this cemetery, and brass accounted for a relatively small proportion of the detected metal objects, indicating that brass has not been used on a large scale. Both their date in the early first millennium CE and their composition with intermediate to high zinc contents indicate that they were made by cementation. The three brass objects analyzed are from different tombs, but they are all hairpins. Brass has the noble golden color of gold and has a good decorative effect. The above indicates that the brass in the cemetery was selectively used to make hairpins whose golden color would have contrasted well with the color of the hair.
All three objects contain a relatively high sulfur content, present as copper and zinc sulfide inclusions. It is possible that the ore used to make these brass objects contained a large amount of residual sphalerite, rather than being “pure” calamine. Although it is theoretically correct to assume that the result of using calamine for cementation is sulfur-free brass, the actual ore formation often produces mixed ore containing sphalerite, which cannot be removed mechanically before the ore is used in cementation. In addition, or alternatively, the sulfur could have entered the brass together with freshly smelted copper, and we note that the bronze objects and coins from the same cemetery have similar or even higher sulfur contents than the brass objects. In any case, it is considered that the existence of these sulfide inclusions indicates that these objects are very close to primary products and did not underwent many re-melting and recycling steps.
Although brass use before the Ming Dynasty spanned a long period of time, it clearly shows three concentrated periods from the early to a later stage. These show the characteristics of the development stages, which mainly refer to accidental smelting of naturally mixed ores in the Central Plains and Haidai areas during prehistory, followed by external introduction of cementation brass in the northwest, beginning from about the Warring States Period, and local production in the northeast, possibly from the late Tang or Northern Song Dynasty onward. The accidental smelting of complex ores to natural alloys is a world-wide phenomenon in the early stages of metallurgy [51]. The establishment of long-distance communication routes, such as the Silk Road network or the spread of religious practices facilitated the inter-continental movement of numerous exotic objects, including brass [33]. This route is characteristically seen in the concentration of such objects in Xinjiang Province and the Hexi Corridor in NW China (see Fig. 8), and the presence of these potentially imported objects in a cemetery with a relatively high proportion of ancestral genetic diversity is noted [37]. The local production of brass is to be expected wherever suitable ores are available, and the potential emergence of this technology in NE China at about the same time as its appearance in Central Europe and the Islamic world is intriguing, and calls for further research. Only the identification of production waste such as cementation crucibles in the archaeological record, or clear historical documents and accounts can offer conclusive evidence for local brass making. In the absence of these, brass objects offer a relatively direct approach to studying the manufacturing process of brass, especially if they did not undergo re-melting and recycling. In particular, their overall zinc content, levels of cadmium as a trace element, and of tin as a contamination from the copper or as an intentionally added alloying element carry important information. Finally, the presence of sulfide and other inclusions can also provide insight into the production process and working history of the brass, as part of the metallographic study of such objects. The lead content can vary widely, and is difficult to interpret with confidence. However, it offers the possibility to provenance the origin of the ore used through lead isotope analyses, which is of importance for decorative objects such as personal ornaments, which can travel widely. However, the mixing of LIA signatures from the copper metal and the zinc ore during cementation can confuse the picture.
Availability of data and materials
The data and materials used during the study are available from the corresponding author on reasonable requests.
Abbreviations
- OM:
-
Optical microscope
- SEM-EDS:
-
Scanning electron microscopy in combination with energy dispersive X-ray analysis
References
Craddock PT. The origins and inspirations of zinc smelting. J Mater Sci. 2009;44:2181–91.
Zhou W, Martinón-Torres M, Chen J, Liu H. Distilling zinc for the Ming dynasty: the technology of large scale zinc production in Fengdu, southwest China. J Archaeol Sci. 2012;39:908–21.
Zhou W, Martinón-Torres M, Chen J, Li Y. Not so efficient, but still distilled: the technology of Qing dynasty zinc production at Dafengmen, Chongqing, southwest China. J Archaeol Sci. 2014;43:278–88.
Craddock PT. Zinc in classical antiquity. In: Craddock PT, editor. 2000 years of zinc and brass. London: British Museum Press; 1998. p. 1–5.
Thornton CP. Of brass and bronze in prehistoric Southwest Asia. In: La Niece S, Hook DR, Craddock P, editors. Metals and Mines Studies in Archaeometallurgy. Archetype Publications: London; 2007. p. 123–35.
Fan X, Zhao X. Prehistoric brass objects and smelting techniques. J Natl Mus China. 2016;8:142–50.
Caley ER. Orichalcum and related ancient alloys origin composition and manufacture, with special reference to the coinage of the roman empire. New York: The American Numismatic Society; 1964.
Craddock PT. The composition of the copper alloys used by the greek, etruscan and roman civilizations: 3. the origins and early use of brass. J Archaeol Sci. 1978;5:1–16.
Rehren Th. Archäometallurgische Untersuchung des Zinktäfelchens, 160–69, und 8. Quellen und Funde zum frühen Zink in Europa, 169-172, in: R. Fellmann, Das ZinkTäfelchen vom Thormebodewald auf der Engehalbinsel bei Bern und seine keltische Inschrift, Archaeologie im Kanton Bern. Fundberichte und Aufsaetze 4A.1999;7:133–75.
Thornton CP, Ehlers CB. Early brass in the ancient near east. Inst Archaeo-Metallurgical Stud. 2003;23:3–8.
Thornton CP, Lamberg-Karlovsky CC, Liezers M, Young SMM. On pins and needles: tracing the evolution of copper-base alloying at Tepe Yahya, Iran, via ICP-MS analysis of common-place items. J Archaeol Sci. 2002;29:1451–60.
Bayley J. Roman brass making in Britain. Hist Metall. 1984;8:42–3.
Bayley J. The production of brass in antiquity with particular reference to Roman Britain. In: Craddock PT, editor. 2000 years of zinc and brass. London: The British Museum; 1998. p. 7–26.
Picon M, Nezet-Celestin ML, Desbat A. Un type particulier de grands récipients en terre réfractaire utilisés pour la fabrication du laiton par cémentation. Société Francaise d’Etude de la Céramique Antique en Gaule, Actes du Congrés de Rouen. 1995;207–15.
Rehren Th. Small size, large scale roman brass production in Germania inferior. J Archaeol Sci. 1999;26:1083–7.
Uda I, Cucini C, Riccardi MR, Tarantino SC. Brass production in Milan–Italy—during the Roman period. Cementation, casting and heat treatment, 2015 Poster presented at the Archaeometallurgy in Europe conference.
Merkel S. Carolingian and ottonian brass production in westphalia. evidence from the crucibles and slag of dortmund and soest. Metalla. 2016;22:21–39.
Craddock PT. The copper alloys of the Medieval Islamic world—inheritors of the classical tradition. World Archaeol. 1979;11:67–79.
Morton V. Brass from the past: Brass made, used and traded from prehistoric times to 1800. Oxford: Archaeopress Publishing; 2019. p. 5–6.
Bourgarit D, Bauchau F. The ancient brass cementation processes revisited by extensive experimental simulation. Archaeotechnology. 2010;62:27–33.
Bourgarit D, Thomas N. From laboratory to field experiments: shared experience in brass cementation. Hist Metall. 2011;45:8–16.
Ma Y. The preliminary research on the smelting technology of “erhuo brass” and “sihuo brass” in Ancient China. Master thesis of University of Science and Technology Beijing. 2007;34–64 (In Chinese).
Sun S, Han R. A preliminary study of early Chinese copper and bronze artefacts. J Archaeol. 1981;3:287–302 (In Chinese).
Fan X, Harbottle G, Gao Q. Brass before bronze? early copper-alloy metallurgy in China. J Anal At Spectrom. 2012;27:821–6.
Fan X, Zhao X, Zhao Z. Comparative experiment to simulate smelting of brass with calamine and malachite. Nonferrous Metal. 2014;12:7–10 (In Chinese).
Zhao K, Zhang H. Textual research and elucidation with simulated tests on various kinds of medicinal “Gold” and “Silver” in ancient Chinese alchemy. Stud Hist Nat Sci. 1987;2:105–22 (In Chinese).
Banpo Museum. Shaanxi Institute of Archaeology (1988) Report on the excavation of the Jiangzhai-Neolithic Site, Heritage Publishing House. 1988 (In Chinese).
Zhao K. Textual research of “Yellow copper” in successive dynasties of China. Stud Hist Nat Sci. 1987;6(4):323–321.
Zhou W. Production and development of brass smelting technology in China. Imperial Palace academic Quarterly. 2000:1-8 (In Chinese).
Yuan X. Scientific analysis of a brass sample from Hanjinxiaohe site in Xinjiang. In: New research on cultural relic protection. Beijing: Heritage Publishing House; 2010. p. 148–51 (In Chinese).
Zhou W. The origin and development of brass in China. Nat Pal Mus Res Quart. 2002;18:67–92 (In Chinese).
Lin H. On the import of toushi into Ancient China. Archaeol Cult Rel.1999;67–76 (In Chinese).
Pollard AM. From alexander the great to the buddha: buddhism and the introduction of brass technology into China. Archaeol Anthropol Sci. 2022;14(6):1–14.
Xiao H, Huang X, Cui J. Local cementation brass production during 12th–13th century CE, North China: evidence from a royal summer palace of jin dynasty. J Archaeol Sci:Rep. 2020;34:1–8.
Liu S. Talk about the age of brass coin in ancient China. J Pal Muse. 2001; 84–86 (In Chinese).
Zhou W, Fan X, He L. Experimental evidence for metallic zinc brass. Stud Hist Nat Sci. 1994;13:60–4 (In Chinese).
Xiong J, Tao Y, Ben M, Yang Y, Du P, Allen E, et al. Uniparental genetic analyses reveal multi-ethnic background of Dunhuang Foyemiaowan population (220–907CE) with typical Han Chinese archaeological culture. Front Ecol Evol. 2022;10:1–14.
Gansu Provincial Institute of Cultural Relics and Archaeology. Excavation Report of Xindiatai Tomb Group in Foye miaowan, Dunhuang in 2015. Gansu: Gansu Education Press. 2021: 825–976 (In Chinese).
Heginbotham A, Bassett J, Bourgarit D, Eveleigh C, Frantz T, Glinsman L, Hook D, et al. The copper CHARM set: a new set of certified reference materials for the standardization of quantitative X-ray fluorescence analysis of heritage copper alloys. Archaeom. 2015;57:856–68.
Wang Y. The Error Analysis and Methodologically Development for SEM-EDS Chemical Investigation of Ancient Porcelain Glaze and Bronze. Master's thesis of Beijing University of Science and Technology. 2020:41.
Martinón-Torres M, Li XJ, Bevan A, Xia Y, Zhao K, Rehren Th. Forty thousand arms for a single emperor: from chemical data to the labor organization behind the bronze arrows of the terracotta army. J Archaeol Meth Theory. 2014;21:534–62.
Baker JM. The Colour and Composition of Early Anglo-Saxon Copper Alloy Jewellery. PhD Thesis Durham University. 2013.
Bayley J. A Saxon brass bar ingot cache from kingsway. Hidden Histories and Records of Antiquity Essays on Saxon and Medieval London for John Clark Curator Emeritus Museum of London LAMAS Special Paper. 2014;17:121–8.
Park J. The key role of zinc, tin and lead in copper-base objects from medieval Talgar in Kazakhstan. J Archaeol Sci. 2009;36:622–8.
Rostoker W, Dvorak JR. Some experiments with cosmelting to copper alloys. Archeomat. 1991;5:5–20.
Goel RP. Thermodynamic considerations in Metal Extraction. Metallurgy. 2008; 1-12.
Willis MG, Toguri JM. Yazawa's D. The AusIMM Metallurgical Society Special Paper. 2009; 1-8.
Rehren Th, Boscher L, Pernicka E. Large scale smelting of speiss and arsenical copper at early bronze age arisman. Iran J Archaeol Sci. 2012;39:1717–27.
Dungworth D. Caley’s ‘zinc decline’ reconsidered. Numis Chron. 1996;156:228–34.
Cowell MR, Cribb J, Bowman S, Shashoua Y. The Chinese cash: composition and production. In: Archibald MM, Cowell MR, editors. Metallurgy and numismatics. London: Royal Numismatic Society Special Publication; 1993. p. 185–98.
Radivojević M, Rehren Th, Kuzmanović-Cvetković J, Jovanović M, Northover JP. Tainted ores and the rise of tin bronzes in Eurasia, c. 6500 years ago. Antiquity. 2013;87:1030–45.
Acknowledgements
ThR gratefully acknowledges support from the A.G. Leventis Foundation; this publication is an output of the A.G. Leventis Chair in Archaeological Sciences at the Cyprus Institute. Thank Gansu Provincial Institute of Cultural Relics and Archaeology for authorizing and providing samples. We would like to thank Mokun Shan, Chenyuan Li and Xueqi Bai from the University of Science and Technology Beijing for revising the English. We also thank the anonymous reviewers and the Editor for their assistance in improving the clarity and focus of this paper; any remaining errors are ours.
Funding
This work was supported by The National Social Science Fund of China (Project No. 17ZDA219). This research was financially supported by the Scientific Research Fund of the University of Science and Technology Beijing and Gansu Provincial Institute of Cultural Relics and Archaeology (Project No. 38430058).
Author information
Authors and Affiliations
Contributions
QB performed the data analyses and wrote the paper, YX and JL performed the experiments and data analysis. HL helped with the excavation and sample collection, XH, ThR, HL and JL reviewed and edited the paper. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Analysis results of brass inclusions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, Q., Li, Y., Rehren, T. et al. Early brass from the Foyemiaowan-Xindiantai cemetery, 265–439 ce: the origin and diffusion of brass in ancient China. Herit Sci 10, 159 (2022). https://doi.org/10.1186/s40494-022-00784-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-022-00784-5