Abstract
The use of non-invasive macroscopic imaging techniques is becoming more prevalent in the field of cultural heritage, especially to avoid invasive procedures that damage valuable artworks. For this purpose, an X-ray powder diffraction scanner (MA-XRPD) capable of visualising crystalline compounds in a highly specific manner was recently developed. Many inorganic pigments present in paintings fall into this category of materials. In this study, the 17th century oil painting Girl with a Pearl Earring (c. 1665) by Johannes Vermeer was analysed with a combination of transmission and reflection mode MA-XRPD. By employing this scanner in reflection mode, the relative sensitivity for compounds that are present at the paint surface could be increased, establishing it as a highly relevant technique for investigating the degradation processes that are ongoing at paint surfaces. Many of the original pigments employed by Vermeer could be identified, along with four secondary alteration products: gypsum (CaSO4·2H2O), anglesite (PbSO4), palmierite (K2Pb(SO4)2) and weddellite (CaC2O4·2H2O). The formation of gypsum was linked to the presence of chalk in the upper glaze layer while the formation of palmierite and weddellite is driven by the presence of lake pigments (and their substrates). In this manner, MA-XRPD can also be used to pinpoint locations relevant for sampling and synchrotron µ-XRPD analysis, which provides information on the microscopic make-up of the paint. A paint cross-section taken from an area rich in palmierite was analysed with synchrotron µ-XRPD, which confirmed the presence of this secondary compound at the interface of the upper paint layer with the ground layer as well as the presence of anglesite in the ground layer. The capacity of MA-XRPD to identify and chart secondary alteration products in a non-invasive manner has only very recently been demonstrated and makes it a highly relevant technique for the assessment of the chemical condition of works of art.
Similar content being viewed by others
Introduction
As a master of light and colour, Johannes Vermeer’s palette was both limited and distinctive. He made extensive use of the costly pigment ultramarine and used several red pigments, including vermilion and red lakes [1, 2]. While the original materials and techniques employed by Vermeer have been extensively studied, questions remain about how those materials might have changed since the work was painted in c. 1665 [3]. Degradation phenomena can cause binding media, varnish layers and original pigments to undergo chemical and physical transformations, leading to discolouration or a deterioration of the structural integrity of the paint material. These processes can be initiated by internal factors, for example the chemical interaction of pigments with organic binders, or by external factors, which can range from environmental conditions (humidity and light) to the presence of airborne pollutants in museums [4,5,6].
Current methods to investigate the occurrence of degradation processes of pigments at or below the painting surface usually require microscopic paint samples to be taken and prepared as cross-sections. Subsequently these samples can be analysed with laboratory-based techniques, such as scanning electron microscopy combined with energy-dispersive X-ray spectroscopy (SEM–EDX) and Fourier-transform infrared (FTIR) spectroscopy [7, 8]. Synchrotron-based methods such as microscopic X-ray powder diffraction (µ-XRPD) and X-ray absorption near edge structure (µ-XANES) also have been employed, for example to reveal the mechanism behind the conversion of red lead to plumbonacrite and to investigate the degradation of cadmium yellow pigments [4, 9].
A major disadvantage of the aforementioned techniques is that invasive sampling of the original painting is required: an undertaking that is often not permitted or limited to locations on the painting that already show signs of damage. Additionally, relying on a limited number of micrometric samples to draw conclusions about the macroscopic composition of a work of art introduces problems about whether these samples are representative of a wider area. For these reasons, several mobile non-invasive macroscopic imaging techniques have been developed in the past decade. Macroscopic X-ray fluorescence (MA-XRF) has been extensively used to acquire information about the elemental composition of a painting [10, 11]. The main limitation of MA-XRF is that it cannot differentiate between compounds with a similar elemental but different molecular composition, as is often the case in degradation processes. More recently, a macroscopic scanning variant of FTIR in reflection mode (MA-rFTIR) has been developed, providing information on the molecular level [12, 13]; however, its application is often limited to paintings without varnish, since the latter absorbs most of the infrared radiation. Visible/near infrared reflectance imaging spectroscopy (RIS) has also been frequently used for the investigation of oil paintings and is capable of delivering distribution images based on molecular features from both organic and inorganic compounds [14, 15].
In recent years, macroscopic X-ray powder diffraction (MA-XRPD) has also been successfully converted from a synchrotron-based imaging technique on the microscopic scale to a non-invasive laboratory-based technique on the macroscopic scale that can be deployed in situ in transmission mode, providing information on the bulk composition of the paint stratigraphy [16]. MA-XRPD was successfully employed in transmission mode to visualise the different types of chrome yellow used in Van Gogh’s Sunflowers (1889, Van Gogh Museum) and to provide information about the orientation of the needle-shaped crystals in his brushstrokes [17]. Furthermore, MA-XRPD has been used to discriminate between pigments applied on the recto or verso side of an illuminated manuscript by considering the small angular diffraction shift [18]. Finally, MA-XRPD has also been used in reflection mode, which is more suitable for superficial analysis, to demonstrate its potential for detecting crystalline compounds present at or close to the surface of a small mock-up painting [19] and to visualise degradation products at the paint surface of several flower still life paintings [20].
In this article, MA-XRPD will be employed in reflection mode to investigate and explain the presence of secondary alteration products in Vermeer’s iconic painting Girl with a Pearl Earring (c. 1665, Mauritshuis). The painting was last examined in 1994 using a combination of non-invasive technical photography methods (including infrared and visual light) and invasive microscopic techniques (including SEM–EDX, high-performance liquid chromatography) in order to better understand the composition of the different paint layers [21, 22]. Given the advances made in both microscopic and macroscopic analytical imaging methods since 1994, a new research project, The Girl in the Spotlight, was initiated. The project The Girl in the Spotlight is a Mauritshuis initiative, and involves a team of internationally recognised specialists working within the collaborative framework of the Netherlands Institute for Conservation, Art and Science (NICAS), with some scientists from other institutions. Within this project, the painting was analysed with a multitude of techniques, including MA-XRF, reflectance imaging spectroscopy and MA-XRPD.
Materials and methods
Macroscopic X-ray powder diffraction
A laboratory MA-XRPD scanner was used to analyse the painting Girl with a Pearl Earring. A monochromatic Cu-Kα (8.04 keV) X-ray source was employed to scan the painting in reflection mode. Due to geometrical limitations dictated by the dimensions of the X-ray source, a primary beam impingement angle of 10° relative to the painting surface was chosen, leading to a beam with an elliptical footprint of approximately 1 × 0.2 mm2 (horizontal × vertical). Low energy X-rays are also more easily attenuated by the sample, making this manner of scanning more sensitive for investigating superficial degradation phenomena. The X-ray source (IμS-CuHB, Incoatec GmbH, DE) generates a photon flux of 2.9 × 108 photons s−1, has a focal diameter of (142 ± 2) µm, a focal distance of (20 ± 1) cm and a divergence of (2.4 ± 0.1) mrad. The painting was placed at a distance of 20 cm from the X-ray source, while the distance between the painting and 2D diffraction detector was kept at 0.5 cm. A PILATUS 200K detector was oriented at an angle of 40° with the painting surface.
X-ray fluorescence data was simultaneously acquired by means of a Vortex-EX silicon drift detector (Hitachi, JP), positioned with an angle of 45° relative to the XY-plane of the painting surface. A set of three motorized stages (25 cm × 10 cm × 10 cm, Newport Corp., US) are responsible for the movements during the scanning procedure. The X-ray source, PILATUS 200K detector and Vortex detector were placed on a motorized platform, capable of moving the setup in the XZ plane. The artwork was placed on a motorized easel, capable of moving the painting in the vertical Y-direction (Fig. 1). To correct for topographical variations and curvatures on the painting surface, the Z-motor stage, coupled to a laser distance sensor (Baumer Hold., CH), automatically adjusts the distance between the X-ray source and the painting surface. Calibration of the setup was performed with a chalk paint layer on a mock-up canvas.
In reflection-mode an area encompassing the entirety of the Girl’s face (200 × 224 mm2) was scanned with an exposure time of 10 s per point and a step size of 2 mm × 2 mm. This step size is larger than the actual beam footprint (1 × 0.2 mm2) but was adopted because of time constraints. The total scan time for this area was approximately 35 h.
The diffraction data was processed using the XRDUA software package and with structural information files obtained from the American Mineralogist Database [23, 24]. The data was fitted using a whole pattern refinement procedure. This fitting procedure has been described in detail elsewhere [18].
Macroscopic X-ray fluorescence imaging
MA-XRF maps of the entire painting were collected in two scan sessions using the Bruker M6 Jetstream [11]. The instrument consists of a measuring head equipped with a 30 W Rhodium-target microfocus X-ray tube, a polycapillary lens, and a 60 mm2 XFlash silicon drift detector (SDD) with a beryllium window. By slowly moving the measuring head on the XY motorised stage, the painting is scanned pixel by pixel. By recording the emitted X-ray fluorescence radiation, the chemical elements present in the paint can be identified. With the Bruker M6, only elements heavier than silicon can be detected. Scans were carried out at 50 kV and a current of 600 μA, with a 400 μm step size, and a dwell time of 125 ms. The distance between the scanning head and the paint surface was set at c. 1 cm, corresponding to an X-ray spot size of c. 300 μm. All data were collected with the Bruker M6 Jetstream software package. The acquired data cubes were then exported as raw files, and processed and stitched using PyMca and the in-house developed Datamuncher software [25, 26].
Microscopic X-ray powder diffraction imaging
A microscopic paint sample collected from a shadow tone of the Girl’s face was used to investigate the presence of secondary alteration products as indicated by the MA-XRPD analysis. The cross-section was embedded in Technovit 2000 LC mounting resin (Heraeus Kulzer GmbH, DE) and polished using a sample holder and Micromesh sheets up to grade 12,000 (Micro-Surface Finishing Products Inc., Wilton, Iowa, USA). A Zeiss Axio Imager.A2m microscope equipped with a Zeiss AxioCam MRc5 digital camera was used to examine the polished cross section, prior to SEM–EDX analysis.
The cross-sectioned sample was analysed with μ-XRPD at beamline P06 (PETRA-III, DESY, DE), a hard X-ray micro- and nano-probe beamline suited for X-ray powder diffraction imaging experiments on the (sub-)micrometric scale. A Kirkpatrick-Baez optical system was used to focus the 21 keV beam to a diameter of 0.5 μm and a flux of 1010 photons s−1. The sample was mounted on a plastic frame that could be moved in the XYZ directions. An EIGER X 4M detector (Dectris Ltd., DE) was used to collect the diffraction signals. The sample was placed at a distance of 18 cm in front of the detector, ensuring a sufficiently wide angular range.
The synchrotron diffraction data has been corrected for attenuation effects and was also processed using XRDUA.
Results and discussion
Identification of original pigments and restorations
Using MA-rXRPD, several original pigments that Vermeer used in the upper layer(s) of the stratigraphy have been identified (Fig. 2). The red pigment vermilion (cinnabar, HgS) was used extensively in the lips of the Girl, and, mixed with lead white, more sparsely in the flesh tones of her face to create a soft pink colour. Vermeer painted the blue headscarf with ultramarine (lazurite), which he also mixed into the dark yellow paint in her jacket and yellow headscarf. In the bright blue part on the left side of her headscarf, the ultramarine was mixed with lead white, which explains the visible contrast in brightness between the left (lit) and right (shadow) sides [27]. Lead white, a mixture of hydrocerussite (2PbCO3·Pb(OH)2) and cerussite (PbCO3), has also been used throughout the entirety of the Girl’s face. In the bright yellow part of her headscarf, MA-rXRPD detected lead white while in the darker parts yellow ochre [Goethite, FeO(OH)] is found. Finally chalk (calcite, CaCO3), together with quartz (SiO2), is mainly present in the dark background surrounding the Girl and in the dark areas of her headscarf. Previous analyses of cross-sections from the Girl have revealed that the dark glaze layer in the background does indeed contain chalk, together with lake pigments (weld and indigo), carbon black and lead white [22]. Because XRPD is restricted to detecting crystalline compounds, the amorphous dyes in the background cannot be identified. However, these dyes are often precipitated on an inert metallic binder such as alum (KAl(SO4)2·12H2O), which can be visualised using MA-rXRPD. Besides original pigments, several non-original pigments and fill materials were also identified. These are from the 1994 restoration, or in some cases earlier treatments [28]. Some damages in the Girl’s face were filled with a putty containing chalk, and a small area was restored with goethite (associated with yellow ochre). Part of the Girl’s lead-white containing collar and several smaller areas in the Girl’s face have been retouched with a mixture containing titanium white (rutile, TiO2) and barite (BaSO4). Finally, three secondary alteration compounds were also identified: gypsum (CaSO4·2H2O), weddellite (CaC2O4·2H2O) and palmierite (K2Pb(SO4)2).
Superficial sensitivity of reflection mode MA-XRPD (MA-rXRPD)
Macroscopic X-ray powder diffraction imaging allows to identify, localise and quantify crystalline compounds in a complex paint matrix. Scanning in reflection mode greatly increases the sensitivity for (thin) surface layers [20]. The X-ray beam impinges upon the painting under a small angle (10°) with the painting surface, resulting in a shallow probing depth. This sampling depth ds varies from 10 µm or less for compounds containing ‘heavy’ elements (Pb) up until 50 µm for compounds consisting of ‘light elements’ (Ca) and was calculated using Eq. 1 for the alteration products encountered with MA-rXRPD in Girl with a Pearl Earring (Table 1).
To illustrate the increased superficial sensitivity of MA-rXRPD, a small region of the painting was also analysed in transmission mode, facilitating a direct comparison between the two modes. In this case a measurement was taken on the Girl’s nose in both reflection and transmission mode (Fig. 3). Transmission mode MA-XRPD shows the presence of four compounds: calcite (chalk, CaCO3), hydrocerussite (lead white, 2PbCO3·Pb(OH)2), cerussite (lead white, PbCO3) and wax. Hydrocerussite and cerussite are both components of the pigment lead white and together with chalk they constitute the ground layer [22]. Finally, beeswax was found as well. In 1960 the back of the painting was wax-resin lined using beeswax to provide extra support for the original canvas [22]. This clearly demonstrates that transmission mode MA-XRPD provides information about the bulk (e.g. ground layer) composition of the painting. Indeed, vermilion used in the pink flesh tones is not visible in the transmission data. In reflection mode multiple other crystalline components appear: palmierite (K2Pb(SO4)2) and weddellite (CaC2O4·2H2O) are two superficial alteration compounds, while vermilion and cerussite-rich lead white are present in the upper paint layer to provide a pink flesh tone together with chalk. No information on the ground layer is obtained, but several superficial pigments and degradation compounds are revealed instead.
Alteration products in Girl with a Pearl Earring
MA-rXRPD is a useful technique for tracking the formation of secondary reaction products, which are often formed at (or close to) the surface of the painting. Three such compounds were identified on the macroscopic scale (Fig. 2): gypsum (CaSO4·2H2O), weddellite (CaC2O4·2H2O) and palmierite (K2Pb(SO4)2). To further investigate the occurrence of each of these compounds a cross-section extracted from an area on the painting rich in palmierite was analysed with µ-XRPD (Fig. 4). With synchrotron µ-XRPD analysis, microscopic information about all of the individual layers of the paint stratigraphy is obtained, which makes it a highly useful method not only for verification of the macroscopic data but also for providing information on layers beneath the surface.
Two secondary lead sulphates were detected with MA-rXRPD and/or µ-XRPD: palmierite and anglesite (Fig. 2, 4). Palmierite has previously been identified as an alteration product in paint fragments from seventeenth-century Dutch paintings by Johannes Vermeer, Jacob Jordaens and Rembrandt, and the authors have also identified it in paintings by Jan Steen, Martinus Nellius and Jan Davidsz de Heem [20, 29,30,31,32,33]. Anglesite has previously been found on an ancient bronze inkwell and in paintings by Van Gogh and Memling [34,35,36].
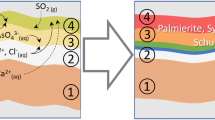
In Girl with a Pearl Earring, palmierite formed in dark areas, such as the background and the shadow tones of the Girl’s face (Fig. 2), and is present in an underlayer between the upper paint layer and the ground layer (Fig. 4). The formation of palmierite requires the supply and migration of three different ions: Pb2+, K+ and SO42−. Figure 5 shows that sulphur (S–K) and potassium (K–K) are localised in the background of Girl with the Pearl Earring and in several areas of the Girl’s face. In the facial areas sulphur is mostly associated with the sulphur-containing pigment vermilion however. The distribution of potassium looks similar to the palmierite distribution of Fig. 2. In these areas lead is present as well. It has been proposed that lead ions can be released from lead white from the ground by a reaction with the oil binding medium and can easily diffuse into the upper paint layer(s) [29, 33]. Additionally lead driers can act as a source of Pb2+ ions [37]. Potassium-containing sources include pigments such as smalt and green earth as well as lake substrates such as alum. Potash alum is a frequently used substrate for red lakes and was identified by MA-rXRPD as a minor component in several parts of the Girl’s face, including her lips, nose and eyes as well as in the shadow (Fig. 2) [38]. In areas where alum is found, there is also a significant presence of palmierite, indicating that potash alum functions here as a source of both potassium and sulphate ions for the local formation of palmierite. However, palmierite was also identified in the background, an area where no red lakes were used but that is nevertheless rich in potassium (Fig. 5). During the 1994 restoration and as part of the current research, the composition of the background was also investigated. The main colourants of the glaze in the background are indigo and weld: blue and yellow dyestuffs that together create a translucent green glaze. While indigo requires no substrate, weld is soluble in oil and therefore needs to be precipitated on a substrate. For yellow lakes in 17th century Dutch paintings a substrate of chalk with a limited amount of alum was often used [22]. The presence of alum in the upper glaze layer was confirmed by FTIR analysis and SEM-EDX [22]. With MA-rXRPD, however, only a limited amount of alum is identified in the background, which could suggest that most of the alum has already reacted away to form palmierite. Anglesite on the other hand was not identified with MA-rXRPD because its formation is restricted to the ground layer (Fig. 4).
Next to palmierite, also gypsum was identified with MA-rXRPD. Historically gypsum has been used in ground layers by Southern European artists, or as an extender or bulking agent for specific pigments (e.g. orpiment) [39, 40]. The superficial presence of gypsum in Girl with a Pearl Earring indicates it is likely a secondary reaction product. There is a very small amount of gypsum present in the ground layer, which consists mostly of chalk and lead white (Fig. 4). Gypsum has often been encountered before as a degradation product in black crusts found on historical buildings and monuments [41, 42] as well as an alteration product in oil paintings [20]. Gypsum can also formed as minor by-product in the manufacturing process of lake pigments on a combined chalk alum substrate [43]. The formation of gypsum requires the presence of both calcium (Ca2+) cations and sulphate (SO42−) anions. Figure 2 shows that gypsum and calcite have a mostly similar distribution, suggesting that chalk from the upper glaze layer (or ground layer) is reacting with sulphate ions, which are supplied by either an external source, for example atmospheric pollutant SO2, or an internal source, such as alum.
Weddellite was identified as a fourth secondary alteration product, alongside gypsum, palmierite and anglesite. Weddellite has been previously encountered as a degradation product on stone sculptures and gilded paintings, sometimes occurring together with its monohydrated analogue whewellite (CaC2O4·H2O) [44, 45]. Furthermore, it has been demonstrated that calcium ions have an extremely high propensity to form oxalate salts over a wide pH range, explaining their common occurrence in works of art [46]. Two sources of oxalate ions for the formation of metal oxalates have been described: biological/microbial and chemical. The former is especially relevant for works of art in outdoor conditions, e.g. sculptures and wall paintings [47]. For indoor easel paintings, the potential for microbial growth is limited, yet cases of bacterial colonisation on traditional oil paintings have been reported [48]. More likely in the case of Girl with a Pearl Earring, a chemical mechanism is at play in which organic materials such as varnishes, binders and dyes release oxalate ions during degradation. Figure 2 shows that weddellite is mostly formed in areas where palmierite has formed as well and where chalk is present. While palmierite requires the presence of the lake substrate alum, the formation of weddellite appears to involve the (degradation of the) organic dye itself and/or the degradation of other organic materials such as binders and varnishes. As part of degradation, the organic compounds release oxalate ions which form the stable calcium oxalate salt by reacting with calcium ions from either the upper glaze or ground layer. In the background area indigo, weld or other organic components used in the translucent green paint are suspected to be responsible for the supply of oxalate ions.
Conclusions
The ability of MA-XRPD to perform non-invasive, superficial and highly specific chemical imaging was demonstrated on a 17th century oil painting made by Johannes Vermeer. Besides the visualisation of a variety of inorganic pigments, several degradation products were identified. A small amount of gypsum was identified in the upper glaze layer of the background, likely the result of chalk reacting with sulphate ions released by internal or external components. Furthermore, two different lead sulphates were encountered, anglesite and palmierite. Anglesite was identified in the ground layer while palmierite was found at the surface in the background and shadow areas. Palmierite is believed to be the reaction product of the lake substrate, alum, and migratory Pb(II) ions. The calcium oxalate weddellite was encountered in similar areas, indicating that possibly the organic dye itself is involved in degradation processes.
The information that is obtained about these degradation compounds using MA- and µ-XRPD warrants further investigation to determine their exact reasons of formation. Especially the role played by organic dyes in the formation of weddellite and palmierite in oil paintings should be examined more closely using complementary non-invasive imaging techniques capable of visualising amorphous organic compounds such as MA-rFTIR or NIR reflectance imaging and by performing accelerated ageing experiments under different environmental conditions.
The chemical distributions shown in this article highlight the added value MA-XRPD brings to the field of cultural heritage for the identification of both inorganic pigments and their secondary reaction products. By combining the data from MA-XRPD with µ-XRPD, superficial information on the macroscopic scale and stratigraphic details on the microscopic scale are acquired, providing insight into both the active area and mechanism of the degradation process. MA-XRPD can therefore be considered as a highly relevant technique for the in situ evaluation of degradation phenomena in a work of art, and could help guide restoration efforts by pinpointing degraded areas suitable for cleaning treatments or sampling.
Availability of data and materials
Not applicable.
Abbreviations
- FTIR:
-
Fourier transform infrared
- MA-rFTIR:
-
macroscopic reflection fourier transform infrared
- MA-rXRPD:
-
macroscopic reflection X-ray powder diffraction
- MA-XRF:
-
macroscopic X-ray fluorescence
- MA-XRPD:
-
macroscopic X-ray powder diffraction
- SEM–EDX:
-
scanning electron microscopy energy dispersive X-ray spectroscopy
- µ-XRPD:
-
microscopic X-ray powder diffraction
- XANES:
-
X-ray absorption near edge structure
References
Kühn H. A study of the pigments and the grounds used by Jan Vermeer. Rep Stud Hist Art. 1968;2:154–75.
Delaney JK, Van Loon A, Vandivere A. Mapping the pigment distribution of Vermeer’s Girl with a Pearl Earring. Her Sci. 2019.
Costaras N. A study of the materials and techniques of Johannes Vermeer. In: Gaskell I, Jonker M, editors. Vermeer studies. Washington: National Gallery of Art; 1998. p. 144–67.
Monico L, Chieli A, De Meyer S, Cotte M, De Nolf W, Falkenberg G, et al. Role of relative humidity and Cd/Zn stoichiometry in the photo-oxidation process of cadmium yellows in oil paintings. Chem Eur J. 2018;24:11584–93.
Monico L, Sorace L, Cotte M, De Nolf W, Janssens K, Romani A, et al. Disclosing the binding medium effects and the pigment solubility in the (Photo)reduction process of chrome yellows. ACS Omega. 2019;4:6607–19.
Worobiec A, Samek L, Krata A, Meel KV, Krupinska B, Stefaniak EA, et al. Transport and deposition of airborne pollutants in exhibition areas located in historical buildings–study in Wawel Castle Museum in Cracow, Poland. J Cult Herit. 2010;11:354–9.
Schreiner M, Melcher M, Uhlir K. Scanning electron microscopy and energy dispersive analysis: applications in the field of cultural heritage. Anal Bioanal Chem. 2007;387:737–47.
Prati S, Joseph E, Sciutto G, Mazzeo R. New advances in the application of FTIR microscopy and spectroscopy for the characterization of artistic materials. Acc Chem Res. 2010;43:792–801.
Vanmeert F, Van der Snickt G, Janssens K. Plumbonacrite identified by X-ray powder diffraction tomography as a missing link during degradation of red lead in a Van Gogh Painting. Angew Chem. 2015;127:3678–81.
Alfeld M, Janssens K, Dik J, De Nolf W, Van der Snickt G. Optimization of mobile scanning macro-XRF systems for the in situ investigation of historical paintings. J Anal At Spectrom. 2011;26:899–909.
Alfeld M, Pedroso JV, van Eikema Hommes M, Van der Snickt G, Tauber G, Blaas J, et al. A mobile instrument for in situ scanning macro-XRF investigation of historical paintings. J Anal At Spectrom. 2013;28:760–7.
Legrand S, Alfeld M, Vanmeert F, De Nolf W, Janssens K. Macroscopic Fourier transform infrared scanning in reflection mode (MA-rFTIR), a new tool for chemical imaging of cultural heritage artefacts in the mid-infrared range. Analyst. 2014;139:2489–98.
Gabrieli F, Dooley KA, Zeibel JG, Howe JD, Delaney JK. Standoff Mid-infrared emissive imaging spectroscopy for identification and mapping of materials in polychrome objects. Angew Chem. 2018;57:7341–5.
Cucci C, Delaney JK, Picollo M. Reflectance hyperspectral imaging for investigation of works of art: old master paintings and illuminated manuscripts. Acc Chem Res. 2016;49:2070–9.
Alfeld M, De Viguerie L. Recent developments in spectroscopic imaging techniques for historical paintings—a review. Spectrochim Acta Part B. 2017;136:81–105.
Vanmeert F, De Nolf W, De Meyer S, Dik J, Janssens K. Macroscopic X-ray powder diffraction scanning, a new method for highly selective chemical imaging of works of art: instrument Optimization. Anal Chem. 2018;90:6436–44.
Vanmeert F, Hendriks E, Van der Snickt G, Monico L, Dik J, Janssens K. Chemical mapping by macroscopic X-ray powder diffraction of van Gogh’s sunflowers: identification of areas with higher degradation risk. Angew Chem. 2018;57:7418–22.
Vanmeert F, De Nolf W, Dik J, Janssens K. Macroscopic X-ray powder diffraction scanning: possibilities for quantitative and depth-selective parchment analysis. Anal Chem. 2018;90:6445–52.
De Meyer S, Vanmeert F, Janssens K, Storme P. A mobile scanner for XRPD-imaging of paintings in transmission and reflection geometry. In: 6th interdisciplinary ALMA conference. Brno: Academy of Fine Arts in Prague; 2017.
Vanmeert F, De Keyser N, Van Loon A, Klaassen L, Noble P, Janssens K. Transmission and reflection mode macroscopic X-ray powder diffraction (MA-XRPD) imaging for the noninvasive visualization of paint degradation in still life paintings by Jan Davidsz. de Heem. Anal Chem. 2019;91:7153–61.
Wadum J. Vermeer illuminated: conservation, restoration and research. The Hague: Mauritshuis: V + K Publishing/Inmerc; 1994.
Groen K, Van der Werf I, Van den Berg K, Boon J. Scientific examination of Vermeer’s “Girl with a Pearl Earring”. In: Gaskell I, Jonker M, editors. Vermeer Studies: Studies in the History of Art 55. New Haven and London: National Gallery of Art, Washington; Yale University Press; 1998. p. 168–83.
De Nolf W, Vanmeert F, Janssens K. XRDUA: crystalline phase distribution maps by two-dimensional scanning and tomographic (micro) X-ray powder diffraction. J Appl Crystallogr. 2014;47:1107–17.
Downs RT, Hall-Wallace M. The American mineralogist crystal structure database. Am Miner. 2003;88:247–50.
Alfeld M, Janssens K. Strategies for processing mega-pixel X-ray fluorescence hyperspectral data: a case study on a version of Caravaggio’s painting Supper at Emmaus. J Anal At Spectrom. 2015;30:777–89.
Solé VA, Papillon E, Cotte M, Walter P, Susini J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim Acta Part B. 2007;62:63–8.
Van Loon A, Vandivere A, Gambardella A, Gonzalez V, Keune K, Haswell R, et al. Out of the blue: Vermeer’s use of ultramarine in Girl with a Pearl Earring. Her Sci; 2019; (in preparation).
Wadum J, Costaras N. Johannes Vermeer’s “Girl with a Pearl Earring” De- & Re-restored. Restauration, Dé-Restauration, Re-Restauration. Paris: ARAAFU; 1995. p. 163–72.
Van Loon A, Noble P, Boon JJ. White hazes and surface crusts in Rembrandt’s Homer and related paintings. In: ICOM committee for conservation 16th triennial meeting. Lisbon; 2011.
Boon JJ. Chemistry underneath the painting surface: palmierite formation in/on a painting by Johannes Vermeer and by Jacob Jordaens using Laboratory- and synchrotron-aided spectroscopic methods. J Microsc Microanal. 2013;19:1408–9.
Van Loon A, Noble P, Krekeler A, Van der Snickt G, Janssens K, Yoshinari A, et al. Artificial orpiment, a new pigment in Rembrandt’s palette. Her Sci. 2017;5:26.
Simoen J, De Meyer S, Vanmeert F, De Keyser N, Avranovich Clerici E, Van der Snickt G, et al. Combined Micro- and Macro scale X-ray powder diffraction mapping of degraded Orpiment paint in a 17th century still life painting by Martinus Nellius. Her Sci. 2019; (Submitted).
Price S, Van Loon A, Keune K, Parsons A, Murray C, Beale A, et al. Unravelling the spatial dependency of the complex solid-state chemistry of Pb in a paint micro-sample from Rembrandt’s Homer using XRD-CT. Chem Commun. 2019;55:1931–4.
Remazeilles C, Conforto E. A buried roman bronze inkwell: chemical interactions with agricultural fertilizers. Stud Conserv. 2008;53:110–7.
Van der Snickt G, Janssens K, Dik J, De Nolf W, Vanmeert F, Jaroszewicw J, et al. Combined use of synchrotron radiation based micro-X-ray fluorescence, micro-X-ray diffraction, micro-X-ray absorption near-edge, and micro-Fourier transform infrared spectroscopies for revealing an alternative degradation pathway of the pigment Cadmium yellow in a painting by Van Gogh. Anal Chem. 2012;84:10221–8.
Janssens K, Legrand S, Van der Snickt G, Vanmeert F. Virtual archaeology of altered paintings: multiscale chemical imaging tools. Elements. 2016;12:39–44.
Cotte M, Checroun E, De Nolf W, Taniguchi Y, De Viguerie L, Burghammer M, et al. Lead soaps in paintings: friends or foes? Stud Conserv. 2016;62:2–23.
Kirby J, Spring M, Higgitt C. The technology of red lake pigment manufacture: study of the dyestuff substrate. Natl Gallery Tech Bull. 2005;26:71–87.
Gettens R, Mrose M. Calcium sulphate minerals in the grounds of Italian paintings. Stud Conserv. 1954;1:174–89.
Keune K, Mass J, Mehta A, Church J, Meirer F. Analytical imaging studies of the migration of degraded orpiment, realgar and emerald green pigments in historic paintings and related conservation issues. Her Sci. 2016;4:10.
Toniolo L, Zerbi CM, Bugini R. Black layers on historical architecture. Environ Sci Pollut Res Int. 2009;16:218–26.
Siedel H. Salt efflorescence as indicator for sources of damaing salts on historic buildings and monuments: a statistical approach. Environ Earth Sci. 2018;77:572.
Hermens E, Wallert A. The Pekstok papers: lake pigments, prisons and paint-mills. In: Hermens E, Ouwerkerk A, Costaras N, editors. Looking through paintings: the study of painting techniques and materials in support of art historical research. London: Archetype; 1998. p. 269–94.
Cariati F, Rampazzi L, Toniolo L, Pozzi A. Calcium oxalate films on stone surfaces: experimental assessment of the chemical formation. Stud Conserv. 2000;45:180–8.
Lluveras A, Boularand S, Rosell-Roqué J, Cotte M, Giraldez P, Vendrell M. Weathering of gilding decorations investigated by SR: development and distribution of calcium oxalates in the case of Sant Benet de Bages (Barcelona, Spain). Appl Phys A. 2008;90:23–33.
Zoppi A, Lofrumento C, Mendes NFC, Castellucci EM. Metal oxalates in paints: a Raman investigation on the relative reactivities of different pigments to oxalic acid solutions. Anal Bioanal Chem. 2010;397:841–9.
Rosado T, Milene G, Mirao J, Candeias A, Caldeira AT. Oxalate biofilm formation in mural paintings due to micro-organisms—a comprehensive study. Int Biodeter Biodegr. 2013;85:1–7.
Seves AM, Sora S, Ciferri O. The microbial colonization of oil paintings. A laboratory investigation. Int Biodeter Biodegr. 1996;37:215–24.
Acknowledgements
The research project The Girl in the Spotlight is a Mauritshuis initiative, led by paintings conservator Abbie Vandivere, with a team of internationally recognised specialists working within the collaborative framework of the Netherlands Institute for Conservation + Art + Science + (NICAS).
The following NICAS partners are actively involved in the research: Rijksmuseum, Delft University of Technology (TU Delft), University of Amsterdam (UvA) and the Cultural Heritage Agency of the Netherlands (RCE). Cooperating in this project are: Shell Technology Centre Amsterdam (STCA), Maastricht University, University of Antwerp, National Gallery of Art Washington DC and Hirox Europe. These institutions have made their equipment, expertise and research time available for the benefit of this project.
NICAS is an interdisciplinary research centre initiated by the division for Physical Sciences of the Netherlands Organisation for Scientific Research (NWO-CEW), within which art history, conservation and restoration, and sciences are united.
This research was part of the activities of the Chair on Advanced Imaging Techniques for the Arts, established by the Baillet Latour fund.
The authors would like to thank staff from beamline P06 at the PETRA-III (DESY) synchrotron facilities, in particular Jan Garrevoet, Matthias Alfeld and Gerald Falkenberg.
Finally, the authors would like to declare that a small subset of the data presented in this article (i.e. the lead white related distributions) has been used in a different article within the context of investigating and quantifying the lead white ratio.
Funding
The authors would like to thank Interreg Vlaanderen—Nederland for funding to help develop the MA-rXRPD scanner.
This project was made possible with support from the Johan Maurits Compagnie Foundation.
This study was supported by Interreg and CALIPSOplus (Grant 730872).
Author information
Authors and Affiliations
Contributions
SDM wrote the manuscript which was revised by KJ, FV, RV, VG, AV and GVDS. The MA-XRPD analysis was performed by SDM and FV with support from GVDS and AV while the data was processed by RV, FV and SDM. The MA-XRF analysis was performed by AvL. The µ-XRPD samples were taken by AvL and analysis was performed by SDM and VG. The overarching project ‘Girl in the Spotlight’ was coordinated by AV. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
De Meyer, S., Vanmeert, F., Vertongen, R. et al. Imaging secondary reaction products at the surface of Vermeer’s Girl with the Pearl Earring by means of macroscopic X-ray powder diffraction scanning. Herit Sci 7, 67 (2019). https://doi.org/10.1186/s40494-019-0309-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-019-0309-3