Abstract
Choroid plexus tumors (CPTs) are intraventricular tumors derived from the choroid plexus epithelium and occur frequently in children. The aim of this study was to investigate the genomic and epigenomic characteristics of CPT and identify the differences between choroid plexus papilloma (CPP) and choroid plexus carcinoma (CPC). We conducted multiomics analyses of 20 CPT patients including CPP and CPC. Multiomics analysis included whole-genome sequencing, whole-transcriptome sequencing, and methylation sequencing. Mutually exclusive TP53 and EPHA7 point mutations, coupled with the amplification of chromosome 1, were exclusively identified in CPC. In contrast, amplification of chromosome 9 was specific to CPP. Differential gene expression analysis uncovered a significant overexpression of genes related to cell cycle regulation and epithelial-mesenchymal transition pathways in CPC compared to CPP. Overexpression of genes associated with tumor metastasis and progression was observed in the CPC subgroup with leptomeningeal dissemination. Furthermore, methylation profiling unveiled hypomethylation in major repeat regions, including long interspersed nuclear elements, short interspersed nuclear elements, long terminal repeats, and retrotransposons in CPC compared to CPP, implying that the loss of epigenetic silencing of transposable elements may play a role in tumorigenesis of CPC. Finally, the differential expression of AK1, regulated by both genomic and epigenomic factors, emerged as a potential contributing factor to the histological difference of CPP against CPC. Our results suggest pronounced genomic and epigenomic disparities between CPP and CPC, providing insights into the pathogenesis of CPT at the molecular level.
Similar content being viewed by others
Introduction
Choroid plexus tumor (CPT) is an intraventricular neoplasm derived from the choroid plexus epithelium [32]. CPT patients are typically diagnosed under the age of five years and account for 2–6% of all pediatric brain tumor patients [38]. The 2021 World Health Organization Classification of Tumors of the Central Nervous System categorizes CPT into three subtypes based on their histological characteristics: choroid plexus papilloma (CPP), atypical choroid plexus papilloma (aCPP) and choroid plexus carcinoma (CPC). CPP shows benign characteristics and is associated with good survival rates, whereas CPC exhibits malignancy, rapid progression, and recurrence [32].
Germline and somatic mutations in TP53, a well-known tumor suppressor gene, are among the most common genetic markers of CPC [31]. Moreover, mutations in TP53 are used to diagnose various diseases, such as Li-Fraumeni syndrome (LFS), which is a cancer predisposition syndrome commonly associated with CPC. [3, 16]. Mutations in TP53 have been previously reported in 44–67% of CPC patients [31, 36, 52, 56] in studies using targeted sequencing approaches such as Sanger sequencing, panel sequencing, or whole-exome sequencing.
According to previous research, CPT harbors multiple copy number alterations [36, 45, 56], and pediatric CPC frequently shows copy number gains of chromosomes 1, 2, and 21q [56]. In addition, chromosome 9p gain and 10q loss were reported to be associated with longer survival in CPC [45]. Recently, Tong et al. identified TAF12, NFYC, and RAD54L, located on chromosome 1, as oncogenes affecting susceptibility to CPC [57].
Several studies examining the epigenomic aspects of CPT classified benign CPP and malignant CPC according to differential CpG methylation profiles [36, 43, 55]. In particular, Pienkowska et al. reported that AK1, PER2, and PLSCR4 were differentially methylated and differentially expressed between CPP and CPC [43].
However, most of the previous studies used single-omics methodologies, which pose limitations in comprehensively characterizing CPT. Although Thomas et al. performed multiomics profiling of CPT using methylation microarray, whole-exome, and RNA sequencing, they focused on the comparison of pediatric and adult CPTs [56]. In this study, we performed comprehensive multiomics analyses of 20 pediatric CPT patients using next-generation sequencing (NGS) techniques encompassing whole-genome sequencing (WGS), whole-transcriptome sequencing (WTS), and methylation sequencing (Methyl-seq) to better understand the genomic and epigenomic characteristics of CPT, focusing on the differences between CPP and CPC.
Materials and methods
Patients and samples
To gain genomic and epigenomic insights into CPT, a total of 20 CPT samples were obtained (Table 1). The primary tumors were pathologically diagnosed as CPT, including 6 CPPs, 2 aCPPs, 1 mixed CPP and papillary ependymoma (mCPP), and 11 CPCs. Normal DNA from peripheral white blood cells was available for 11 patients. The patient cohort included 11 females and 9 males, and the average age of the patients was 5.2 years. Thirteen patients had lateral ventricle tumors. Five patients developed lesions of the fourth ventricle. In two cases, the tumors were in the third ventricle. Regarding leptomeningeal seeding (LMS) status, there was no LMS in patients with CPP. However, LMS was found in 5 of the 11 patients with CPC: three patients were diagnosed with LMS preoperatively and LMS was confirmed on spine MRI one month after surgery. All patients with LMS died except for one patient living abroad whose survival could not be confirmed after follow-up.
Clinical data were abstracted from medical records. Human specimens were obtained from patients undergoing surgical resection with informed consent for their usage for research purposes in accordance with the guidelines of the Institutional Review Board of Seoul National University Hospital, which approved this study (IRB No. 1507-047-687).
For DNA extraction from samples, a Qiagen QIAamp DNA kit (Qiagen, Valencia, CA) was used. For RNA isolation, a RNeasy Plus Mini Kit (Qiagen) was employed. The concentration and purity of the DNA and RNA were assessed using a spectrophotometer (Denovix Inc., Wilmington, DE). The quality of the purified RNA was validated with a Nanodrop 2000 Spectrophotometer and an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Cell cultures
Cells were isolated from fresh CPT tissues (2 CPP and 3 CPC) within 4 h of collection. Briefly, the tissues were minced with a surgical knife and dispersed into small aggregates. After enzymatic dissociation into single cells, the cells were filtered and the subsequent processing was performed as described previously [6, 41]. Tumor cells were incubated in Dulbecco's Modified Eagle's Medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum Opti-Gold (GenDEPOT, Katy, TX) and penicillin–streptomycin (× 1 final concentration; Invitrogen). All cells were maintained at 37 °C with 5% CO2 in a humidified atmosphere, and only early-passage (p4) cells were used for the validation test.
Whole-genome sequencing
WGS data were generated from 20 tumor samples and 11 available matched blood samples. WGS libraries were constructed with an input of 0.1–0.5 μg of fragmented DNA using the TruSeq Nano DNA kit (Illumina, Inc., San Diego, CA) following the manufacturer's protocol. The libraries were subjected to an Agilent 2100 Bioanalyzer to estimate the quality and were loaded onto the Illumina NovaSeq 6000 (Theragen Bio, Seongnam, Korea) according to the manufacturer’s recommendations.
Sequencing reads were mapped to the human reference genome (version GRCh38) using Burrows-Wheeler Alignment tool (version 0.7.15) with the “-M” option [28]. Mapped BAM files were sorted and indexed using SAMtools (version 1.9) [7]. Duplicate reads were removed using Picard (version 2.9.0) with the MarkDuplicates module (http://broadinstitute.github.io/picard/). Mapping quality was recalibrated using the BaseRecalibrator and ApplyBQSR tools of the Genome Analysis Toolkit (GATK) (version 4.1.7.0) [34].
Germline single nucleotide variations (SNVs) and short insertions and deletions (INDELs) were called using HaplotypeCaller in GATK (version 4.1.0.0) with the “-ERC GVCF” option and jointly genotyped using the GenotypeGVCFs tool in GATK [34]. The called variants were separated into SNVs and INDELs and were recalibrated using the VariantRecalibrator and ApplyRecalibration tools. Only variants labeled as PASS were used for further analysis and were annotated with a custom pipeline based on ensemble-vep (version 108) [35]. Somatic SNVs and INDELs were called using Mutect2 in GATK (version 4.1.0.0) [34] with the “-af-of-alleles-not-in-resource 0.00003125” and “-max-mnp-distance 0” options and panel of normal constructed 11 normal samples. Only variants labeled as PASS by the “FilterMutectCalls” option were used for further analysis. The remaining variants were annotated with a custom pipeline based on ensemble-vep (version 108) [35]. For somatic mutation analysis, samples with matched normal were detected by paired mode and tumor-only mode, and samples without matched normal were detected by tumor-only mode. Each analysis method was based on the guideline of GATK.
Since matched whole-blood normal samples were not available for some samples, we were limited in distinguishing whether the mutations detected in the tumor are somatic or germline. To account for this, we built a machine learning model to predicted as somatic or germline mutations. After that, mutations with an allele frequency lower than 0.001 in large databases, including the 1000 Genomes Project [14], gnomAD [22], and Korea1K [20] databases, were defined as rare variants.
Somatic copy number alterations (SCNAs) were called by CNVkit (version 0.9.6) with the “-method wgs” and “-target-avg-size 100,000” options for WGS data [53]. Since there were no matched normal data for some patients (9 out of 20 patients), we used a pooled reference that combined all normal samples, as proposed by CNVkit. After utilizing CNVkit, blacklisted regions (centromere regions, with an interval padding of 500,000 bp on each side; telomere regions, 5000 bp on each side) were removed by referring to the University of California Santa Cruz (hg38) blacklist file. Regions with significant focal and arm-level copy number alterations were identified with Genome Identification of Significant Targets in Cancer (GISTIC) [37] using the above copy number alteration profile (Q value < 0.05). The oncogene and tumor suppressor gene annotation of significant focal SCNA regions followed the cancer gene consensus (version 90) [50].
Predicting somatic mutations by machine learning
Predicting somatic point mutations first involves a preprocessed input file constructed with Mutect2 in GATK (version 4.1.0.0) [34] and, allele frequency from the Korea1K database [20]. In addition to these features, we demonstrated the novel feature VAF_z, the z-score of the variant allele fraction value between the block (\(\pm 1000\text{bp}\)) of the mutation.
Among 102 features as input data, 23 features are defined as important by feature importance evaluation using AutoGluon-Tabular package [9].
Generally, somatic mutations occur rarely compared to germline mutations. Due to this phenomenon, our input data were highly imbalanced. To solve this problem, the WeightedEnsemble L2 model combines 13 classifiers (CatBoost, RandomForestEntr, XGBoost, RandomForestGini, LightGBMLarge, LightGBM, ExtraTreesEntr, ExtraTreesGini, LightGBMXT, NeuralNetTorch, KNeighborsUnif, KNeighborsDist, NeuralNetFastAI). Validation of the model is implemented by fivefold cross validation. We measure the robustness of this model with F1-score and Matthews Correlation Coefficient.
Clonality analysis
The clonality of the tumor samples was analyzed using FastClone, a state-of-the-art probabilistic tool designed for deconvoluting tumor heterogeneity in bulk-sequencing samples [61]. FastClone is notable for its ability to efficiently and accurately identify subclonal structures by deconvoluting subclones based on its somatic mutations and SCNAs. By modeling the relationships between mutations and the underlying clonal structure with FastClone, we estimated the proportion of cells with a specific set of mutations. Patients without matched blood normal data were excluded since the necessary somatic mutations and SCNAs information could not be accurately provided for clonality analysis.
Whole-transcriptome sequencing
WTS data were generated from 20 CPT primary tissue samples. WTS libraries were prepared for 151 bp paired-end sequencing using a TruSeq stranded mRNA Sample Preparation Kit (Illumina). mRNA molecules were purified and fragmented from 1 μg of total RNA using oligo (dT) magnetic beads. The fragmented mRNAs were synthesized as single-stranded cDNAs through random hexamer priming. By applying this cDNA as a template for second strand synthesis, double-stranded cDNA was prepared. After a sequential process of end repair, A-tailing and adapter ligation, cDNA libraries were amplified with PCR. The quality of these cDNA libraries was evaluated with the Agilent 2100 BioAnalyzer (Agilent, Santa Clara, CA). The libraries were also quantified with the KAPA library quantification kit (Kapa Biosystems, Wilmington, MA) according to the manufacturer’s library quantification protocol. Following cluster amplification of denatured templates, paired-end sequencing (2 × 151 bp) was performed using an Illumina NovaSeq 6000 (Illumina) instrument.
Gene-level expression values were calculated using a custom pipeline using RSEM (version 1.3.0) [26] with GENCODE (version 33) [12] as a human transcriptome reference (version GRCh38). Differentially expressed gene (DEG) analysis was performed using DESeq2 (version 1.26.0) [33] with the default settings. DEGs with a adjusted P value < 0.01 and absolute log2(fold change) > 1 were considered significantly up- or downregulated between CPP and CPC, and these genes were used for subsequent analyses. The DEG analysis between the groups with and without LMS was performed equally with DESeq2, genes with − log10(padj) > 5 and absolute log2 (fold change) > 1 were considered significantly up- or downregulated. principal component analysis (PCA) was performed on the significant DEGs with default parameters. Gene set enrichment analysis was conducted using Enrichr [5, 24, 62], and enriched terms with adjusted P values < 0.01 were identified based on the Gene Ontology Biological Process [2, 13], and Molecular Signatures Database [51].
Methylation sequencing
Methyl-seq data were generated from 20 CPT primary tissue samples. DNA quantification and DNA quality control were performed using Qubit, NanoDrop, and gel electrophoresis. The electrophoresis run was performed on a 0.7% agarose gel for 45 min at 100 V, with 30 ng of DNA loaded. Library preparations were performed according to the SureSelect XT Methyl-Seq Target Enrichment System Kit Protocol (Agilent). Briefly, 3 μg of gDNA was randomly sheared and then DNA fragments of 100–175 bp were extracted. Sheared DNA fragments were end-repaired and purified using AMPure XP beads. The quality of these DNA fragments was evaluated with an Agilent 2100 BioAnalyzer (Agilent). They were quantified with the KAPA library quantification kit (Kapa Biosystems) according to the manufacturer’s library quantification protocol. Following cluster amplification of denatured templates, paired-end sequencing (2 × 151 bp) was performed using Illumina NovaSeq 6000 (Illumina).
Sequencing reads were mapped to the human reference genome (version GRCh38) using Bismark (version 0.18.1) with default options [23]. Mapped BAM files were sorted using SAMtools (version 1.9) [7]. Methylation levels for each CpG site were calculated using MethylKit (version 1.12.0) [1]. To analyze differentially methylated site (DMS), we used calculateDiffMeth in MethylKit and considered CpG sites with a -log Q value > 40 and absolute differential methylation value > 55 as significant DMSs. We defined the promoter region as the ~ 1000 bp from the transcription start site using GENCODE (version 33) and calculated the mean beta value of CpG sites within the promoter regions of each gene. PCA was performed on significant DMSs with default settings. GENCODE (version 33), ENCODE Candidate Cis-Regulatory Elements, and RepeatMasker were used to label the CpG sites based on the identity of the genomic regions in which they were located in. The mean beta value of each genomic region was calculated for each sample [10].
Reverse transcription and quantitative real-time PCR
cDNAs were synthesized from high-quality RNA using the RNA to cDNA EcoDry Premix kit (TAKARA, Shiga, Japan) according to the manufacturer’s instructions. quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis was carried out by a TaqMan assay on an ABI 7500 system (Applied Biosystems, Foster City, CA) using purchased TaqMan probes (CDC20, LRP2, TMEM265, DDTL, L1TD1, and GABBR1) and TaqMan® Gene Expression Master Mix (Invitrogen). The relative expression levels in each sample were quantified using the 2-DDCT method. The value of each control sample was set to one and was used to calculate the fold change in target gene expression. The results were normalized to that of GAPDH and were presented relative to the CPP. All data were obtained from three independent experiments, each performed in triplicate.
Results
Sequencing statistics
To characterize the genetic and epigenetic variances among the subtypes of CPT, we analyzed 20 CPT patients using WGS, WTS, and Methyl-seq. We produced 31 WGS, 20 WTS, and 20 Methyl-seq data, with average read depths of 43X, 53X, and 131X, respectively. The percentage of sequenced reads mapped to the target region was 99.9%, 97.4%, and 75.6%, respectively for WGS, WTS, and Methyl-seq (Supplementary Table 1).
Point mutations of TP53 and EPHA7 are unique and mutually exclusive in CPC
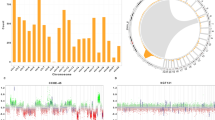
To identify the genetic variations responsible for CPT, we analyzed point mutations in the WGS data. Notably, rare TP53 and EPHA7 variants were discovered only in CPCs (Fig. 1a, Supplementary Table 2). Somatic or rare germline TP53 mutations occurred in 9 (81.9%) of 11 CPC patients; of these, eight were missense mutations and one was a nonsense mutation. Among them, one TP53 variant was a somatic mutation that was not detected in the matched blood sample, and the others were rare germline mutations with allele frequencies of less than 0.001 in healthy populations such as Korea1K [20], gnomAD [22], and 1000 Genomes Project [14]. Notably, all these variants were located in the p53 DNA binding domain. Six patients (including one patient with somatic mutation) had variants that were already reported as pathogenic or likely-pathogenic (c.743G > A, c.374C > A, c.652G > A, c.742C > T, and c.476C > T) [11, 25, 29]. Among them, one patient (CPC-1) was diagnosed with LFS with a multiple family history of cancer. Another patient (CPC-2) had a grandmother with breast cancer. Although no family history of cancer was revealed in the medical records of the other four patients, many of these cases might correspond to LFS or Li-Fraumeni-like syndrome. Previous studies have reported a significant association between TP53 mutations and the development of CPT, particularly CPC [36, 52, 57, 63]. In line with previous studies, TP53 mutations were observed only in CPC patients in our data. To predict the functional importance of each variant, we performed multiple sequence alignment (MSA). All seven variants occurred at highly conserved positions (Fig. 1b).
An overview of sample information and genetic events from WGS data. a Summary of sample information and genetic events of 20 CPT patients. b, c Multiple sequence alignment diagrams of regions where point mutations occurred within TP53 (b) and EPHA7 (c), respectively, in several vertebrate species. A red exclamation mark indicates a perfect match across all species examined. A yellow asterisk indicates an 80% or better match across species, but not a perfect match. A green asterisk indicates more than 50% and less than 80% match across species
Interestingly, CPC patients without TP53 mutations harbor rare EPHA7 mutations. EPHA7 has been identified as a tumor suppressor in various tumors including lymphoma, melanoma, tongue squamous cell carcinoma, and prostate cancer [17, 27, 30, 40, 54]. The two variants identified in this study have not been reported previously. Of the two patients with EPHA7 mutations, one patient was a long-term survivor, but the other had LMS at the time of diagnosis and died due to rapid progression of the disease. To predict the potential impact associated with the mutations in EPHA7, we also performed MSA using the EPHA7 sequences of several vertebrate species. The sites of the variants in EPHA7 were highly conserved, implying that the identified mutations in EPHA7 may impair its function (Fig. 1c).
Large-scale SCNA reveals distinct differences between CPP and CPC
To identify SCNAs in CPT, we analyzed the WGS data of CPT patients. Whole chromosome 12 gain was commonly observed in all subtypes. Whole chromosome 9 gain was repeatedly observed almost exclusively in CPP with 66.7% (4/6) of CPP samples and one CPC sample showing only chromosome 9p gain (Fig. 2a, b). Conversely, whole chromosome 1 gain was detected exclusively in CPC, with 72.7% (8/11) of CPC samples, but not in CPP. The observed whole chromosome 12 gain in CPC is concordant with previous research reporting that more than 70% of CPT patients had chromosome 12 gain [45, 56]. CPP-specific chromosome 9 gain and CPC-specific chromosome 1 gain were also confirmed in previous reports [45, 46, 56]. In addition, losses of chromosomes 16p, 17q, 19, and 22p were commonly found in CPP and CPC. There was no CPP-specific arm-level loss event. Chromosomes 17p, and 22q loss were detected specifically in CPC (Fig. 2c and Supplementary Fig. 1A, B).
There were 6 gains and 28 losses of focal SCNA cytobands commonly detected in both CPP and CPC samples (Supplementary Fig. 1C), which led to amplification of 2 known oncogenes (GNAS and PTK6) and deletion of 13 known tumor suppressor genes (ATM, B2M, BAZ1A, CBFB, CDH1, CLTC, CTCF, KNL1, N4BP2, RHOA, RHOH, SETD2, and WT1). In addition, 15 gains and 43 losses of focal SCNA cytobands were detected uniquely in CPP samples, including 1 oncogene amplification (CCND2) and 12 tumor suppressor gene deletions (ARID1A, ARNT, ASXL1, BLM, BRIP1, CAMTA1, FES, KAT6B, NFKB2, RPL5, SUFU, and TET1). On the other hand, 15 gains and 12 losses of focal SCNA cytobands were specifically detected in CPC, including four specific oncogene amplifications (MAFB, MYCN, PLCG1, and SRC) and three tumor suppressor gene deletions (EP300, PRKAR1A, and RFWD3) (Fig. 2a, d, Supplementary Table 3).
CPC has higher intratumoral heterogeneity than CPP
To characterize the genetic clonal structure of CPT subtypes, clonality was predicted for 11 samples with matched blood normal samples based on somatic point mutations and SCNAs profiles. All CPP (n = 2) and aCPP (n = 1) patients were predicted to have one clone, but 62.5% (5 out of 8) of CPC patients (n = 8) were predicted to have two or more clones, with an average of 1.75 clusters (Fig. 1a). Moreover, there was a significant difference in the number of clones between CPP/aCPP and CPC (p = 0.019, Supplementary Fig. 2), suggesting more vigorous tumor evolution in CPC than CPP.
Cell cycle- and epithelial-mesenchymal transition-related genes are overexpressed in CPC
To identify genes with different expression levels in CPP and CPC, we performed DEG analysis based on expression data, excluding aCPP and mCPP. There were 2,262 genes that showed a significant difference (adjusted P value < 0.01, absolute log2 (fold change) > 1) in expression levels between the histological subtypes, of which 1,288 genes were upregulated, and 974 genes were downregulated in CPC (Fig. 3a). We performed a hierarchical clustering analysis based on the expression of these DEGs and confirmed the clustering according to the CPP and CPC subtypes (Fig. 3b). The gene expression profiles of aCPP and mCPP were intermediate between those of CPP and CPC. The expression pattern of aCPP resembled that of the CPCs, while that of mCPP was more similar to that of the CPPs.
Expression profile of DEGs between the subtypes of CPT. a Volcano plot showing genes that are significantly up- and downregulated between CPP and CPC. b Heatmap of DEGs between CPP and CPC. Hierarchical clustering was performed by gene (column) and sample (row) according to expression pattern. The top of the figure is colored according to the pathway to which the gene belongs. c Enrichment test of significant DEGs and their associated pathways
Additionally, we performed clustering analysis of the DEGs above using the PCA method, and the resulting clusters showed distinct expression profiles by histological subtype (Supplementary Fig. 3A).
Enrichment analysis of the lists of genes upregulated and downregulated between the two histological subtypes was performed. The gene sets significantly upregulated in CPC were associated with the ‘G2-M Checkpoint’, ‘E2F Targets’, ‘Epithelial-Mesenchymal Transition’ and ‘Mitotic Spindle’ pathways (Fig. 3c). The pathway analysis results identified characteristics associated with cell division and migration, indicating the malignant features of CPC. In contrast, the gene set downregulated in CPC was not meaningfully enriched in any pathways.
There were 141 genes that were included in both the significant SCNAs and the DEGs of CPP and CPC. The enrichment analysis of these genes revealed that the ‘G2-M Checkpoint’ pathway was the most significant. Of the 13 genes in this pathway, 10 of them, including CDC20, were located on chromosome 1 (Supplementary Table 4). To confirm the expression levels of CDC20 in CPP and CPC, we performed RT-qPCR. The expression level of CDC20 was significantly higher in the CPC tissue samples than in the CPP samples. Primary cultured cells could not be tested for statistical significance because the number of samples for CPP is less than 3, but there still seems to be a notable difference in the expression levels (Supplementary Fig. 3B). In a previous study, RAD54L, TAF12, and NFYC, which are co-located on human chromosome 1p, were shown to be involved in the initiation and proliferation of CPC [57]. However, in our data RAD54L was the only one of these three genes to satisfy the threshold for classification as a DEG (Supplementary Fig. 3C, Supplementary Table 5).
All CPC patients with LMS died, whereas CPC patients without LMS remain alive. LMS was therefore the defining factor in determining the survival outcome of CPC. For this reason, we also performed DEG analysis of CPC patients based on LMS status. Six genes (HLA-DRA, TMEM265, DDTL, L1TD1, GABBR1, and LRP2) showed significantly different expression levels depending on LMS status (-log10 (padj) > 5, Fig. 4A). HLA-DRA and LRP2 were downregulated in the group with LMS, while TMEM265, DDTL, L1TD1, and GABBR1 were upregulated in the group with LMS (Supplementary Table 6). In particular, L1TD1 and GABBR1 have been previously associated with tumor metastasis and progression in other cancers. L1TD1 is highly expressed in seminoma, embryonal carcinoma, and medulloblastoma cancer cells, and its upregulation is associated with poor clinical outcome and metastasis of medulloblastoma. L1TD1 knockdown results in downregulation of pluripotency factors and reduced proliferation, as well as decreased migration and invasion capacity of medulloblastoma cells [39, 47]. There is accumulating evidence implicating GABBR1 in the cancer cell growth and metastasis of high-grade chondrosarcoma, breast cancer, and renal cell carcinoma [18, 21, 59]. Furthermore, previous methylation analysis studies of CPT have demonstrated that GABA receptor signaling is the most enriched pathway in CPC [43]. We further validated the differential expression of those genes in tissues through RT-qPCR. Among the six genes, five genes (LRP2, TMEM265, DDTL, L1TD1, and GABBR1) showed concordant expression patterns, and TMEM265 showed a statistically significant difference in its expression levels (Fig. 4b).
Expression profile of DEGs between LMS (−) and LMS (+) CPC. a Volcano plot showing genes that are significantly up- and downregulated depending on LMS status in CPC. b PCR validation of DEGs that are significantly up- and downregulated between LMS (−) and LMS (+) CPC. Significant differences between groups are indicated by asterisks. *P < 0.05, **P < 0.01, ***P < 0.001 as calculated by the Wilcoxon rank-sum test
CPC and CPP show differential methylation patterns in repeat regions
To identify distinct methylation patterns in CPP and CPC, we performed a DMS analysis. There were 2,139 significantly different CpG sites (-log Q value > 40, absolute differential methylation value > 55), including 1,709 hypermethylation and 430 hypomethylation sites. When clustering was performed with CpG sites with significant methylation differences, two clusters formed, one for each CPT subtype (Fig. 5a, b). In addition, aCPPs were clustered with CPCs, but mCPP was grouped with CPPs. In PCA with the corresponding sites, samples of the same subtype of CPT clustered closely together (Supplementary Fig. 4A).
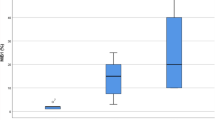
Methylation profile of DMSs between CPT subtypes. a Volcano plot showing sites that are significantly hyper- and hypomethylated sites between CPP and CPC. b Heatmap of DMSs between CPP and CPC. Hierarchical clustering is performed by CpG site (column) and sample (row) according to the methylation pattern. The top of the figure indicates the genomic regions in which each site is located. c Boxplot of the degree of methylation in four types of repeat regions, grouped by CPT subtype. Significant differences between groups are indicated by asterisks.: *P < 0.05, **P < 0.01 as calculated by t test. d Copy number ratio, expression, and methylation of AK1 in different CPT subtypes. Significant differences between groups are indicated by asterisks.: *P < 0.05, **P < 0.01, ***P < 0.001 as calculated by the Wilcoxon rank-sum test
We examined differences in methylation according to genomic region, including protein-coding regions, cis-regulatory elements, and repeat regions. There was no significant difference in the degree of methylation in the genomic regions except for the repeat regions (Supplementary Fig. 4B). Interestingly, the degree of methylation in major repeat regions such as long interspersed nuclear elements (LINEs), short interspersed nuclear elements (SINEs), long terminal repeats (LTRs), and DNA transposon regions was significantly lower in CPC than in CPP (Fig. 5c).
In the promoter region, we identified 746 hypermethylation sites involving 112 genes and 332 hypomethylation sites involving 83 genes. Notably, we found that the promoter regions of p53-related genes were hypermethylated, while those related to angiogenesis were hypomethylated in CPC. There were only significantly enriched pathways in each methylation status. Specifically, AK1, KIF13B, PLXNB2, and RALGDS were found to be involved in the ‘p53 pathway’, whereas PDGFA and OLR1 were involved in ‘angiogenesis’. These results suggest the tumorigenic characteristics of CPC. (Supplementary Fig. 4C).
The differential expression of AK1 driven by genomic and epigenomic factors in CPP
To identify the genes whose expression was affected by methylation, we compared the gene lists from the DMS analysis and the DEG analysis. As a result, we discovered four overlapping genes (FABP3, PDE6G, FAM24B, and CNN3) between the hypomethylated and upregulated genes and 16 overlapping genes (SYNE1, APOBEC4, SLC20A2, GAS2L2, AL031710.1, AC027449.1, AK1, AKNA, SPAG8, RALGDS, TCTEX1D4, AP000842.2, MIR600HG, DDO, TRIM29, and FAM102A) between the hypermethylated and downregulated genes.
Interestingly, among the genes identified through the DMS pathway analysis, AK1 showed significant differences in its expression level (Fig. 5d). Previous studies have identified AK1 as a methylation signature in CPC, and its downregulation is documented not only in CPC but also in various other cancer types [19, 43, 58]. Additionally, recent research on colorectal cancer has revealed an increased expression of the adenylate kinase (AK) genes in benign polyps, followed by a decrease in expression levels of AK genes in cancer [44]. Although CPP does not seem to develop into CPC, the significant difference in expression of AK1 may cause the differences in histological characteristics between the two CPT subtypes. We observed that the amplification of chromosome 9 resulted in the increased copy number of AK1 in CPP. Therefore, we hypothesized that both the copy number gain and the hypomethylation of AK1 in CPP may have driven the overexpression of AK1, proposing that AK1 be one of the candidates driving the difference between CPC and CPP.
Discussion
The primary objective of this study was to investigate the genomic and epigenomic characteristics of CPT and discern the differences between CPP and CPC. To achieve this, we conducted comprehensive multiomics analyses of 20 pediatric CPT patients, employing NGS techniques, including WGS, WTS, and Methyl-seq.
Our findings revealed distinctive TP53 alterations in CPC, along with novel EPHA7 point mutations specifically in TP53-wild-type CPC. Both genes, recognized for their tumor suppressor roles, may play a crucial role in the pathogenesis of CPC. TP53 mutations in CPC have been reported in several CPT studies [36, 52, 63]. On the other hand, EPHA7 has not been studied in choroid plexus tumors but has been identified as a tumor suppressor in various other cancers [17, 27, 30, 40, 54]. Our study implies that mutation of EPHA7 may have resulted in the loss of its tumor suppressor properties and the promotion of carcinoma progression in choroid plexus tumor. However, further research is required to understand the specific mechanism of the anti-tumor effect of wild-type EPHA7 and the oncogenesis of mutant EPHA7 in choroid plexus tumors. Due to small numbers of patients with an EPHA7 mutation, it is difficult to determine the association between the EPHA7 mutation and the prognosis of CPC. Among the two patients with an EPHA7 mutation, one patient is a long-term survivor, but the other had LMS at the time of diagnosis and died of rapid progression of disease.
SCNAs at the arm level emerged as characteristic features for each CPT subtype. Chromosome 12 gain was prevalent in CPT patients, while chromosome 9 and chromosome 1 gains were exclusively associated with CPP and CPC, respectively. Our findings suggest that SCNAs play an important role in CPT development and progression. Kaishi Satomi et al., although not analyzing CPT patients, observed that chromosome 12 gain is the most frequently copy number alteration in central nervous system germ cell tumors [48]. CPP-specific chromosomal 9 gain and CPC-specific chromosomal 1 gain were also previously reported [45, 46, 56]. Chromosome 9 gain has been associated with good survival in CPC [45]. However, chromosome 9 gain was mainly not observed in CPC whereas it was identified dominantly in CPP patients in our results. One CPC patient with chromosome 9 gain had LMS and expired due to disease progression.
Furthermore, a significantly higher number of clones were observed in CPC compared to CPP, indicating a higher intratumoral heterogeneity. As far as we know, this is the first analysis to perform clonality analysis of CPT with WGS data. We showed that significantly more clusters were predicted in CPC than in CPP, and these results may reflect the tendency for relapse and therapeutic resistance in CPC.
Notably, overexpression of cell cycle-related genes located on chromosome 1 was identified in CPC, contributing to carcinoma characteristics. According to a previous study, some genes located on chromosome 1p were involved in the initiation and proliferation of CPC, but our study found that some of these genes did not satisfy our thresholds in the DEG analysis between CPP and CPC [57]. Especially, DEG analysis between CPC patients with different LMS status showed that CPC patients with LMS had significantly higher expression levels of genes including L1TD1 and GABBR1, which are known to be associated with tumor migration and invasion in other solid tumors [4, 8, 15, 42, 47, 49, 60, 64]. RT-qPCR experiments confirmed that the expression patterns of DEGs between LMS + and LMS- CPCs were generally concordant with WTS results although only TMEM265 showed statistical significance. This may be due to the small sample size used in the experiments.
Additionally, to the best of our knowledge, our study is the first to report hypomethylation in various types of repeat regions in CPC, implying the potential influence of the loss of epigenetic silencing of transposable elements on CPC development. We also highlighted the differential expression of AK1 as a distinguishing factor between CPP and CPC, potentially influenced by both genomic and epigenomic factors. Downregulation of AK1 has been reported in several cancers [19, 58], and hypermethylation and downregulation of the AK1 promoter region in choroid plexus tumors have already been reported [43]. Our results are consistent with this, and furthermore, our analysis suggests that AK1 upregulation in CPP compared to CPC is induced by the amplification of chromosome 9 as well as the hypomethylation of AK1.
Although aCPP and mCPP have generally intermediate properties based on their gene expression and methylation profiles, a closer look at the clustering results revealed that aCPP was closer to CPC while mCPP was more similar to CPP. However, due to the small sample size, the results should be interpreted with caution.
There are some limitations in this study, including a relatively small sample size due to the rarity of pediatric CPT. Continuous efforts to secure additional samples from multiple centers are crucial. Additionally, there was a lack of age-matched normal choroid plexus tissues and normal blood samples for some patients limited our ability to conduct a comprehensive comparison. To address this, we utilized large-scale healthy population-based variant databases as controls.
Conclusions
In conclusion, our comprehensive multiomics analysis of 20 pediatric CPT patients using various NGS techniques provides valuable insights into the difference of CPT subtypes. We suggest that the difference between CPP and CPC arise during the initial stages of progression and develop benign and malignant characteristics, respectively (Fig. 6). The identified genomic and epigenomic characteristics could contribute to the understanding of CPT pathogenesis. In addition, this study may provide insight for the development of novel therapeutic strategies aimed at addressing the specific molecular intricacies associated with CPT.
Availability of data and materials
All the raw data generated and used in this study have been deposited in the Sequence Read Archive (SRA, https://www.ncbi.nlm.nih.gov/sra) under the submission ID, PRJNA1107711 (link for reviewer: https://dataview.ncbi.nlm.nih.gov/object/PRJNA1107711?reviewer=njv4q0jujhbdcrf50sanjikgsj). We have also deposited our processed data at GEO (WTS: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE267874, BisulfiteSeq: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE267876).
Abbreviations
- CPT:
-
Choroid plexus tumor
- CPP:
-
Choroid plexus papilloma
- aCPP:
-
Atypical choroid plexus papilloma
- mCPP:
-
Mixed CPP and papillary ependymoma
- CPC:
-
Choroid plexus carcinoma
- LFS:
-
Li-Fraumeni syndrome
- NGS:
-
Next-generation sequencing
- WGS:
-
Whole-genome sequencing
- WTS:
-
Whole-transcriptome sequencing
- Methyl-seq:
-
Methylation sequencing
- LMS:
-
Leptomeningeal seeding
- GATK:
-
Genome analysis toolkit
- SNV:
-
Single nucleotide variation
- INDEL:
-
Insertion and deletion
- SCNA:
-
Somatic copy number alteration
- GISTIC:
-
Genome identification of significant targets in cancer
References
Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE (2012) methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol 13:R87. https://doi.org/10.1186/gb-2012-13-10-r87
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT et al (2000) Gene ontology: tool for the unification of biology. The gene ontology. Consort Nat Genet 25:25–29. https://doi.org/10.1038/75556
Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, Belotti M, Gauthier-Villars M, Stoppa-Lyonnet D, Consolino E, Brugieres L et al (2015) Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol 33:2345–2352. https://doi.org/10.1200/JCO.2014.59.5728
Chakroborty D, Emani MR, Klen R, Bockelman C, Hagstrom J, Haglund C, Ristimaki A, Lahesmaa R, Elo LL (2019) L1TD1—a prognostic marker for colon cancer. BMC Cancer 19:727. https://doi.org/10.1186/s12885-019-5952-2
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A (2013) Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform 14:128. https://doi.org/10.1186/1471-2105-14-128
Choi SA, Koh EJ, Kim RN, Byun JW, Phi JH, Yang J, Wang KC, Park AK, Hwang DW, Lee JY et al (2020) Extracellular vesicle-associated miR-135b and -135a regulate stemness in Group 4 medulloblastoma cells by targeting angiomotin-like 2. Cancer Cell Int 20:558. https://doi.org/10.1186/s12935-020-01645-6
Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM et al (2021) Twelve years of SAM tools and BCF tools. Gigascience. https://doi.org/10.1093/gigascience/giab008
Dunne MR, Michielsen AJ, O’Sullivan KE, Cathcart MC, Feighery R, Doyle B, Watson JA, O’Farrell NJ, Ravi N, Kay E et al (2017) HLA-DR expression in tumor epithelium is an independent prognostic indicator in esophageal adenocarcinoma patients. Cancer Immunol Immunother 66:841–850. https://doi.org/10.1007/s00262-017-1983-1
Erickson N, Mueller J, Shirkov A, Zhang H, Larroy P, Li M, Smola A (2020) Autogluon-tabular: robust and accurate automl for structured data. arXiv:200306505
Espinet E, Gu Z, Imbusch CD, Giese NA, Buscher M, Safavi M, Weisenburger S, Klein C, Vogel V, Falcone M et al (2021) Aggressive PDACs show hypomethylation of repetitive elements and the execution of an Intrinsic IFN program linked to a ductal cell of origin. Cancer Discov 11:638–659. https://doi.org/10.1158/2159-8290.CD-20-1202
Fortuno C, Lee K, Olivier M, Pesaran T, Mai PL, de Andrade KC, Attardi LD, Crowley S, Evans DG, Feng BJ et al (2021) Specifications of the ACMG/AMP variant interpretation guidelines for germline TP53 variants. Hum Mutat 42:223–236. https://doi.org/10.1002/humu.24152
Frankish A, Diekhans M, Jungreis I, Lagarde J, Loveland JE, Mudge JM, Sisu C, Wright JC, Armstrong J, Barnes I et al (2021) Gencode 2021. Nucl Acids Res 49:D916–D923. https://doi.org/10.1093/nar/gkaa1087
Gene Ontology C (2021) The gene ontology resource: enriching a GOld mine. Nucleic Acids Res 49:D325–D334. https://doi.org/10.1093/nar/gkaa1113
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA et al (2015) A global reference for human genetic variation. Nature 526:68–74. https://doi.org/10.1038/nature15393
Gonias SL, Karimi-Mostowfi N, Murray SS, Mantuano E, Gilder AS (2017) Expression of LDL receptor-related proteins (LRPs) in common solid malignancies correlates with patient survival. PLoS ONE 12:e0186649. https://doi.org/10.1371/journal.pone.0186649
Guha T, Malkin D (2017) Inherited TP53 mutations and the Li-Fraumeni syndrome. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a026187
Guo Y, Shi W, Fang R (2021) miR-18a-5p promotes melanoma cell proliferation and inhibits apoptosis and autophagy by targeting EPHA7 signaling. Mol Med Rep. https://doi.org/10.3892/mmr.2020.11717
Huang D, Alexander PB, Li QJ, Wang XF (2023) GABAergic signaling beyond synapses: an emerging target for cancer therapy. Trends Cell Biol 33:403–412. https://doi.org/10.1016/j.tcb.2022.08.004
Jan YH, Lai TC, Yang CJ, Huang MS, Hsiao M (2019) A co-expressed gene status of adenylate kinase 1/4 reveals prognostic gene signature associated with prognosis and sensitivity to EGFR targeted therapy in lung adenocarcinoma. Sci Rep 9:12329. https://doi.org/10.1038/s41598-019-48243-9
Jeon S, Bhak Y, Choi Y, Jeon Y, Kim S, Jang J, Jang J, Blazyte A, Kim C, Kim Y et al (2020) Korean Genome Project: 1094 Korean personal genomes with clinical information. Sci Adv 6:eaaz7835. https://doi.org/10.1126/sciadv.aaz7835
Kanbara K, Otsuki Y, Watanabe M, Yokoe S, Mori Y, Asahi M, Neo M (2018) GABA(B) receptor regulates proliferation in the high-grade chondrosarcoma cell line OUMS-27 via apoptotic pathways. BMC Cancer 18:263. https://doi.org/10.1186/s12885-018-4149-4
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alfoldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP et al (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581:434–443. https://doi.org/10.1038/s41586-020-2308-7
Krueger F, Andrews SR (2011) Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27:1571–1572. https://doi.org/10.1093/bioinformatics/btr167
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A et al (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucl Acids Res 44:W90-97. https://doi.org/10.1093/nar/gkw377
Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Jang W et al (2018) ClinVar: improving access to variant interpretations and supporting evidence. Nucl Acids Res 46:D1062–D1067. https://doi.org/10.1093/nar/gkx1153
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform 12:323. https://doi.org/10.1186/1471-2105-12-323
Li D, Xiang B, Ying XX, Ying XX, Dong H (2014) Correlation analysis of EphA7 expression with clinico-pathological parameters and prognosis in tongue squamous cell carcinoma. Shanghai Kou Qiang Yi Xue 23:575–579
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25:1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, Tsimberidou AM, Vnencak-Jones CL, Wolff DJ, Younes A et al (2017) Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the association for molecular pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn 19:4–23. https://doi.org/10.1016/j.jmoldx.2016.10.002
Li S, Wu Z, Ma P, Xu Y, Chen Y, Wang H, He P, Kang Z, Yin L, Zhao Y et al (2017) Ligand-dependent EphA7 signaling inhibits prostate tumor growth and progression. Cell Death Dis 8:e3122. https://doi.org/10.1038/cddis.2017.507
Li Y, Liu H, Li T, Feng J, He Y, Chen L, Li C, Qiu X (2021) Choroid plexus carcinomas with TP53 germline mutations: management and outcome. Front Oncol 11:751784. https://doi.org/10.3389/fonc.2021.751784
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M et al (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. https://doi.org/10.1101/gr.107524.110
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F (2016) The Ensembl variant effect predictor. Genome Biol 17:122. https://doi.org/10.1186/s13059-016-0974-4
Merino DM, Shlien A, Villani A, Pienkowska M, Mack S, Ramaswamy V, Shih D, Tatevossian R, Novokmet A, Choufani S et al (2015) Molecular characterization of choroid plexus tumors reveals novel clinically relevant subgroups. Clin Cancer Res 21:184–192. https://doi.org/10.1158/1078-0432.CCR-14-1324
Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G (2011) GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol 12:R41. https://doi.org/10.1186/gb-2011-12-4-r41
Naeini RM, Yoo JH, Hunter JV (2009) Spectrum of choroid plexus lesions in children. AJR Am J Roentgenol 192:32–40. https://doi.org/10.2214/ajr.08.1128
Narva E, Rahkonen N, Emani MR, Lund R, Pursiheimo JP, Nasti J, Autio R, Rasool O, Denessiouk K, Lahdesmaki H et al (2012) RNA-binding protein L1TD1 interacts with LIN28 via RNA and is required for human embryonic stem cell self-renewal and cancer cell proliferation. Stem Cells 30:452–460. https://doi.org/10.1002/stem.1013
Oricchio E, Nanjangud G, Wolfe AL, Schatz JH, Mavrakis KJ, Jiang M, Liu X, Bruno J, Heguy A, Olshen AB et al (2011) The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell 147:554–564. https://doi.org/10.1016/j.cell.2011.09.035
Phi JH, Choi SA, Kim SK, Wang KC, Lee JY, Kim DG (2015) Overcoming chemoresistance of pediatric ependymoma by inhibition of STAT3 signaling. Transl Oncol 8:376–386. https://doi.org/10.1016/j.tranon.2015.08.001
Piao XM, Kang HW, Jeong P, Byun YJ, Lee HY, Kim K, Seo SP, Kim WT, Lee JY, Ha YS et al (2021) A prognostic immune predictor, HLA-DRA, plays diverse roles in non-muscle invasive and muscle invasive bladder cancer. Urol Oncol 39:237.e21-237.e29. https://doi.org/10.1016/j.urolonc.2020.11.017
Pienkowska M, Choufani S, Turinsky AL, Guha T, Merino DM, Novokmet A, Brudno M, Weksberg R, Shlien A, Hawkins C et al (2019) DNA methylation signature is prognostic of choroid plexus tumor aggressiveness. Clin Epigenet 11:117. https://doi.org/10.1186/s13148-019-0708-z
Rebane-Klemm E, Reinsalu L, Puurand M, Shevchuk I, Bogovskaja J, Suurmaa K, Valvere V, Moreno-Sanchez R, Kaambre T (2023) Colorectal polyps increase the glycolytic activity. Front Oncol 13:1171887. https://doi.org/10.3389/fonc.2023.1171887
Rickert CH, Wiestler OD, Paulus W (2002) Chromosomal imbalances in choroid plexus tumors. Am J Pathol 160:1105–1113. https://doi.org/10.1016/S0002-9440(10)64931-0
Ruland V, Hartung S, Kordes U, Wolff JE, Paulus W, Hasselblatt M (2014) Choroid plexus carcinomas are characterized by complex chromosomal alterations related to patient age and prognosis. Genes Chromosomes Cancer 53:373–380. https://doi.org/10.1002/gcc.22148
Santos MC, Silva PB, Rodini CO, Furukawa G, Marco Antonio DS, Zanotto-Filho A, Moreira JC, Okamoto OK (2015) Embryonic stem cell-related protein L1TD1 is required for cell viability, neurosphere formation, and chemoresistance in medulloblastoma. Stem Cells Dev 24:2700–2708. https://doi.org/10.1089/scd.2015.0052
Satomi K, Takami H, Fukushima S, Yamashita S, Matsushita Y, Nakazato Y, Suzuki T, Tanaka S, Mukasa A, Saito N et al (2022) 12p gain is predominantly observed in non-germinomatous germ cell tumors and identifies an unfavorable subgroup of central nervous system germ cell tumors. Neuro Oncol 24:834–846. https://doi.org/10.1093/neuonc/noab246
Shi J-w, Huang Y (2019) Screen and classify genes on bladder cancer associated with metastasis. Gene Reports 16:100430
Sondka Z, Bamford S, Cole CG, Ward SA, Dunham I, Forbes SA (2018) The COSMIC cancer gene census: describing genetic dysfunction across all human cancers. Nat Rev Cancer 18:696–705. https://doi.org/10.1038/s41568-018-0060-1
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550. https://doi.org/10.1073/pnas.0506580102
Tabori U, Shlien A, Baskin B, Levitt S, Ray P, Alon N, Hawkins C, Bouffet E, Pienkowska M, Lafay-Cousin L et al (2010) TP53 alterations determine clinical subgroups and survival of patients with choroid plexus tumors. J Clin Oncol 28:1995–2001. https://doi.org/10.1200/JCO.2009.26.8169
Talevich E, Shain AH, Botton T, Bastian BC (2016) CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol 12:e1004873. https://doi.org/10.1371/journal.pcbi.1004873
Theocharis S, Klijanienko J, Giaginis C, Alexandrou P, Patsouris E, Sastre-Garau X (2014) Ephrin receptor (Eph) -A1, -A2, -A4 and -A7 expression in mobile tongue squamous cell carcinoma: associations with clinicopathological parameters and patients survival. Pathol Oncol Res 20:277–284. https://doi.org/10.1007/s12253-013-9692-3
Thomas C, Sill M, Ruland V, Witten A, Hartung S, Kordes U, Jeibmann A, Beschorner R, Keyvani K, Bergmann M et al (2016) Methylation profiling of choroid plexus tumors reveals 3 clinically distinct subgroups. Neuro Oncol 18:790–796. https://doi.org/10.1093/neuonc/nov322
Thomas C, Soschinski P, Zwaig M, Oikonomopoulos S, Okonechnikov K, Pajtler KW, Sill M, Schweizer L, Koch A, Neumann J et al (2021) The genetic landscape of choroid plexus tumors in children and adults. Neuro Oncol 23:650–660. https://doi.org/10.1093/neuonc/noaa267
Tong Y, Merino D, Nimmervoll B, Gupta K, Wang YD, Finkelstein D, Dalton J, Ellison DW, Ma X, Zhang J et al (2015) Cross-species genomics identifies TAF12, NFYC, and RAD54L as choroid plexus carcinoma oncogenes. Cancer Cell 27:712–727. https://doi.org/10.1016/j.ccell.2015.04.005
Vasseur S, Malicet C, Calvo EL, Dagorn JC, Iovanna JL (2005) Gene expression profiling of tumours derived from rasV12/E1A-transformed mouse embryonic fibroblasts to identify genes required for tumour development. Mol Cancer 4:4. https://doi.org/10.1186/1476-4598-4-4
Wei B, Zhu Y, Yang P, Han Y, Wang S, Wang X, Xia S, Song X, Zhang Z, Wang S et al (2021) GABA(B1e) promotes the malignancy of human cancer cells by targeting the tyrosine phosphatase PTPN12. iScience 24:103311. https://doi.org/10.1016/j.isci.2021.103311
Wu W, Chang F, Zhang J, Tang S, Chen F (2022) GABBR1 as a predictor of prognosis and its correlation with immune infiltration and dysfunction in clear cell renal cell carcinoma
Xiao Y, Wang X, Zhang H, Ulintz PJ, Li H, Guan Y (2020) FastClone is a probabilistic tool for deconvoluting tumor heterogeneity in bulk-sequencing samples. Nat Commun 11:4469. https://doi.org/10.1038/s41467-020-18169-2
Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, Lachmann A, Wojciechowicz ML, Kropiwnicki E, Jagodnik KM et al (2021) Gene set knowledge discovery with Enrichr. Curr Protoc 1:e90. https://doi.org/10.1002/cpz1.90
Zaytseva M, Valiakhmetova A, Yasko L, Samarin A, Papusha L, Shekhtman A, Usman N, Voronin K, Karachunskiy A, Novichkova G et al (2023) Molecular heterogeneity of pediatric choroid plexus carcinomas determines the distinctions in clinical course and prognosis. Neuro Oncol 25:1132–1145. https://doi.org/10.1093/neuonc/noac274
Zhao S, Dong X, Ni X, Li L, Lu X, Zhang K, Gao Y (2021) Exploration of a novel prognostic risk signature and its effect on the immune response in nasopharyngeal carcinoma. Front Oncol 11:709931. https://doi.org/10.3389/fonc.2021.709931
Acknowledgements
We thank the patients and their families for their participation. We also appreciate Prof. Eunjung Lee for helpful comments.
Funding
This work was supported by the Seoul National University Hospital Research Fund (03–20220260 to SK.K.) and SNUH Kun-hee Lee Child Cancer & Rare Disease Project, Republic of Korea (22A-017-0100 to SK.K.). This work is also supported by the Ulsan 10K Genomes Project (1.180017.01) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A3A13045998 to Y.C. and NRF-2018R1A6A1A03025810 to S.L.).
Author information
Authors and Affiliations
Contributions
These authors contributed equally: Yeonsong Choi, Seung Ah Choi, Eun Jung Koh, Ilsun Yun. SKK and SL conceptualized the study. Data were collected and curation of data was conducted by SAC, EJK, JHP, SHP, DSK, SHK, JWC, JWL, TYJ, SKK. Bioinformatics analyses were performed by YC, IY, SP, SP, DW. The original draft was written by YC, SAC, EJK, IY, SL, SKK, and all co-authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Choi, Y., Choi, S.A., Koh, E.J. et al. Comprehensive multiomics analysis reveals distinct differences between pediatric choroid plexus papilloma and carcinoma. acta neuropathol commun 12, 93 (2024). https://doi.org/10.1186/s40478-024-01814-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-024-01814-y