Abstract
Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal dominant epigenetic disorder with highly variable muscle involvement and disease progression. Ongoing clinical trials, aimed at counteracting muscle degeneration and disease progression in FSHD patients, increase the need for reliable biomarkers. Muscle magnetic resonance imaging (MRI) studies showed that the appearance of STIR-positive (STIR+) lesions in FSHD muscles represents an initial stage of muscle damage, preceding irreversible adipose changes. Our study aimed to investigate fibrosis, a parameter of muscle degeneration undetectable by MRI, in relation to disease activity and progression of FSHD muscles. We histologically evaluated collagen in FSHD1 patients’ (STIR+ n = 27, STIR− n = 28) and healthy volunteers’ (n = 12) muscles by picrosirius red staining. All patients (n = 55) performed muscle MRI before biopsy, 45 patients also after 1 year and 36 patients also after 2 years. Fat content (T1 signal) and oedema/inflammation (STIR signal) were evaluated at baseline and at 1- and 2-year MRI follow-up. STIR+ muscles showed significantly higher collagen compared to both STIR− (p = 0.001) and healthy muscles (p < 0.0001). STIR− muscles showed a higher collagen content compared to healthy muscles (p = 0.0194). FSHD muscles with a worsening in fatty infiltration during 1- (P = 0.007) and 2-year (P < 0.0001) MRI follow-up showed a collagen content of 3.6- and 3.7-fold higher compared to FSHD muscles with no sign of progression. Moreover, the fibrosis was significantly higher in STIR+ muscles who showed a worsening in fatty infiltration in a timeframe of 2 years compared to both STIR− (P = 0.0006) and STIR+ muscles with no sign of progression (P = 0.02). Fibrosis is a sign of muscle degeneration undetectable at MRI never deeply investigated in FSHD patients. Our data show that 23/27 of STIR+ and 12/28 STIR− muscles have a higher amount of collagen deposition compared to healthy muscles. Fibrosis is higher in FSHD muscles with a worsening in fatty infiltration thus suggesting that its evaluation with innovative non-invasive techniques could be a candidate prognostic biomarker for FSHD, to be used to stratify patients and to evaluate the efficacy of therapeutic treatments.
Similar content being viewed by others
Introduction
Facioscapulohumeral muscular dystrophy (FSHD) is the second most prevalent muscular dystrophy in the adult population, and affects approximately 870,000 people worldwide [1]. The most common form of the disease is type 1 (FSHD1), which is associated with a contraction of the D4Z4 macrosatellite repeat on the subtelomeric region of chromosome 4q35. This shortening, combined with a permissive allele, allows the inappropriate transcription of the double homeobox 4 (DUX4) gene, considered the primary drive for muscle degeneration in patients [2]. FSHD is a slowly progressive disease with an autosomal dominant inheritance, characterized by an extreme variability in age at onset and clinical severity, even in the same family, and by an unpredictable rate of disease progression in different muscles of a single individual [3, 4]. Approximately 20% of patients’ relatives remain asymptomatic although sharing the same FSHD-causing genetic background of the affected probands [3]. Muscle magnetic resonance imaging (MRI) has become the “gold-standard” technique to evaluate and follow muscle involvement and degeneration in FSHD patients [5, 6]. It is a non-invasive method able to detect fatty infiltration, which is a sign of terminal muscle degeneration, on T1 weighted (T1w) sequences, and oedema, which is a sign of inflammation, on short tau inversion recovery (STIR) sequences [7]. MRI studies in FSHD patients showed that muscle degeneration is an asynchronous process as apparently unaffected muscles coexist with affected muscles thus showing various degrees of fatty replacement in the same patient. Recent longitudinal studies pointed out that the appearance of STIR-positive (STIR+) lesions represents an initial stage of muscle damage, preceding and accelerating irreversible adipose changes [8, 9]. Therefore, STIR+ lesions are considered a biomarker of an active phase of the disease characterized by inflammation, faster progression towards fatty replacement, and higher likelihood to express DUX4 targets [9,10,11,12,13]. There is a wealth of literature about the interrelation among inflammation, the impairment of non-myogenic mesenchymal stromal cells and fibrosis in driving muscle degeneration in muscular disorders [14,15,16,17]. In FSHD muscles, the histological identification and characterization of the inflammatory changes dates back to 1995, and both initial and subsequent pathology studies showed the presence of intramuscular T and B lymphocytes [18,19,20]. Our group has recently demonstrated impaired in vitro properties of non-myogenic mesenchymal stromal cells (historically called fibroadipogenic progenitors) isolated from FSHD patients’ muscles [21]. These cells accumulate in FSHD STIR+ muscles and this expansion correlate to the progressive fibrous-fatty replacement of muscles [21]. These data were in agreement with the results obtained in a FSHD mouse model [22], suggesting a possible involvement of non-myogenic mesenchymal stromal cells in the pathogenesis of the disease. However, detailed studies investigating muscle fibrosis, another feature of dystrophic muscle degeneration, in FSHD patients are lacking. Precisely, collagen deposition was quantified and found increased in muscles of an inducible-DUX4 mouse model [22] and qualitatively described as a part of FSHD patients’ muscle myophatic changes [12, 20, 23]. Notably, current available MRI scanning protocols are unable to confidently assess muscular collagen deposition [7]. In our study, we aimed at investigating fibrosis in a large cohort of FSHD patients’ muscles, in relation to their radiological features, and to evaluate whether it could be a predictive marker of disease progression. Our data showed that 23/27 STIR+ and 12/28 STIR− FSHD muscles had increased collagen content compared to control muscles. In addition, the amount of fibrosis positively correlated with the lymphocyte infiltration and the progression in fatty replacement, thus distinguishing between progressive and non-progressive FSHD STIR+ muscles in a timeframe of two years.
Materials and methods
Patient enrollment and sample collection
We retrospectively analyzed muscle specimens from 55 genetically confirmed FSHD1 patients and 12 healthy controls (Additional file 1: Table S1). Controls were healthy volunteers, including unaffected relatives of FSHD patients, that were age-matched [mean age controls ± standard deviation (SD): 46 ± 16 years, mean age FSHD patients ± SD: 48 ± 14 years], and no past and current history of systemic inflammatory disease (Additional file 1: Table S1). All patients underwent muscle MRI before biopsy, according to previously published protocol [24]. Biopsy procedure was MRI-informed to include muscles with either normal (T1−) or hyperintense (T1+) signals on T1w sequences and muscles with either normal (STIR−) or hyperintense (STIR+) signals on STIR-sequences. A second and third muscle MRI, performed 12 and 24 months after biopsy, were available in 45 and 36 FSHD patients, respectively. Clinical severity was assessed at baseline by the 10-grade clinical severity scale (CSS) [25]. Conchotome muscle biopsy [26] was performed within 2 weeks from the first MRI scan after obtaining signed informed consent. Two neurologists with experience in muscle imaging (E. Ricci; S.B.) assessed all the scans independently. Baseline and follow-up T1w sequences were first evaluated to assign a semi-quantitative score of fatty infiltration of single muscles (named T1-score) using a 5-point semi-quantitative scale (0 = normal appearance; 1 = traces of increased signal intensity; 2 = increased signal intensity with beginning confluence in less than 50% of the muscle; 3 increased signal intensity in more than 50% of an examined muscle; 4 = the entire muscle is replaced by increased signal intensity) [27]. To detect more subtle changes not identified by the former assessment, the observers performed a direct visual comparison between the baseline and the follow-up scan of each patient on the same computer monitor as previously described [9]. Radiological progression in single muscles was defined as an increase in fatty replacement.
Skeletal muscle sections
Transverse skeletal muscle cryosections of healthy, FSHD STIR+ and FSHD STIR− muscles were obtained from fresh frozen muscle biopsies mounted in optimal cutting temperature compound (OCT 4583, Tissue-Tek®, Sakura) and frozen in isopentane (106056, Merck Millipore) cooled in liquid nitrogen. Seven μm sections were cut with a cryostat at − 25 °C and 2 different sections were processed in duplicates for each patient. The slides with muscle cryosections of the 3 aforementioned groups were used for picrosirius red staining. An immunohistochemistry analysis of inflammation was performed according to the amount of muscle specimens.
Picrosirius red staining
Collagen detection was performed using 0.1% picrosirius red solution (Sigma Direct Red 80 365548, Sigma Picric Acid Solution P6744) which stains collagens type I and III in red and muscle fibers in pale yellow. Slides were fixed in 4% paraformaldehyde (Sigma) for 10 min, washed twice in tap water for 5 min, and incubated for 1 h with 0.1% picrosirius red solution. Then, the slides were washed 3 times in water with 0.5% acetic acid (64197, J.T. Baker) for 5 min, dehydrated in ethanol (64175, VWR chemicals) and fixed in xylene (1330207, VWR chemicals). Coverslips were mounted with Eukitt mounting medium (SIC). Stained muscle sections were observed under the optical microscope Olympus BH-2 (Olympus Life Science) and non-overlapping 20× images covering the entire surface of each section were acquired with the software Jenoptik-Gryphax V1.1.10.6. The evaluation of fibrosis was performed with ImageJ software (1.53 version) by measuring the total collagen deposition (red) between the muscle fibers (soft yellow) with color subtraction and reported in percentage area [% of muscle occupied by collagen/field].
Immunohistochemistry
The slides were fixed in cold acetone (VWR chemicals) for 10 min and then washed 3 times in 1× Phosphate Buffered Saline (PBS, Gibco) for 5 min. The slides were incubated for 1 h at room temperature in 1× PBS with 5% of BSA (Bovine Serum Albumin, Sigma) blocking solution. The specific primary antibody [1:100 CD8 (Dako M7103), 1:500 CD4 (Dako M7310), 1:500 CD20 (Dako M0755), 1:50 BDCA1 (Clone AD5-8E7, Miltenyi Biotec)] was incubated overnight at 4 °C in PBS/1% BSA. After 3 washes in 1X PBS for 5 min, horseradish peroxidase-conjugated secondary antibody (MP-7405-15, VectorLaboratories) was added for 1 h at room temperature. Diaminobenzidine (Sigma) was used to reveal the secondary antibody. The slides were washed in tap and distilled water before being dehydrated in ethanol (64175, VWR chemicals), fixed in xylene (1330207, VWR chemicals) and coverslips were mounted with Eukitt mounting medium (SIC). Stained muscle sections were observed under an optical microscope Olympus BH-2 (Olympus Life Science) and 10× non-overlapping images covering the entire surface of each section were acquired with the software Jenoptik-Gryphax V1.1.10.6. The count of immune cell infiltrates (total, endomysial and perivascular immune cells) was performed on the aforementioned images, normalized per mm2 of analyzed surface area and reported as number of immune cells/mm2 of surface. The total count immune cell infiltrate was calculated only for the muscle specimens analyzed for the presence of all the listed immune markers.
Statistical analysis
Data were analyzed using GraphPad Prism software (8.4 version; San Diego, CA, USA) and were presented as mean ± SD. Statistical significance level was set for p values < 0.05. After checking for normality by the D’Agostino-Pearson omnibus test, differences in variables between groups were analyzed using Mann–Whitney test or Kruskal–Wallis test with Dunn’s post-hoc analysis. Correlations were estimated by Pearson’s correlation test.
Results
Fibrosis in FSHD patients’ muscles
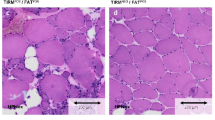
In order to obtain a comprehensive characterization of fibrosis in the context of dystrophic degeneration, we employed picrosirius red staining to quantify the deposition of collagen on muscle sections obtained from MRI-informed muscle biopsies in a cohort of 55 FSHD patients. Specimens were obtained from 27 muscles with (STIR+) and from 28 muscles without (STIR−) signs of disease activity. Data were compared to muscle sections obtained from 12 age-matched healthy volunteers. Information regarding patients, healthy controls, and sampled muscles were summarized in Additional file 1: Table S1. Collagen was significantly higher in FSHD muscles compared to control muscles (Fig. 1a, b), with FSHD STIR+ muscles showing the greatest amount of collagen deposition (Fig. 1a, b). FSHD muscles with (T1+) and without (T1−) MRI features of fatty infiltration did not show significant differences in the amount of intramuscular fibrosis (Fig. 1b), and the amount of fibrosis did not correlate with the T1-score of FSHD muscles at baseline (Fig. 1c). Considering the mean ± 2 standard deviation (SD) of collagen measured in healthy muscles as the upper normal limit threshold (3.6% of collagen/field), 85.2% (23/27) of FSHD STIR+ and 42.9% (12/28) of FSHD STIR− muscles displayed a higher-than-normal extent of collagen accumulation as index of fibrotic muscle degeneration (Table 1).
Collagen content of healthy and FSHD muscles. a Representative pictures of picrosirius red staining in healthy, FSHD STIR− and FSHD STIR+ muscle sections (20× magnification). b The graph shows the percentage of fibrosis per field in healthy (n = 12), FSHD STIR− (n = 28) and FSHD STIR+ (n = 27) muscles. The results are reported as mean ± SD and were compared using Kruskal–Wallis test (§§§§p < 0.0001) followed by Dunn’s test (*p < 0.05, ***p < 0.001, ****p < 0.0001). c Pearson correlation between the percentage of fibrosis and the MRI T1-score of sampled FSHD muscles
Composition of immune cell infiltrates in FSHD muscles
The presence of T and B lymphocytes and myeloid dendritic cells was assessed and compared among healthy, FSHD STIR− and FSHD STIR+ muscle sections by immunohistochemistry analysis (Fig. 2a). FSHD STIR+ muscles showed the highest count of CD4+ T lymphocytes (Fig. 2b), CD8+ T lymphocytes (Fig. 2c), CD20+ B lymphocytes (Fig. 2d) and BDCA1+ myeloid dendritic cells (Fig. 2e), compared to both FSHD STIR− and healthy muscles. FSHD STIR− muscles showed a slight not statistically significant increase in CD8+ T lymphocytes (Fig. 2c) and BDCA1+ myeloid dendritic cells (Fig. 2e) compared to healthy muscles. We then analyzed the immune cell infiltrates considering their localization between the muscle fibers (endomysial) or close to blood vessels (perivascular). CD4+ T lymphocytes were distributed equally between the endomysium and the vessels in healthy, FSHD STIR− and FSHD STIR+ muscles (Additional file 2: Fig. S1a). The number of CD8+ T lymphocytes was higher at the endomysium in all the muscles analyzed, compared with the perivascular site (Additional file 2: Fig. S1b). CD20+ B lymphocytes (Additional file 2: Fig. S1c) and BDCA1+ myeloid dendritic cells (Additional file 2: Fig. S1d) were found mostly at the endomysium in healthy and FSHD STIR− muscles while both cell populations were found equally distributed between the endomysium and perivascular regions in FSHD STIR+ muscles. To further analyze the differences in immune cells abundance in FSHD muscles, the total count of immune cells per sample was calculated as the sum of all immune cell infiltrates found in each sample (total immune cells/mm2). The mean ± 2SD total count of infiltrating immune cells in healthy muscles was used as the threshold (5.3 immune cells/mm2) to determine an upper control limit for muscle immune cell infiltrates. Over ninety percent (92.3%, 12/13) of FSHD STIR+ and 35.7% (5/14) of FSHD STIR− muscles showed an above threshold number of immune cells/mm2, and the former had a mean value of 3.9-fold higher compared to the latter (Table 2).
Evaluation of immune cell infiltrates in healthy and FSHD muscles. a Representative pictures of peroxidase staining of CD4+, CD8+, CD20+ and BDCA1+ cells in healthy, FSHD STIR− and FSHD STIR+ muscle sections (20× magnification). The graphs show the total distribution of CD4+ cells/mm2 (b) in healthy (n = 6), FSHD STIR− (n = 16) and FSHD STIR+ (n = 15) muscles, CD8+ cells/mm2 (c) in healthy (n = 6), FSHD STIR− (n = 14) and FSHD STIR+ (n = 15) muscles, CD20+ cells/mm2 (d) in healthy (n = 6), FSHD STIR− (n = 16) and FSHD STIR+ (n = 15) muscles and BDCA1+ cells/mm2 (e) in healthy (n = 6), FSHD STIR− (n = 17) and FSHD STIR+ (n = 15) muscles. The results are reported as mean ± SD. Groups were compared using Kruskal–Wallis test (§ < 0.05, §§ < 0.01, §§§p < 0.001) followed by Dunn’s multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001)
Evaluation of muscle fibrosis in relation to immune cell infiltrates and MRI fat degeneration
We then evaluated the possible correlation between collagen deposition and immune cell infiltration in FSHD muscles. The correlation analysis showed that the amount of collagen significantly correlated with the number of CD4+ T lymphocytes (Fig. 3a), CD8+ T lymphocytes (Fig. 3b) and CD20+ B lymphocytes (Fig. 3c), but not with the number of BDCA1+ myeloid dendritic cells (Fig. 3d). To assess the relationship between muscle fibrosis and progression of fatty replacement, the sampled muscles were evaluated by MRI at the 1- and 2-year follow-up visits in 45 and 36 FSHD patients, respectively. FSHD muscles which showed a worsening in fatty infiltration at 1 year MRI follow-up had a collagen content at baseline of 3.6-fold significantly higher compared to FSHD muscles without T1 MRI signs of progression (Fig. 4a). Likewise, FSHD muscles with a worsening in fatty infiltration at 2 year MRI follow-up showed a collagen content at baseline of 3.7-fold significantly higher compared to FSHD muscles without sign of progression on T1w sequences (Fig. 4b). The 1-year MRI follow-up showed that 41.7% (10/24) FSHD STIR+ muscles and 0% (0/21) FSHD STIR− muscles exhibited progression of fatty infiltration on T1w sequences (Additional file 1: Table S1). FSHD STIR+ muscles with progression showed a significantly higher amount of collagen at baseline compared to FSHD STIR− muscles and a moderate albeit not significant increase compared to FSHD STIR+ muscles without progression (Fig. 4c). The 2-year MRI follow-up showed that 83.3% (15/18) FSHD STIR+ and 0% (0/18) FSHD STIR− muscles exhibited changes in fatty infiltration on T1w sequences (Additional file 1: Table S1). FSHD STIR+ muscles with progression showed a significantly higher content of collagen at baseline, compared to both FSHD STIR− and FSHD STIR+ muscles without progression (Fig. 4D). Notably, seven of nine (78%) of the STIR+ muscles without progression at 1 year which showed an increased fat content during the second year of MRI follow-up (Fig. 4d, Additional file 1: Table S1 samples FSHD#35, FSHD#37, FSHD#42, FSHD#44, FSHD#47, FSHD#51, FSHD#56), had the highest value of collagen at baseline (above the mean value of 8% of this group) (Fig. 4c, Additional file 1: Table S1).
Muscle fibrosis and immune cell infiltration. Pearson correlations between the extent of fibrosis [fibrosis (%)/field] and the number of CD4+ (a), CD8+ (b), CD20+ (c) and BDCA1+ (d) cells in FSHD muscles. Pearson correlation coefficient (r value) and p value (p) are reported when statistically significant
Collagen content and MRI fat degeneration. The graphs show the percentage of fibrosis in FSHD muscles without and with changes in fatty infiltration on T1w sequences in a 1-year MRI follow-up (no progression, n = 35; with progression, n = 10) and in b 2-year MRI follow-up (no progression, n = 21; with progression, n = 15). The graphs show the percentage of fibrosis in FSHD STIR− muscles without (n = 21), FSHD STIR+ muscles without (n = 14) and with (n = 10) changes in fatty infiltration on T1w sequences in c 1-year MRI follow-up and d 2-year MRI follow-up [FSHD STIR− muscles without (n = 18); FSHD STIR+ muscles without (n = 3); with (n = 15) progression]. The results are reported as mean ± SD. Comparison between two groups were done using Mann–Whitney test (###p < 0.001, ####p < 0.0001). Groups were compared using Kruskal–Wallis test (§§§p < 0.001) followed by Dunn’s test (*p < 0.05, ***p < 0.001)
Immune cell infiltrates and fat degeneration in FSHD muscles
To investigate whether the presence of immune cell infiltrates correlated with the progression of muscle fat accumulation, we compared the number of T and B lymphocytes and myeloid dendritic cells between FSHD muscles with and without progression in fatty infiltration during the 1- and 2-year MRI follow-up. FSHD muscles with progression at the 1-year MRI follow-up showed a significantly high number of both BDCA1+ myeloid dendritic cells (Fig. 5a) and CD8+ T lymphocytes (Fig. 5b), but not of CD20+ B lymphocytes (Fig. 5c) and CD4+ T lymphocytes (Fig. 5d), compared to FSHD muscles without progression. FSHD muscles with progression at the 2-year MRI follow-up showed a significantly high number of BDCA1+ myeloid dendritic cells (Fig. 6a), CD8+ T lymphocytes (Fig. 6b) and CD20+ B lymphocytes (Fig. 6c), but not of CD4+ T lymphocytes (Fig. 6d), compared to FSHD muscles without progression.
Immune infiltrates and fat degeneration in FSHD muscles at 1 year. The graphs show the a BDCA1+ (without progression n = 24, with progression n = 4), b CD8+ (without progression n = 22, with progression n = 4), c CD20+ (without progression n = 23, with progression n = 4) and d CD4+ (without progression n = 23, with progression n = 4) cells/mm2 in FSHD muscle sections without (orange framed bar) and with (burgundy framed bar) changes in fatty infiltration on T1w sequences at the 1-year MRI follow-up. The results are reported as mean ± SD and were compared using Mann–Whitney test (*p < 0.05 **p < 0.01)
Immune infiltrates and fat degeneration in FSHD muscles at 2 years. The graphs show the a BDCA1+ (without progression n = 13, with progression n = 7), b CD8+ (without progression n = 12, with progression n = 8), c CD20+ (without progression n = 12, with progression n = 7) and d CD4+ (without progression n = 12, with progression n = 7) cells/mm2 in FSHD muscle sections without (orange framed bar) and with (burgundy framed bar) changes in fatty infiltration on T1w sequences at the 2-year MRI follow-up. The results are reported as mean ± SD and were compared using Mann–Whitney test (*p < 0.05 **p < 0.01)
Discussion
Here we performed a retrospective cohort study focused on the evaluation of fibrosis in FSHD patients’ muscles with the final aim of identifying a candidate tissue biomarker with predictive value on muscle degeneration in FSHD patients. In recent years, MRI has turned out to be more sensitive than clinical tests in describing muscle involvement in muscular dystrophies, including FSHD, contributing to the comprehension of disease progression in single muscles [5,6,7,8,9, 11, 24]. However, differently from fatty replacement (a sign of terminal muscle degeneration determined by assessing the T1w sequences) and oedema/inflammation (a biomarker of disease activity in FSHD muscles with a prognostic value, evaluated by the presence of hyperintense signal on STIR-sequences), fibrosis, another relevant feature of muscle degeneration [28], cannot be determined by the commonly used MRI protocols. A link between the primary muscle disease and fibrosis was established years ago in muscular dystrophies [29] especially in Duchenne Muscular Dystrophy (DMD) [30]. Indeed, where fibrosis was found as the only myopathologic parameter significantly correlated with poor motor outcome, as evaluated in 10 year clinical follow-up study of DMD patients [31]. An impaired muscle regeneration potential is a complex process accountable for a dysregulation among myoblasts, satellite cells, non-satellite cells with myogenic potential, non-myogenic mesenchymal stromal cells and immune cells within the muscle niche [14,15,16,17]. In a FSHD mouse model, the inducible expression of DUX4 caused a high pro-fibrotic state of muscles associated to an expansion of fibro-adipogenic progenitors and an increased inflammatory response [22, 32]. Accordingly, we have recently demonstrated a positive correlation between the expansion of non-myogenic mesenchymal cells and the amount of fibrosis in FSHD patients’ muscles [21] although an association of this finding with an altered DUX4 signature has not been clarified yet. Until now, however, the correlation between the disease progression (in terms of MRI fatty infiltration), collagen deposition (as an indicator of fibrosis), and immune cell infiltration has not been established for FSHD patients. In this study, we histologically determined the extent of fibrosis in a large cohort of FSHD muscles by evaluating their collagen content. Our data showed that 85.2% of FSHD STIR+ and 42.9% of FSHD STIR− muscles had higher collagen deposition compared to healthy muscles. Moreover, the evaluation of fibrosis in relation to MRI progression of fatty infiltration at the 1- and 2-year follow-up showed that the extent of collagen positively correlated with fatty replacement of FSHD muscles. These results may suggest that, as observed in other muscular dystrophies [29,30,31], an excessive collagen deposition is a primary event that precedes muscle degeneration also for the FSHD. Moreover, fibrosis proved to be an independent prognostic biomarker of muscle degeneration in FSHD because it was able to distinguish between progressive and not progressive FSHD STIR+ muscles in a timeframe of 2 years. A limitation to this study could be the different numbers of the two categories of muscles analyzed [not progressive STIR+ muscles (n = 3) versus progressive STIR+ muscles (n = 15), respectively], although they significantly differ in the extent of fibrosis. There are numerous publications supporting the interdependence between inflammatory response and fibrosis [33, 34], even in muscular dystrophies [35], although it has to be deepened for FSHD. We evaluated immune infiltration in FSHD muscles and consistently with previous reports [3, 13, 18, 19], we found that the most prevalent immune cell infiltrates in our cohort of FSHD muscles were T lymphocytes (both CD4+ and CD8+), and the FSHD STIR+ muscles presented the highest amount of immune cells infiltrating the tissue. We observed a low-grade of immune cell recruitment in 5/14 FSHD STIR− muscles. The total immune cells found in the 5 FSHD STIR− muscles differed to the ones observed in FSHD STIR+ muscles (50.1 immune cells/mm2 compared to 12.9 immune cells/mm2, respectively) and in spite of this low-grade immune cell recruitment, the 5 FSHD STIR− muscles did not show MRI signs of disease progression in the 2-year timeframe, as the other FSHD STIR− muscles analyzed. In line with these findings, a recent RNA-sequencing study demonstrated the upregulation of inflammatory genes in both STIR+ and STIR− FSHD muscle biopsies, even in the absence of DUX4 and its target genes expression [12]. In the light of different studies supporting the causative role of inflammation in driving fibrosis in skeletal muscle [28, 36, 37], we evaluated a possible correlation among the immune cells found in FSHD muscles and the amount of fibrosis. Although the specific interrelation between immune cells and fibrosis in FSHD remains to be investigated, we observed a positive correlation between fibrosis and the presence of T and B lymphocytes, suggesting a possible specific role of these infiltrates in stimulating the accumulation of collagen in FSHD muscles like described in DMD [35, 38]. However, the observed inflammatory patterns in FSHD share similarities with other muscular dystrophies [35, 39–42] raising the possibility that a mechanism similar to what is seen in inflammatory idiopathic myopathies could also be occurring in FSHD. In the context of B lymphocytes, although there is no direct evidence regarding their action in FSHD, infiltrating plasma cells have been observed in patients with polymyositis [43]. Furthermore previous studies have reported in FSHD the existence of sarcolemmal complement deposits in non-necrotic muscle fibers [44], an increased expression of complement system proteins [10] and elevated levels of circulating inflammatory cytokines [19] but we did not extensively investigate the presence of specific inflammatory subsets or the activation of the complement system because our interest focused on investigating the immune cell infiltration in relation to fibrosis.
In conclusion, this study introduces a promising new biomarker in FSHD, besides MRI-derived parameters [45]. Here we show that muscle fibrosis, a parameter undetectable by qualitative MRI, has a predictive value towards muscle degeneration in FSHD muscles, regardless of the STIR signal. Although current clinical trials for FSHD utilize skeletal muscle biopsies to evaluate therapy efficacy [46], we hope our study will increase the interest in fibrosis in FSHD. This, in turn, may accelerate the development and the adoption of strategies to non-invasively detect and quantify fibrosis in FSHD patients.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its Additional files].
References
Shatzl T, Kaiser L, Deigner HP (2021) Facioscapulohumeral muscular dystrophy: genetics, gene activation and downstream signalling with regard to recent therapeutic approaches: an update. Orphanet J Rare Dis 16(1):129
Lemmers RJLF, van der Vliet PJ, Klooster R, Sacconi S, Camaño P, Dauwerse JG et al (2010) A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 329:1650–1653
Statland JM, Tawil R (2014) Risk of functional impairment in facioscapulohumeral muscular dystrophy. Muscle Nerve 49:520–527
Tawil R (2018) Facioscapulohumeral muscular dystrophy. Handb Clin Neurol 148:541–548
Leung DG (2018) Magnetic resonance imaging in facioscapulohumeral muscular dystrophy. Muscle Nerve 57(6):872
Voermans NC, Vriens-Munoz Bravo M, Padberg GW, Laforêt P, FSHD European Trial Network workshop study group, van Alfen N, Attarian S et al (2021) 1st FSHD European Trial Network workshop: working towards trial readiness across Europe. Neuromuscul Disord 31(9):907–918
Warman-Chardon J, Diaz-Manera J, Tasca G, Straub V (2020) “247th ENMC International Workshop: muscle magnetic resonance imaging—implementing muscle MRI as a diagnostic tool for rare genetic myopathy cohorts”. Hoofddorp, The Netherlands, September 2019. Neuromuscul Disord 30(11):938–947
Dahlqvist JR, Andersen G, Khawajazada T, Vissing C, Thomsen C, Vissing J (2019) Relationship between muscle inflammation and fat replacement assessed by MRI in facioscapulohumeral muscular dystrophy. J Neurol 266(5):1127–1135
Monforte M, Laschena F, Ottaviani P, Bagnato MR, Pichiecchio A, Tasca G et al (2019) Tracking muscle wasting and disease activity in facioscapulohumeral muscular dystrophy by qualitative longitudinal imaging. J Cachexia Sarcopenia Muscle 10(6):1258–1265
Tasca G, Pescatori M, Monforte M, Mirabella M, Iannaccone E, Frusciante R et al (2012) Different molecular signatures in magnetic resonance imaging-staged facioscapulohumeral muscular dystrophy muscles. PLoS ONE 7(6):e38779
Tawil R, Padberg GW, Shaw DW, van der Maarel SM, Tapscott SJ (2016) Clinical trial preparedness in facioscapulohumeral muscular dystrophy: clinical, tissue, and imaging outcome measures. Neuromuscul Disord 26(2):181–186
Wang LH, Friedman SD, Shaw D, Snider L, Wong CJ, Budech CB et al (2019) MRI-informed muscle biopsies correlate MRI with pathology and DUX4 target gene expression in FSHD. Hum Mol Genet 28(3):476–486
Wong CJ, Wang LH, Friedman SD, Shaw D, Campbell AE, Budech CB et al (2020) Longitudinal measures of RNA expression and disease activity in FSHD muscle biopsies. Hum Mol Genet 29(6):1030–1043
Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S (2011) Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124(Pt 21):3654–3664
Farup J, Madaro L, Puri PL, Mikkelsen UR (2015) Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis 6:e1830–e1913
Molina T, Fabre P, Dumont NA (2021) Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases. Open Biol 11(12):210110
Theret M, Rossi FMV, Contreras O (2021) Evolving roles of muscle-resident fibro-adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Front Physiol 12:673404
Arahata K, Ishihara T, Fukunaga H, Orimo S, Lee JH, Goto K et al (1995) Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): immunocytochemical and genetic analyses. Muscle Nerve Suppl 2:S56-66
Frisullo G, Frusciante R, Nociti R, Tasca G, Renna R, Iorio R et al (2011) CD8+ T cells in facioscapulohumeral muscular dystrophy patients with inflammatory features at muscle MRI. J Clin Immunol 31(2):155–166
Statland JM, Shah B, Henderson D, Van Der Maarel S, Tapscott SJ, Tawil R (2015) Muscle pathology grade for facioscapulohumeral muscular dystrophy biopsies. Muscle Nerve 52(4):521–526
Di Pietro L, Giacalone F, Ragozzino E, Saccone V, Tiberio F, De Bardi M et al (2022) Non-myogenic mesenchymal cells contribute to muscle degeneration in facioscapulohumeral muscular dystrophy type 1 patients. Cell Death Dis 13(9):793
Bosnakovski D, Shams AS, Yuan C, da Silva MT, Ener ET, Baumann CW, Lindsay AJ et al (2020) Transcriptional and cytopathological hallmarks of FSHD in chronic DUX4-expressing mice. J Clin Invest 130(5):2465–2477
Statland JM, Odrzywolski KJ, Shah B, Henderson D, Fricke AF, van der Maarel SM, Tapscott SJ, Tawil R (2015) Immunohistochemical characterization of facioscapulohumeral muscular dystrophy muscle biopsies. J Neuromuscul Dis 2(3):291–299
Tasca G, Monforte M, Ottaviani P, Pelliccioni M, Frusciante R, Laschena F et al (2016) Magnetic Resonance Imaging in a large cohort of facioscapulohumeral muscular dystrophy patients: pattern refinement and implications for clinical trials. Ann Neurol 79(5):854–864
Ricci E, Galluzzi G, Deidda G, Cacurri S, Colantoni L, Merico B et al (1999) Progress in the molecular diagnosis of facioscapulohumeral muscular dystrophy and correlation between the number of KpnI repeats at the 4q35 locus and clinical phenotype. Ann Neurol 45(6):751–757
Patel H, Syddall HE, Martin HJ, Cooper C, Stewart C, Sayer AA (2011) The feasibility and acceptability of muscle biopsy in epidemiological studies: findings from the Hertfordshire Sarcopenia Study (HSS). J Nutr Health Aging 15:10–15
Fischer D, Kley RA, Strach K, Meyer C, Sommer T, Eger K et al (2008) Distinct muscle imaging patterns in myofibrillar myopathies. Neurology 71(10):758–765
Mahdy MAA (2019) Skeletal muscle fibrosis: an overview. Cell Tissue Res 375(3):575–588
Duance VC, Stephens HR, Dunn M, Bailey AJ, Dubowitz V (1980) A role for collagen in the pathogenesis of muscular dystrophy? Nature 284(5755):470–472
Bell CD, Conen PE (1968) Histopathological changes in Duchenne muscular dystrophy. J Neurol Sci 7(3):529–544
Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C (2009) Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol 68(7):762–773
Bosnakovski D, Chan SSK, Recht OO, Hartweck LM, Gustafson CJ, Athman LL et al (2017) Muscle pathology from stochastic low level DUX4 expression in an FSHD mouse model. Nat Commun 8(1):550
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214(2):199–210
Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18(7):1028–40
Madaro L, Bouché M (2014) From innate to adaptive immune response in muscular dystrophies and skeletal muscle regeneration: the role of lymphocytes. Biomed Res Int 2014:438675
Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P (2011) Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1(1):21
Smith LR, Barton ER (2018) Regulation of fibrosis in muscular dystrophy. Matrix Biol 68–69:602–615
Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D’Antona G, Gavina M, Ottoboni L, Constantin G, Bottinelli R, Torrente Y (2007) T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol 213(2):229–238
Becker N, Moore SA, Jones KA (2022) The inflammatory pathology of dysferlinopathy is distinct from calpainopathy, Becker muscular dystrophy, and inflammatory myopathies. Acta Neuropathol Commun 10(1):17
Vattemi G, Mirabella M, Guglielmi V, Lucchini M, Tomelleri G, Ghirardello A et al (2014) Muscle biopsy features of idiopathic inflammatory myopathies and differential diagnosis. Auto Immun Highlights 5(3):77–85
Rayavarapu S, Coley W, Kinder TB, Nagaraju K (2013) Idiopathic inflammatory myopathies: pathogenic mechanisms of muscle weakness. Skelet Muscle 3(1):13
Argyriou A, Horuluoglu B, Galindo-Feria AS, Diaz-Boada JS, Sijbranda M, Notarnicola A et al (2023) Single-cell profiling of muscle-infiltrating T cells in idiopathic inflammatory myopathies. EMBO Mol Med. 31:e17240
McIntyre D, Zuckerman NS, Field M, Mehr R, Stott DI (2014) The V(H) repertoire and clonal diversification of B cells in inflammatory myopathies. Eur J Immunol 44(2):585–596
Spuler S, Engel AG (1998) Unexpected sarcolemmal complement membrane attack complex deposits on nonnecrotic muscle fibers in muscular dystrophies. Neurology 50(1):41–46
Monforte M, Attarian S, Vissing J, Diaz-Manera J, Tasca G (2023) “265th ENMC workshop participants. 265th ENMC International Workshop: muscle imaging in facioscapulohumeral muscular dystrophy (FSHD): relevance for clinical trials”. 22–24 April 2022, Hoofddorp, The Netherlands. Neuromuscul Disord 33(1):65–75
Mellion ML, Ronco L, Berends CL, Pagan L, Brooks S, van Esdonk MJ et al (2021) Phase 1 clinical trial of losmapimod in facioscapulohumeral dystrophy: safety, tolerability, pharmacokinetics, and target engagement. Br J Clin Pharmacol 87(12):4658–4669
Acknowledgements
The authors acknowledge Antonietta Silini for her scientific contribution and editing, Manuela Papacci and Andrea Sabino for their help with sample preparation, Paola Rostagno for scheduling medical appointment and are extremely grateful to the FSHD Italia Onlus association, the patients and their families for the support provided to the research activities.
Funding
E. Ragozzino was supported by a 4-year fellowship by the FSHD Italia Onlus association. Financial support was also received from the Italian Ministry of Health (GR-2018-12366350).
Author information
Authors and Affiliations
Contributions
ERagozzino, GT conceptualisation and design. ERagozzino acquisition, analysis and interpretation of data, manuscript writing. SB, MM, GT and ER enrollment of patients, specimen collection and clinical investigation. LDP, AP, OP, ER data analysis and manuscript editing. GT and ER funding acquisition. ER supervision and final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All protocols were conducted according to the European Good Clinical Practice guidelines after the approval by the Ethical Committee of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS (protocol ID 1524), and upon obtaining the written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Informative data of healthy volunteers, FSHD patients and muscle biopsies. M: male, F: female, CSS: clinical severity score, /: not assessed. Changes in T1w sequences were assessed at 1- and 2-year MRI follow-up. NO means no changes, YES means an increase in fatty replacement on T1 MRI signal. Immune cell infiltrates (CD4, CD8, CD20, BDCA1) and total count of immune cell infiltrates reported as positive cells/mm2.
Additional file 2: Fig. S1.
Evaluation of endomysial and perivascular immune infiltrates in healthy and FSHD muscles: The graphs show the endomysial and perivascular distribution of CD4+ cells/mm2 (A) in healthy (n = 6), FSHD STIR− (n = 16) and FSHD STIR+ (n = 15) muscles, CD8+ cells/mm2 (B) in healthy (n = 6), FSHD STIR− (n = 16) and FSHD STIR+ (n = 15) muscles, CD20+ cells/mm2 (C) in healthy (n = 6), FSHD STIR− (n = 14) and FSHD STIR+ (n = 15) muscles and BDCA1+ cells/mm2 (D) in healthy (n = 6), FSHD STIR− (n = 17) and FSHD STIR+ (n = 15) muscles. The results are reported as mean ± SD. Groups were compared using Kruskal–Wallis test (§ <0.05, §§ <0.01, §§§p < 0.001,) followed by Dunn’s multiple comparison test (*p < 0.05, **p < 0.01). Comparison between two groups were done using Mann-Whitney test (#p < 0.05, ##p < 0.01).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ragozzino, E., Bortolani, S., Di Pietro, L. et al. Muscle fibrosis as a prognostic biomarker in facioscapulohumeral muscular dystrophy: a retrospective cohort study. acta neuropathol commun 11, 165 (2023). https://doi.org/10.1186/s40478-023-01660-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-023-01660-4