Abstract
Background
In clinical practice, thyroid tumor size plays a critical role in the staging of thyroid malignancies and in the selection of nodules that should undergo ultrasound-guided fine-needle aspiration. Thyroid tumor size is influenced by the elapsed time since the beginning of oncogenesis and by the presence of somatic mutations driving growth, such as BRAFV600E mutations, associated with aggressive phenotypes, and RAS-like mutations, associated with more indolent behavior. Although large nodules are often considered to be more alarming, the true impact of tumor size on prognosis remains controversial. The aim of this study was to assess the relationship between mutational status, tumor size and aggressiveness, with emphasis on BRAFV600E and RAS-like mutations.
Method
We conducted a multicentric retrospective chart review in Montréal, Canada, of all patients who underwent thyroid surgery between January 2016 and December 2020, with well-differentiated thyroid cancer on final pathology, and who had undergone molecular testing revealing the presence of BRAFV600E mutations or RAS-like mutations (NRAS, HRAS or KRAS).
Results
We included 214 cases. There were 117 (54.7%) cases of BRAFV600E and 97 (45.3%) cases of RAS-like mutations. The BRAFV600E group was statistically associated with a smaller mean tumor size when compared with the RAS group of 1.55 cm and 2.04 cm, respectively. In a multivariate model, tumors with BRAFV600E mutations were also more likely to display aggressive pathological features, including extra-thyroidal extension, lymph node metastasis, columnar cell features, tall cell histology, or hobnail histology (OR 26.69; 95% CI 11.15–70.81). In contrast, tumor size was not associated with pathologic aggressive features on multivariate analysis (OR 0.81; 95% CI 0.54–1.22).
Conclusion
This study demonstrates that thyroid tumors expressing BRAFV600E mutations correlate with aggressive pathologic features more than tumors expressing RAS-like mutations. When comparing tumors with BRAFV600E and RAS-like mutations, the former were found to be smaller. As a result of this finding, this study suggests that molecular alterations may better predict aggressive pathologic features than the size of the tumor.
Graphical abstract

Similar content being viewed by others
Introduction
Thyroid cancer is the most prevalent endocrine neoplasm worldwide [1], with papillary thyroid carcinoma being the most common subtype [2]. Thyroid ultrasound is commonly used as an initial diagnostic tool for thyroid nodules. Different sonographic features such as shape, echogenicity, margins, the presence of microcalcifications and size are used in many risk-stratification systems [3]. The Thyroid Imaging Reporting and Data System (TI-RADS) risk stratification tool recommends performing an ultrasound-guided fine-needle aspiration (USFNA) on mildly (TR3), moderately (TR4) and highly (TR5) suspicious nodules that are respectively larger than 2.5 cm, 1.5 cm, and 1 cm [4]. The Bethesda classification system is subsequently used to classify the cytology of the biopsied nodules according to their risk of malignancy [5].
After USFNA of thyroid nodules, cytologic diagnosis remains indeterminate (Bethesda Category III, IV, and V) in about 20–25% of cases [6,7,8,9]. For this reason, molecular testing has received extensive attention for its ability to improve risk stratification of indeterminate thyroid nodules [10], and avoid unnecessary diagnostic surgery [9]. Two of the most important and common mutations found in thyroid cancer are BRAFV600E mutations and RAS-like mutations, which involve a family of three highly homologous isoforms (NRAS, KRAS and HRAS). The BRAFV600E mutation is associated with more aggressive tumor behavior with papillary architecture, tall cell features, invasiveness, and frequent nodal metastasis [11,12,13,14]. On the other hand, RAS-like mutations are more commonly associated with indolent behavior, follicular growth, encapsulation, and a lower incidence of nodal metastasis [6].
In clinical practice, thyroid tumor size has been considered an important prognostic marker and plays a critical role in the selection of nodules that should undergo USFNA and the staging of thyroid malignancies [9, 15]. Although large nodules are often considered more alarming, the true impact of tumor size on prognosis remains controversial. Current data on the association between tumor size and prognosis is heterogeneous [16,17,18,19,20,21]. The impact of BRAFV600E and RAS-like mutations on tumor size is also controversial, with some studies reporting smaller tumors when RAS mutations are present, and others reporting the contrary [22].
The aim of this study was to determine the impact of mutational status, specifically BRAFV600E and RAS-like mutations, on tumor size. The second aim of this study was to compare the association between mutational status, size and pathological aggressiveness.
Materials and methods
Study design
A retrospective chart review was performed at the Jewish General Hospital (JGH) and McGill University Health Center (MUHC) in Montréal, Québec, Canada. Patients’ sociodemographic characteristics and oncologic characteristics (pre-operative cytology results, molecular testing results and final pathology results) were recorded. Ethics approval was obtained from both the McGill University Health Centre and CIUSSS West-Central Research Ethics Board in Montréal, QC, Canada (MP-37-2021-7517).
Patient selection
Patients were included in the study if they had undergone thyroid surgery at the JGH or MUHC between January 2016 and December 2020, had a confirmed diagnosis of well-differentiated thyroid malignancy on final pathology, and had undergone molecular testing revealing the presence of BRAFV600E mutations or RAS-like mutations (NRAS, HRAS or KRAS). Overall, 3 patients underwent Afirma™ testing, 157 underwent ThyGenext™ testing and 54 underwent ThyroseqV3™ testing. All these tests were shown to have equivalent diagnostic accuracies in previous studies [23]. Patients with other molecular mutations or with ≥ 2 concomitant mutations identified on molecular testing were excluded. The flow diagram of the patient selection process is provided in Additional file 1. Data collection results are provided in Additional file 2.
Sample collection
After obtaining informed consent, specimen collection for molecular testing was performed with USFNA. The specimen was handled as per the company’s specific requirements and then sent to the appropriate lab for analysis.
Pathology
Tumors were considered to be aggressive if they exhibited at least one of the following features on final postoperative pathology: extra-thyroidal extension (ETE), lymph node metastasis (LN+), or worrisome features/variants on pathology (columnar cell, tall cell or hobnail). All positive lymph nodes were incidental nodes seen as part of a prophylactic dissection, and micrometastases were excluded. An experienced thyroid pathologist reported on the final surgical specimens, highlighting the presence of any of the aforementioned features. Thyroid nodules were considered to exhibit tall cell or hobnail histology when > 30% of the tumor cells demonstrated tall cell or hobnail features. This cut-off was chosen based on the morphologic criteria reported in the 2017 WHO Classification of Tumors of Endocrine Organs [24, 25].
Data analysis
The data was stratified into two groups according to the results of molecular testing (BRAFV600E mutations group and RAS mutations group). Univariate analysis (Chi-square’s test) was used to compare Bethesda score distribution among the two groups. A threshold of P < 0.05 was determined for statistical significance. Simple logistic regression was performed to study the association of variables such as age, sex, McGill Thyroid Nodule Score (MTNS), pathological size and mutational status with aggressive features. The MTNS is a combined scoring system, used as a predictor for thyroid carcinoma, given clinical, radiological, and pathological findings of a certain nodule [26]. Subsequently, with the help of a multivariate model, we determined the association between aggressive pathological features and the following variables: age, sex, McGill Thyroid Nodule Score (MTNS), pathological size and mutational status. The fit and accuracy of the multivariate model was examined using the Hosmer–Lemeshow test. The association between pathological size and mutational status was assessed with a simple logistic analysis. All tests were performed using R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 1651 thyroid surgeries were performed in the targeted timeframe. Among them, 344 underwent molecular testing prior to their surgery and obtained positive results for the presence of suspicious molecular mutations. There were 130 cases excluded due to inconclusive final pathology (benign lesions or non-invasive follicular thyroid neoplasm with papillary like nuclear features) or due to the absence of isolated BRAFV600E or RAS-like mutations on molecular testing. In total, 214 cases with either BRAFV600E mutations or RAS-like mutations on pre-operative molecular testing were included in the study (Additional file 1: Fig. S1).
Baseline characteristics
All the clinicopathological information available on medical records were analyzed. Baseline information including gender, age and the Bethesda score reflecting the USFNA biopsy results were collected for the 214 patients included in this study. There were 117 (54.7%) patients with BRAFV600E mutation and 97 (45.3%) patients with RAS-like mutations (including KRAS, NRAS and HRAS). The mean age in the BRAFV600E group was 45.7 years (SD 13.5) and 49.52 years (SD 13.6) in the RAS group. 22.2% of patients in the BRAFV600E group were male compared to 25.0% in the RAS group. The distribution of Bethesda scores among both groups was statistically different. The majority of tumors with a BRAFV600E mutation had higher Bethesda categories (5.1% category III, 0.9% category IV, 19.7% category V and 74.4% category VI), whereas most tumors harboring RAS mutations had lower Bethesda scores (34.0% category III, 39.2% category IV, 17.5% category V and 7.2% category VI; P < 0.0001). In the BRAF group all tumors were papillary thyroid cancers (PTC). In the RAS group, 95.9% of the cases were PTCs (93/97), 2.1% were follicular cell carcinomas (2/97) and 2.1% were Hurthle cell carcinomas (2/97) (Table 1).
Aggressive features and pathological size
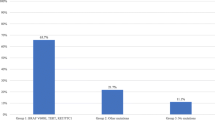
In patients with BRAFV600E mutations, 76.1% (89/117) had at least one aggressive feature on final pathology report. 56.4% (66/117) of tumors in the BRAFV600E mutation group had lymph node metastasis, 27.4% (32/117) had extra-thyroidal extension, 35.0% (41/117) were tall cell variants, 1.7% (2/117) exhibited columnar histology, and 8.5% (10/117) exhibited hobnail histology. In contrast, only 9.3% (9/97) of patients in RAS-like mutation group had aggressive features: lymph node metastasis (8.2%, 8/97) and extra-thyroidal extension (1.0%, 1/97). Importantly, no patients with RAS-like mutation were tall cell, columnar or hobnail variants (Table 1 and Fig. 1). The number of aggressive features in each tumor was also compared. 45/117 (38.5%) of tumors with BRAFV600E mutation had more than one aggressive feature. In comparison, in the RAS group, all aggressive tumors had only one aggressive feature (Table 1 and Fig. 2). Overall, BRAFV600E tumors were found to have qualitatively and quantitatively more aggressive features than RAS-like tumors. The BRAFV600E mutation group was significantly associated with smaller tumors. The BRAFV600E group was associated with a mean tumor size of 1.55 cm (SD 0.84), compared to the RAS group that was associated with a mean tumor size of 2.04 cm (SD 0.31) (Table 1).
Logistic regression (aggressiveness)
On simple logistic regression, the presence of BRAFV600E mutation (OR 31.08, 95% CI 14.51–73.78) and MTNS (OR 1.24, 95% CI 1.14–1.36) were associated with pathological aggressiveness. However, pathological size (OR 0.81, 95% CI 0.61–1.08), age (OR 0.98, 95%CI 0.96–1.00) and gender (OR 0.82, 95% CI 0.43–1.55) were not significantly associated with pathological aggressiveness. On multivariate analysis, mutational status was once again a significant predictor of aggressive pathological features (OR 26.69, 95% CI 11.15–70.81). In contrast, the association between pathological size and tumor aggressiveness was statistically insignificant (OR 1.00, 95% CI 0.68–1.49) (Table 2).
Logistic regression (pathological size)
The association between mutational status and pathological size was assessed via two different methods: 1-A simple logistic regression with pathological size as an independent continuous variable, and in a simple logistic regression model with pathological size as an independent categorical variable (separated in 4 subgroups: 0–1.00 cm, 1.01–1.50 cm, 1.51–2.00 cm and > 2.01 cm. In the first method, for every additional 1.00 cm increase in size, the odds of presence of a BRAFV600E mutation decreased by 0.61 (95% CI 0.45–0.82). In the second method, we can see an increase in the OR for decreasing size categories (Table 3).
Discussion
Substantial developments in clinical translational research have occurred in the past 5 years involving thyroid cancer. Molecular markers, such as BRAFV600E and RAS mutations are outstanding examples in how a prognostic genetic marker can improve risk stratification and hence tailored management of patients with thyroid cancer, including those with conventionally low risks. The primary aim of this study was to determine the impact of mutational status, specifically BRAFV600E and RAS-like mutations, on tumor size. It was found that BRAFV600E-positive tumors were smaller in size, when compared with RAS-like positive tumors. The second aim of this study was to compare the association between mutational status, size and pathological aggressiveness. BRAFV600E-positive tumors were more often presenting with aggressive features, such as nodal disease, extra-thyroidal extension and aggressive histological features/variants than RAS-like positive tumors. Although 0.49 cm is a clinically modest size difference, the multivariate analysis clearly demonstrated the strong correlation of mutational status with pathological aggressiveness, when compared to tumor size. In this model, the elevated odds ratio (26.69) and the wide confidence interval (11.15–70.81) could be explained by the wide gene expression variation noted across tumors with BRAFV600E-like mutations, in the literature. In a study published by The Cancer Genome Atlas Research Network (TGCA) in 2014, when comparing the BRAFV600E-like group with the RAS-like group, the former was overall predominantly less-differentiated, however their data also indicated that BRAFV600E-like tumors represent a diverse group with at least four major molecular subtypes with variable degrees of histological differentiation [27]. Therefore, despite the overall tendencies of BRAFV600E-like tumors to display more aggressive features, the wide diversity of genomic expression may account for the range of differences observed in our statistical analysis and the uncertainty regarding the prognostic and predictive power of BRAFV600E mutations in the literature [28].
Previous studies examining the impact of BRAFV600E and RAS-like mutations on thyroid cancer size have shown inconsistent results. Similarly, to the present study, a study by Kakarmath et al. reported a smaller average size of BRAFV600E-positive tumors when compared with tumors exhibiting RAS-like mutations, of 1.8 cm and size 2.5 cm, respectively [29, 30]. On the other hand, Al-Salam et al. found that thyroid tumors exhibiting BRAFV600E mutations were associated with an increased size in a cohort of 90 adult patients in the United Arab Emirates [22]. A meta-analysis from Lee et al. found that the mean size of tumors exhibiting BRAF mutations ranged from 2.3 to 2.9 cm, whereas, in the absence of BRAF mutations, the mean size of tumors ranged from 1.8 to 2.7 cm [31]. Other studies were not able to find a statistically significant difference in size between BRAFV600E-associated tumors and RAS-associated tumors [9, 32, 33]. These discrepancies in the literature may be due to the differences in availability of high-resolution ultrasonography in different centers around the world. For instance, Al-Salam et al. reported that BRAFV600E positive thyroid tumors were associated with a larger tumor size in a cohort from the United Arab Emirates [22]. This was not reflected in the context of the present study, which was conducted in Canada, a country with a publicly funded healthcare system, where patients with thyroid nodules have access to ultrasound without the need to pay. As a result, thyroid nodules may be picked at smaller sizes in a public healthcare system when compared to systems with financial barriers to medical imaging.
Primary tumor diameter has been described as a predictor of poor oncological outcomes in differentiated thyroid cancer [34]. Previous research has shown that larger thyroid tumor size is associated with an increased risk of recurrence, ETE, bilaterality, vascular invasion, lymph node metastases and distant metastases [35, 36]. For this reason, tumor size has traditionally been a key element in the staging, prognosis and management of thyroid tumors, with larger tumors often being considered more alarming than smaller ones. Moreover, thyroid tumor size plays a critical role in the selection of nodules that should undergo ultrasound-guided fine-needle aspiration, as suggested by many professional guidelines and risk stratification systems [37, 38]. Furthermore, treatment planning, including choice of surveillance and the appropriate extent of thyroid surgery (i.e., hemithyroidectomy versus total thyroidectomy and prophylactic central neck dissection) and staging of thyroid cancer often heavily rely on tumor size [37]. However, over the past few years, multiple major studies have suggested a progressive shift towards a new pathological classification of thyroid lesions, accounting for genotypic differences and consideration of individualized management depending on mutational profile [39,40,41]. Our findings indeed support this point of view, in regard to the current role and interpretation of tumor size in the management of thyroid nodules.
These results may also reflect the radiological and histological features of tumors with these mutations. Indeed, BRAFV600E-positive tumors are more commonly associated with suspicious ultrasound findings and lymph node involvement than tumors involving RAS-like mutations [33, 42]. These suspicious ultrasound findings may prompt earlier USFNA and earlier diagnosis of thyroid cancer. Moreover, it is also known that BRAFV600E-positive tumors are more likely to present with a higher Bethesda category on fine needle aspirate cytology, while tumors with RAS-like mutations are more commonly associated with lower Bethesda category [10]. Therefore, immediate surgical intervention is more likely to happen in a BRAFV600E-positive tumor, whereas tumors with RAS mutations are more likely to be managed with surveillance, with surgical removal only occurring in the presence of significant growth.
The results of the present study highlight the important role of molecular testing on predicting pathological aggressiveness, as smaller tumors may very well be more aggressive. These findings may be explained by the Knudson “two-hit hypothesis” [43]. While RAS-like mutations may stimulate clonal growth of follicular thyroid cells, this isolated mutation may be insufficient in itself to bring about the changes required for aggressive tumor behavior. In this case, a second molecular ‘’hit’’, including TERT or p53 mutation, may be required for RAS-positive nodules to acquire these features [29]. Isolated BRAFV600E mutations, on the other hand, may be sufficient to lead to aggressive tumor behavior by themselves. In this study, we excluded thyroid nodules exhibiting > 1 mutation from this study so that the effect of each mutation alone could be better compared.
There are several limitations in this study that should be recognized. First, the age and gender were the only demographic information collected in our database, which limits the accuracy of the multivariate model. Next, this paper was a cross-sectional study that only allowed us to evaluate aggressivity in terms of pathological features. Therefore, further longitudinal studies would be required to assess long-term oncological behavior associated with each of these mutations. Song et al. [44] and Yip et al. [41] displayed, respectively, higher recurrence rates and higher 5-year risk of distant metastasis with isolated BRAFV600E-positive tumors. Also, we chose to compare only patients with documented BRAFV600E and RAS-like mutations, thereby introducing a selection bias. This approach however allows for direct comparison of the clinical effect of these mutations, alone. Given that thyroid molecular testing was not reimbursed by the Quebec’s provincial public medical insurance (Régie de l'assurance maladie du Québec, RAMQ) at the time of data collection, all patients who underwent molecular testing had to cover the cost either via private insurances, at a personal expense or other, which induces a selection bias. Lastly, while the different molecular tests used in this study may have similar diagnostic performances according to the current literature, the lack of uniformity should be considered in the interpretation of these results.
Conclusion
While USFNA has revolutionized thyroid malignancy diagnostics, the elevated rate of indeterminate results complicates clinical decision-making. In the era of molecular testing, the additional information gained can further inform on tumor behavior. In this study, we found that the BRAFV600E mutation was associated with more aggressive pathological features than RAS-like mutations. Interestingly the mean tumor size of the BRAFV600E group was smaller. This apparent discrepancy may be explained by the fact that BRAFV600E positive nodules often show aggressive sonographic features and a higher Bethesda category on USFNA, thereby prompting more diligent work-up. Therefore, according to this study, size in isolation is not as good a surrogate marker for pathological aggressiveness, as previously thought. Mutational status, on the other hand, is a strong predictor of pathological aggressiveness and can thus be useful as a prognostic factor in combination with other clinical, pathological or radiological findings. Further long-term studies are required to solidify the impact of mutational status on other prognostic factors such as rates of recurrence and disease-free survival.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- TI-RADS:
-
Thyroid imaging reporting and data system
- USFNA:
-
Ultrasound-guided fine-needle aspiration
- JGH:
-
Jewish General Hospital
- MUHC:
-
McGill University Health Center
- ETE:
-
Extra-thyroidal extension
- LN + :
-
Lymph node metastasis
- MTNS:
-
McGill thyroid nodule score
References
Pacini F, Castagna MG. Approach to and treatment of differentiated thyroid carcinoma. Med Clin North Am. 2012;96(2):369–83.
Abubaker J, Jehan Z, Bavi P, Sultana M, Al-Harbi S, Ibrahim M, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93(2):611–8.
Shen Y, Liu M, He J, Wu S, Chen M, Wan Y, et al. Comparison of different risk-stratification systems for the diagnosis of benign and malignant thyroid nodules. Front Oncol. 2019;9:378.
Russ G, Bigorgne C, Royer B, Rouxel A, Bienvenu-Perrard M. The thyroid imaging reporting and data system (TIRADS) for ultrasound of the thyroid. J Radiol. 2011;92(7–8):701–13.
Melo-Uribe MA, Sanabria Á, Romero-Rojas A, Pérez G, Vargas EJ, Abaúnza MC, et al. The Bethesda system for reporting thyroid cytopathology in Colombia: correlation with histopathological diagnoses in oncology and non-oncology institutions. J Cytol. 2015;32(1):12–6.
Patel SG, Carty SE, McCoy KL, Ohori NP, LeBeau SO, Seethala RR, et al. Preoperative detection of RAS mutation may guide extent of thyroidectomy. Surgery. 2017;161(1):168–75.
Sosa JA, Hanna JW, Robinson KA, Lanman RB. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery. 2013;154(6):1420–6.
Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–6.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
Marco Mascarella MP, Veronique-Isabelle Forest, et al. Association of Bethesda category and molecular mutation in patients undergoing thyroidectomy. Authora. March 21, 2021.
Chen Y, Sadow PM, Suh H, Lee KE, Choi JY, Suh YJ, et al. BRAF(V600E) is correlated with recurrence of papillary thyroid microcarcinoma: a systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid. 2016;26(2):248–55.
Elisei R, Viola D, Torregrossa L, Giannini R, Romei C, Ugolini C, et al. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012;97(12):4390–8.
Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab. 2012;97(12):4559–70.
Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–99.
Wong RM, Bresee C, Braunstein GD. Comparison with published systems of a new staging system for papillary and follicular thyroid carcinoma. Thyroid. 2013;23(5):566–74.
Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–28.
Pelizzo MR, Boschin IM, Toniato A, Piotto A, Pagetta C, Gross MD, et al. Papillary thyroid carcinoma: 35-year outcome and prognostic factors in 1858 patients. Clin Nucl Med. 2007;32(6):440–4.
Schindler AM, van Melle G, Evequoz B, Scazziga B. Prognostic factors in papillary carcinoma of the thyroid. Cancer. 1991;68(2):324–30.
Simpson WJ, McKinney SE, Carruthers JS, Gospodarowicz MK, Sutcliffe SB, Panzarella T. Papillary and follicular thyroid cancer. Prognostic factors in 1578 patients. Am J Med. 1987;83(3):479–88.
Shah JP, Loree TR, Dharker D, Strong EW, Begg C, Vlamis V. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164(6):658–61.
Shaha AR, Shah JP, Loree TR. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol. 1996;3(6):534–8.
Al-Salam S, Sharma C, Afandi B, Al Dahmani K, Al-Zahrani AS, Al Shamsi A, et al. BRAF and KRAS mutations in papillary thyroid carcinoma in the United Arab Emirates. PLOS ONE. 2020;15(4):e0231341.
Partyka KL, Trevino K, Randolph ML, Cramer H, Wu HH. Risk of malignancy and neoplasia predicted by three molecular testing platforms in indeterminate thyroid nodules on fine-needle aspiration. Diagn Cytopathol. 2019;47(9):853–62.
Inoshita N, Nishioka H. The 2017 WHO classification of pituitary adenoma: overview and comments. Brain Tumor Pathol. 2018;35(2):51–6.
Lopes MBS. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134(4):521–35.
Sands NB, Karls S, Amir A, Tamilia M, Gologan O, Rochon L, et al. McGill thyroid nodule score (MTNS): “rating the risk,” a novel predictive scheme for cancer risk determination. J Otolaryngol Head Neck Surg. 2011;40(Suppl 1):S1-13.
Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159(3):676–90.
Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14):1493–501.
Kakarmath S, Heller HT, Alexander CA, Cibas ES, Krane JF, Barletta JA, et al. Clinical, Sonographic, and pathological characteristics of RAS-positive versus BRAF-positive thyroid carcinoma. J Clin Endocrinol Metab. 2016;101(12):4938–44.
Changjiao Y, Meiling H, Xin L, Ting W, Rui L. Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocr Connect. 2019;8(7):988–96.
Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110(1):38–46.
Ming J, Liu Z, Zeng W, Maimaiti Y, Guo Y, Nie X, et al. Association between BRAF and RAS mutations, and RET rearrangements and the clinical features of papillary thyroid cancer. Int J Clin Exp Pathol. 2015;8(11):15155–62.
Krasner JR, Alyouha N, Pusztaszeri M, Forest V-I, Hier MP, Avior G, et al. Molecular mutations as a possible factor for determining extent of thyroid surgery. J Otolaryngol Head Neck Surg. 2019;48(1):1–7.
Edge SBMD, Compton CCMDP. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Tran B, Roshan D, Abraham E, Wang L, Garibotto N, Wykes J, et al. The prognostic impact of Tumor size in papillary thyroid carcinoma is modified by age. Thyroid. 2018;28(8):991–6.
Zhang TT, Li CF, Wen SS, Huang DZ, Sun GH, Zhu YX, et al. Effects of tumor size on prognosis in differentiated thyroid carcinoma smaller than 2 cm. Oncol Lett. 2019;17(5):4229–36.
Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103(11):2269–73.
Nguyen XV, Roy Choudhury K, Tessler FN, Hoang JK. Effect of tumor size on risk of metastatic disease and survival for thyroid cancer: implications for biopsy guidelines. Thyroid. 2018;28(3):295–300.
Bible KC, Ryder M. Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat Rev Clin Oncol. 2016;13(7):403–16.
Chin PD, Zhu CY, Sajed DP, Fishbein GA, Yeh MW, Leung AM, et al. Correlation of thyroseq results with surgical histopathology in cytologically indeterminate thyroid nodules. Endocr Pathol. 2020;31(4):377–84.
Yip L, Gooding WE, Nikitski A, Wald AI, Carty SE, Karslioglu-French E, et al. Risk assessment for distant metastasis in differentiated thyroid cancer using molecular profiling: a matched case-control study. Cancer. 2021;127(11):1779–87.
Kabaker AS, Tublin ME, Nikiforov YE, Armstrong MJ, Hodak SP, Stang MT, et al. Suspicious ultrasound characteristics predict BRAF V600E-positive papillary thyroid carcinoma. Thyroid. 2012;22(6):585–9.
Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE, Abeloff MD. Abeloff's clinical oncology. Philadelphia, PA: Elsevier; 2020. Available from: https://www.clinicalkey.com/#!/browse/book/3-s2.0-C20150054004, https://www.sciencedirect.com/science/book/9780323476744.
Song YS, Lim JA, Choi H, Won JK, Moon JH, Cho SW, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer. 2016;122(9):1370–9.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The original idea was conceived, and the study was designed by RP. RP, VF, MH, MP, MT were responsible for creating and completing the database. The original manuscript was created by KS and GM and was reviewed and approved by all authors. AP, SD, VF, MH, MP, MT and RP all made valuable contributions and changes to the manuscript leading to its completion. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study (MP-37-2021-7517) was approved by the Research Ethics Committee at the McGill University Health System and Jewish General Hospital, Montréal, Quebec. All of the research meets the ethics guidelines, including adherence to the legal requirements of Canada.
Consent for publication
Not applicable.
Competing interests
We declare that we have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
. Flow diagram of patient selection process. *NIFTP: Non-invasive follicular thyroid neoplasm with papillary-like nuclear features
Additional file 2
. Database used for analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Semsar-Kazerooni, K., Morand, G.B., Payne, A.E. et al. Mutational status may supersede tumor size in predicting the presence of aggressive pathologic features in well differentiated thyroid cancer. J of Otolaryngol - Head & Neck Surg 51, 9 (2022). https://doi.org/10.1186/s40463-022-00559-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40463-022-00559-9